Abstract
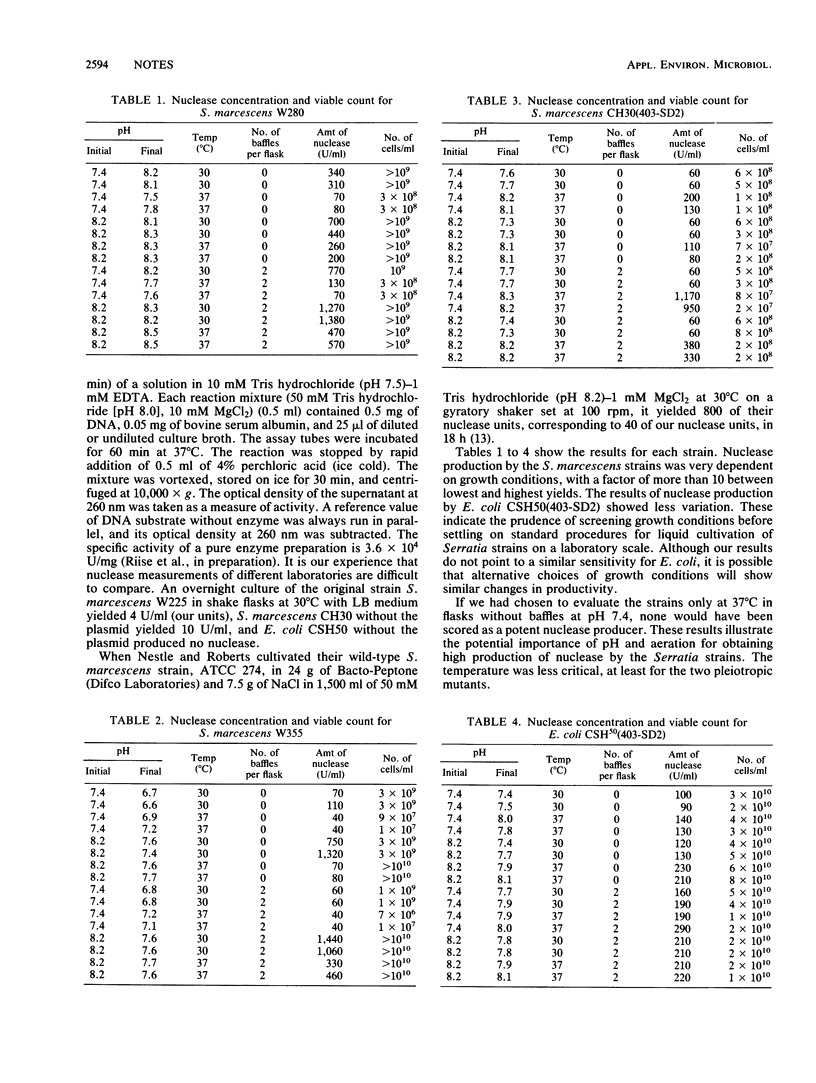
Two high-nuclease-yielding mutants of Serratia marcescens, derived by chemical mutagenesis (W280, W355), and two strains with the pBR322 plasmid 403-SD2, carrying a nuclease gene and a chloramphenicol resistance gene [Escherichia coli CSH50(403-SD2) and S. marcescens CH30(403-SD2)] were investigated for nuclease production in a factorial shake flask experiment, with temperature (30 and 37 degrees C), pH (with or without CaCO3 tablets), and aeration (with or without baffles) as variable conditions. Yields varied 10-fold depending on the conditions investigated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chia W., Scott M. R., Rigby P. W. The construction of cosmid libraries of eukaryotic DNA using the Homer series of vectors. Nucleic Acids Res. 1982 Apr 24;10(8):2503–2520. doi: 10.1093/nar/10.8.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Hohn B. Cosmids: a type of plasmid gene-cloning vector that is packageable in vitro in bacteriophage lambda heads. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4242–4246. doi: 10.1073/pnas.75.9.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORSCHACK R. G., WICKERHAM L. J. Production of extracellular and total invertase by Candida utilis, Saccharomyces cerevisiae, and other yeasts. Appl Microbiol. 1961 Jul;9:291–294. doi: 10.1128/am.9.4.291-294.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAVES G. N., JEFFRIES C. D. EFFECT OF PH ON THE FORMATION OF EXOCELLULAR NUCLEASE IN AGING BROTH CULTURES OF SERRATIA MARCESCENS. J Bacteriol. 1963 Jun;85:1194–1196. doi: 10.1128/jb.85.6.1194-1196.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iusupova D. V., Gareishina A. Z., Beliaeva M. I. Vliianie sostava pitatel'noi sredy na rost i dezoksiribonukleaznuiu aktivnost' pigmentnykh i bespigmentnykh shtammov Serratia marcescens. Prikl Biokhim Mikrobiol. 1972 Nov-Dec;8(6):922–926. [PubMed] [Google Scholar]
- Kovacevic S., Veal L. E., Hsiung H. M., Miller J. R. Secretion of staphylococcal nuclease by Bacillus subtilis. J Bacteriol. 1985 May;162(2):521–528. doi: 10.1128/jb.162.2.521-528.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle M., Roberts W. K. An extracellular nuclease from Serratia marcescens. I. Purification and some properties of the enzyme. J Biol Chem. 1969 Oct 10;244(19):5213–5218. [PubMed] [Google Scholar]
- Takata R., Aoyagi M. Isolation of a Serratia marcescens mutant which is an efficient recipient for the E. coli episome. Mol Gen Genet. 1984;197(3):517–518. doi: 10.1007/BF00329954. [DOI] [PubMed] [Google Scholar]
- Timmis K., Winkler U. Gene dosage studies with pleiotropic mutants of Serratia marcescens superactive in the synthesis of marcescin A and certain other exocellular proteins. Mol Gen Genet. 1973 Aug 17;124(3):207–217. doi: 10.1007/BF00293092. [DOI] [PubMed] [Google Scholar]
- Winkler U., Timmis K. Pleiotropic mutations in Serratia marcescens which increase the synthesis of certain exocellular proteins and the rate of spontaneous prophage induction. Mol Gen Genet. 1973 Aug 17;124(3):197–206. doi: 10.1007/BF00293091. [DOI] [PubMed] [Google Scholar]
- Yonemura K., Matsumoto K., Maeda H. Isolation and characterization of nucleases from a clinical isolate of Serratia marcescens kums 3958. J Biochem. 1983 May;93(5):1287–1295. doi: 10.1093/oxfordjournals.jbchem.a134262. [DOI] [PubMed] [Google Scholar]



