Abstract
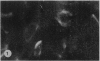
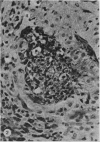
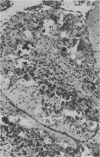
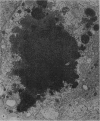
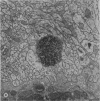
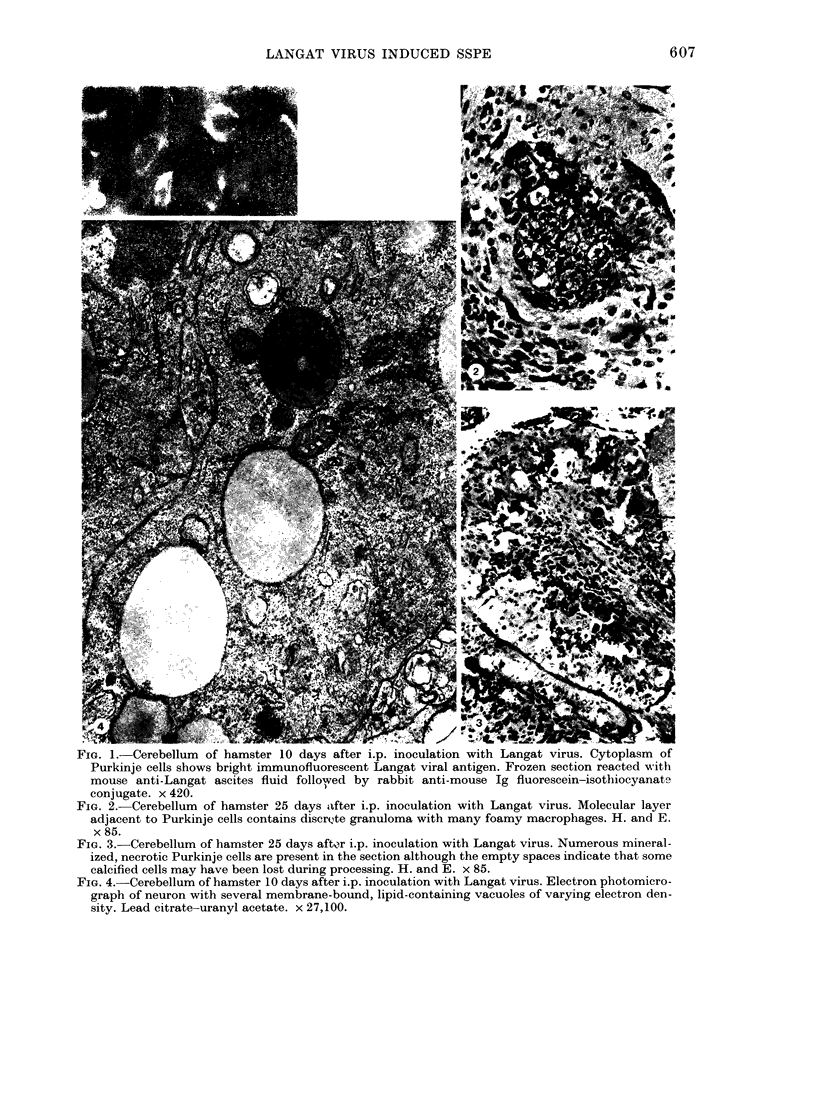
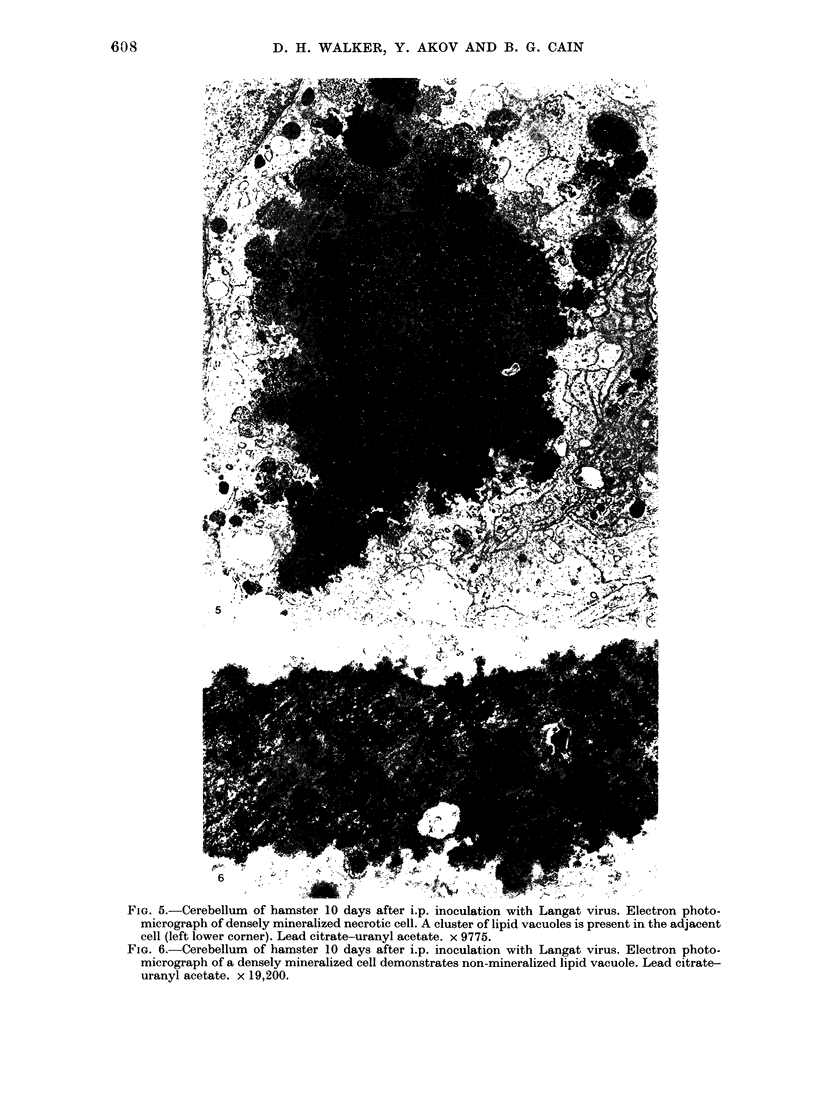
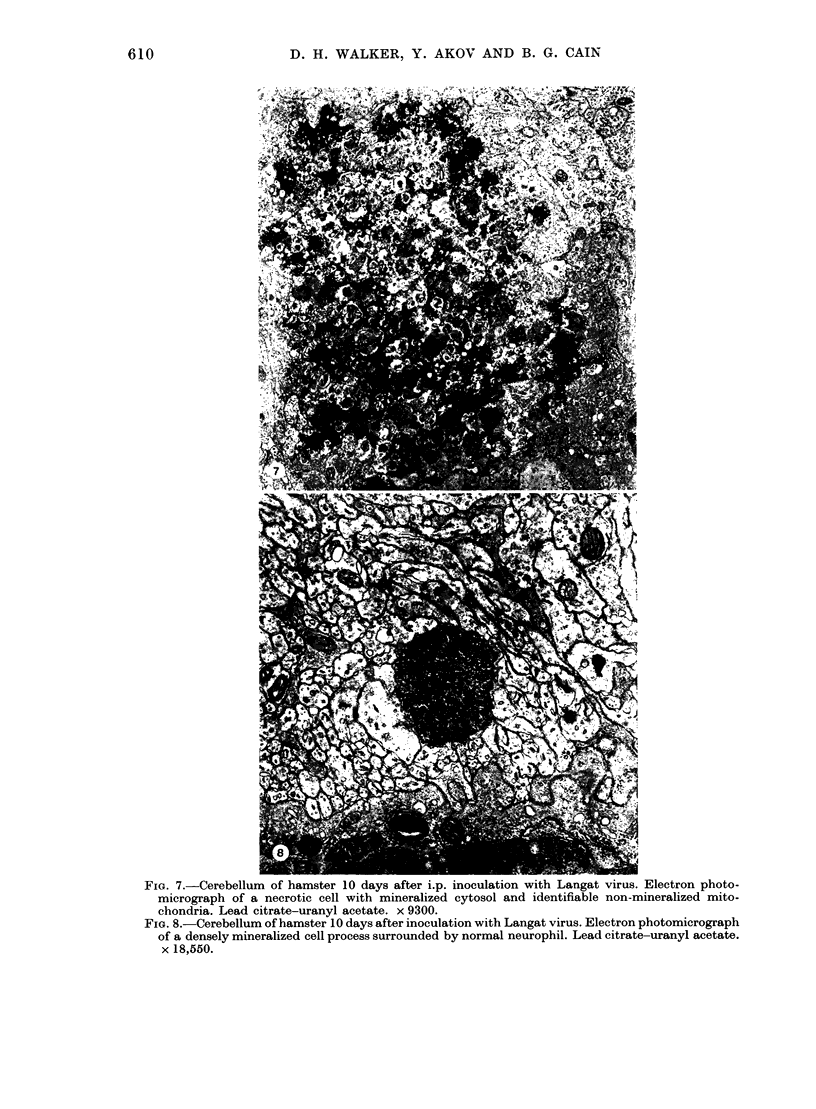
The morphology of subacute sclerosing panencephalitis (SSPE) lesions in hamsters following i.p. inoculation of Langat virus was studied by light microscopy and electron microscopy. The lesions' temporal relationship to virus presence and antibody production was studied by immunofluorescence, virus isolation from brains and brain cell cultures, and by antibody assay of serum and spinal fluid. All 10-day-old hamsters infected with Langat virus developed SSPE lesions, most prominently in the cerebellum, without any overt signs. The lesions appeared 10 days after infection, progressed in severity until Day 21 and persisted unaltered at least 3 months. They consisted of neuronal degeneration, calcification, and intracellular lipid accumulation. Virus was isolated from Days 3 to 15 and disappeared on Day 21. Demonstration by cerebellar immunofluorescence of viral antigen was observed 10 days after inoculation. Antibody appeared in the serum on Day 6, rose to very high titres by Day 21, and thereafter declined. Cell-associated virus was not demonstrable in negative brain-cell cultures. These findings suggest that SSPE in hamsters, associated with Langat virus infection, is biologically different from measles SSPE. Purkinje-cell lipid accumulation and mineralization sparing mitochondria were manifestations of the response of neurons to cell injury at the threshold of irreversibility.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen T. T., Watanabe I., Zeman W., Mealey J., Jr Subacute sclerosing panencephalitis: propagation of measles virus from brain biopsy in tissue culture. Science. 1969 Mar 14;163(3872):1193–1194. doi: 10.1126/science.163.3872.1193. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Viral pathogenesis and molecular biology. Bacteriol Rev. 1977 Dec;41(4):811–821. doi: 10.1128/br.41.4.811-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. T., Johnson K. P., Edmonds C. J. Virus-induced hydrocephalus: development of aqueductal stenosis in hamsters after mumps infection. Science. 1967 Sep 1;157(3792):1066–1067. doi: 10.1126/science.157.3792.1066. [DOI] [PubMed] [Google Scholar]
- Katz M., Oyanagi S., Koprowski H. Subacute sclerosing panencephalitis: structures resembling myxovirus nucleocapsids in cells cultured from brain. Nature. 1969 May 31;222(5196):888–890. doi: 10.1038/222888a0. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- Masters C., Alpers M., Kakulas B. Pathogenesis of reovirus type 1 hydrocephalus in mice. Significance of aqueductal changes. Arch Neurol. 1977 Jan;34(1):18–28. doi: 10.1001/archneur.1977.00500130038008. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Parker J. C., Jr, Klintworth G. K., Graham D. G., Griffith J. F. Uncommon morphologic features in subacute sclerosing panencephalitis (SSPE). Report of two cases with virus recovery from one autopsy brain specimen. Am J Pathol. 1970 Nov;61(2):275–292. [PMC free article] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V., Itabashi H. H. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med. 1969 Sep 11;281(11):585–589. doi: 10.1056/NEJM196909112811103. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Byington D. P., Johnson K. P. Experimental subacute sclerosing panencephalitis in the hamster. Ultrastructure of the chronic disease. Lab Invest. 1974 Oct;31(4):355–368. [PubMed] [Google Scholar]
- SMITH C. E. G. A virus resembling Russian spring-summer encephalitis virus from an ixodid tick in Malaya. Nature. 1956 Sep 15;178(4533):581–582. doi: 10.1038/178581a0. [DOI] [PubMed] [Google Scholar]
- Spector W. G., Heesom N. The production of granulomata by antigen-antibody complexes. J Pathol. 1969 May;98(1):31–39. doi: 10.1002/path.1710980105. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandvik B., Norrby E. Oligoclonal IgG antibody response in the central nervous system to different measles virus antigens in subacute sclerosing panencephalitis. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1060–1063. doi: 10.1073/pnas.70.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil M. L., Itabashi H., Cremer N. E., Oshiro L., Lennette E. H., Carnay L. Chronic progressive panencephalitis due to rubella virus simulating subacute sclerosing panencephalitis. N Engl J Med. 1975 May 8;292(19):994–998. doi: 10.1056/NEJM197505082921903. [DOI] [PubMed] [Google Scholar]
- Zlotnik I., Grant D. P., Carter G. B., Batter-Hatton D. Subacute sclerosing encephalitis in adult hamsters infected with Langat virus. Br J Exp Pathol. 1973 Feb;54(1):29–39. [PMC free article] [PubMed] [Google Scholar]
- Zlotnik I., Grant D. P. Further observations on subacute sclerosing encephalitis in adult hamsters: the effects of intranasal infections with Langat virus, measles virus and SSPE-measles virus. Br J Exp Pathol. 1976 Feb;57(1):49–66. [PMC free article] [PubMed] [Google Scholar]
- Zlotnik I., Grant D. P. The occurrence of vacuolated neurons in the brains of hamsters affected with subacute sclerosing encephalitis following measles or Langat virus infection. Br J Exp Pathol. 1975 Feb;56(1):72–76. [PMC free article] [PubMed] [Google Scholar]
- de Estable-Puig R. F., Estable-Puig J. F. Intraneuronal lipid droplets in irradiated nervous tissue. Virchows Arch B Cell Pathol. 1973 Nov 28;14(2):117–125. doi: 10.1007/BF02889181. [DOI] [PubMed] [Google Scholar]