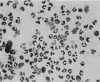
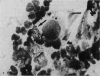Abstract
A strain of Chlamydia trachomatis isolated in McCoy cells from the urethra of a patient suffering from non-gonococcal urethritis was inoculated into the vagina of 8 female marmosets. Chlamydiae were isolated repeatedly for 10-42 days from the lower genital tract of 7 of the marmosets. Six of the infected animals developed an acute inflammatory reaction in the genital tract and chlamydial inclusions in epithelial cells were seen in smears from 2 of them. In addition, each of 6 infected marmosets examined developed humoral antibodies to C. trachomatis. In contrast, 3 control animals inoculated intravaginally with chlamydia-free McCoy cells showed no evidence of chlamydial infection. Since the marmoset is small and easily bred in captivity, it should provide a useful model for studying the mechanisms of chlamydial pathogenicity.
Full text
PDF
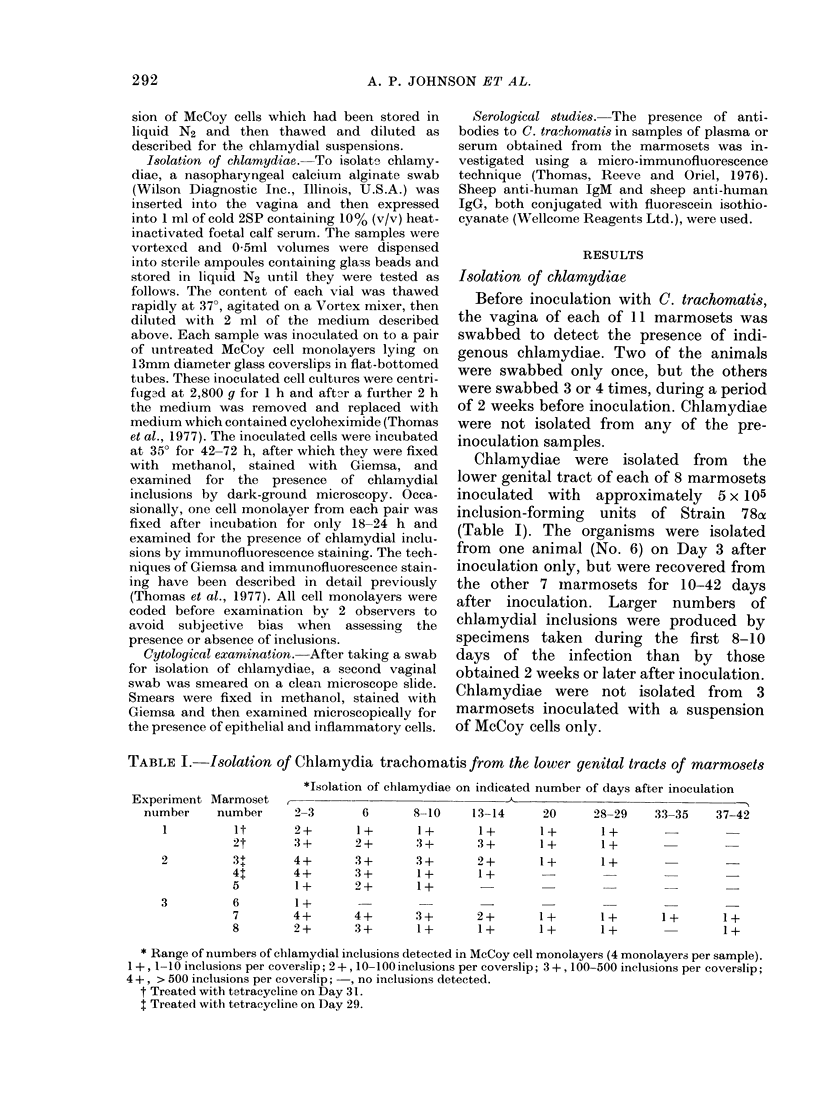
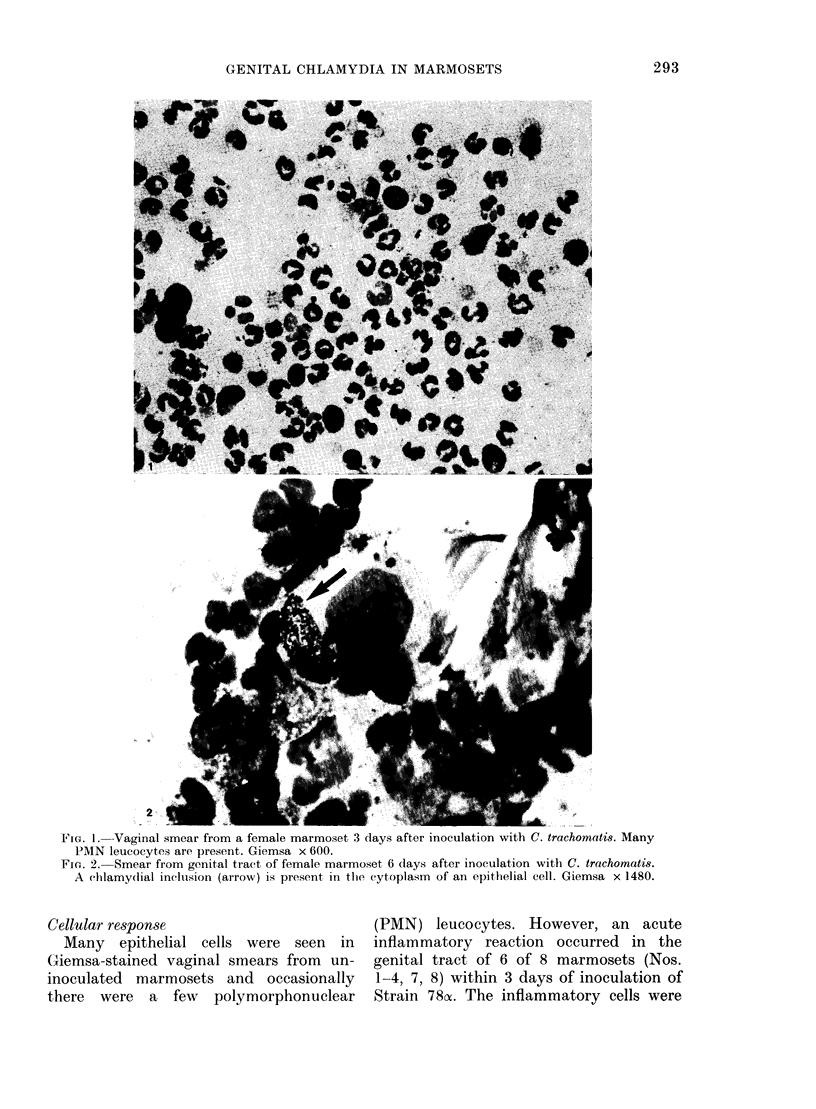


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans R. T., Taylor-Robinson D. Comparison of various McCoy cell treatment procedures used for detection of Chlamydia trachomatis. J Clin Microbiol. 1979 Aug;10(2):198–201. doi: 10.1128/jcm.10.2.198-201.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr P. M., Taylor-Robinson D., Hetherington C. M. The occurrence of ureaplasmas in marmosets. Lab Anim. 1976 Oct;10(10):393–398. doi: 10.1258/002367776780956944. [DOI] [PubMed] [Google Scholar]
- Jacobs N. F., Jr, Arum E. S., Kraus S. J. Experimental infection of the chimpanzee urethra and pharynx with Chlamydia trachomatis. Sex Transm Dis. 1978 Oct-Dec;5(4):132–136. doi: 10.1097/00007435-197810000-00002. [DOI] [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Gordon F. B., Quan A. L. Fluorescent antibody responses to chlamydial infection in patients with lymphogranuloma venereum and urethritis. J Immunol. 1974 Jun;112(6):2126–2134. [PubMed] [Google Scholar]
- Schachter J. Chlamydial infections (third of three parts). N Engl J Med. 1978 Mar 9;298(10):540–549. doi: 10.1056/NEJM197803092981005. [DOI] [PubMed] [Google Scholar]
- Thomas B. J., Evans R. T., Hutchinson G. R., Taylor-Robinson D. Early detection of chlamydial inclusions combining the use of cycloheximide-treated McCoy cells and immunofluorescence staining. J Clin Microbiol. 1977 Sep;6(3):285–292. doi: 10.1128/jcm.6.3.285-292.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B. J., Reeve P., Oriel J. D. Simplified serological test for antibodies to Chlamydia trachomatis. J Clin Microbiol. 1976 Jul;4(1):6–10. doi: 10.1128/jcm.4.1.6-10.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]