Abstract
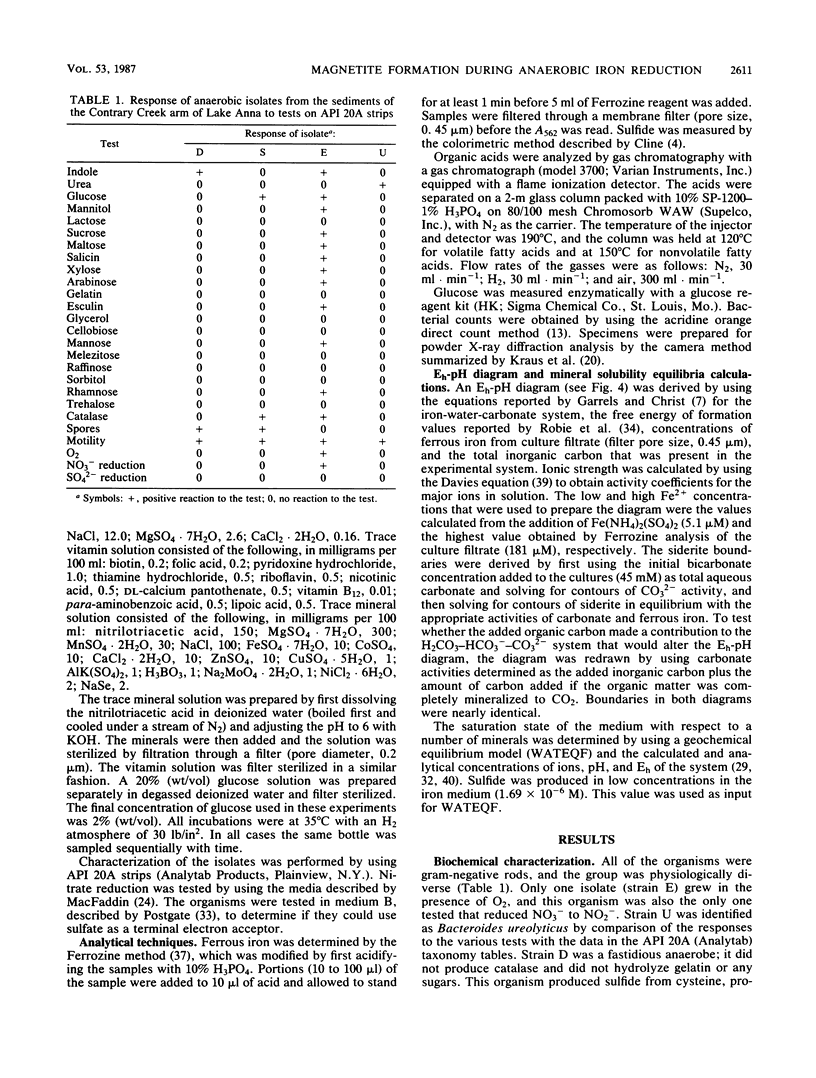
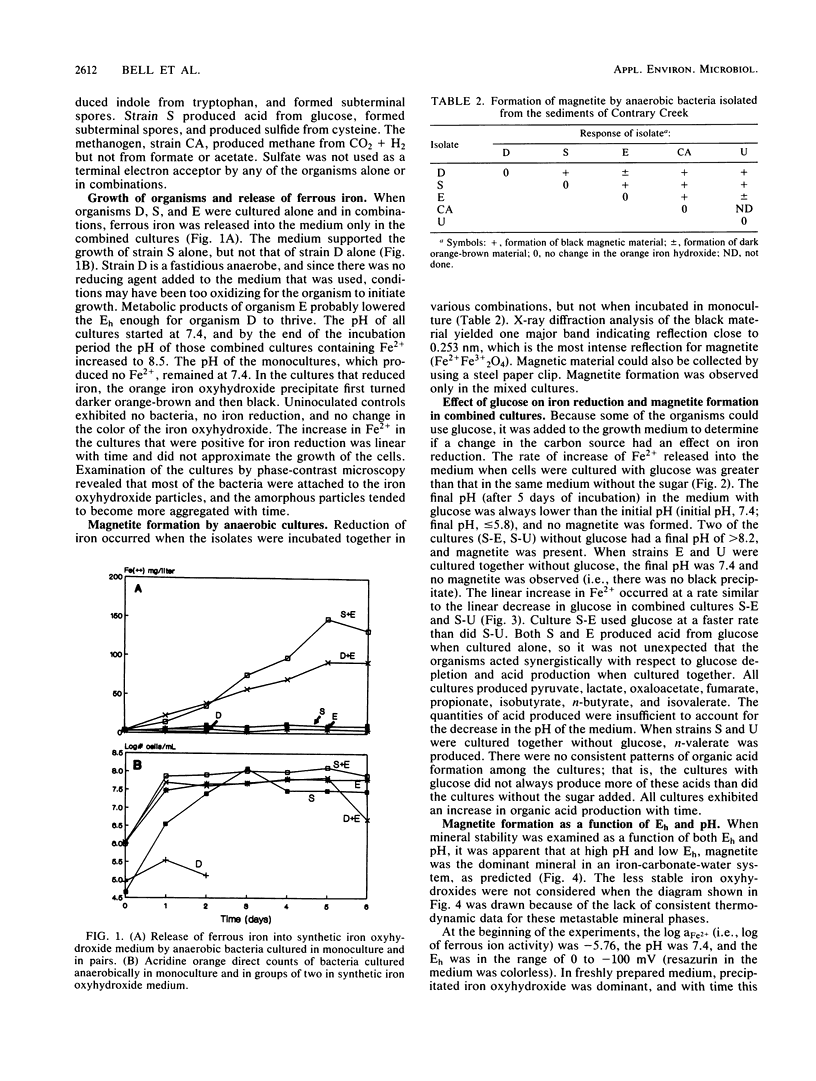
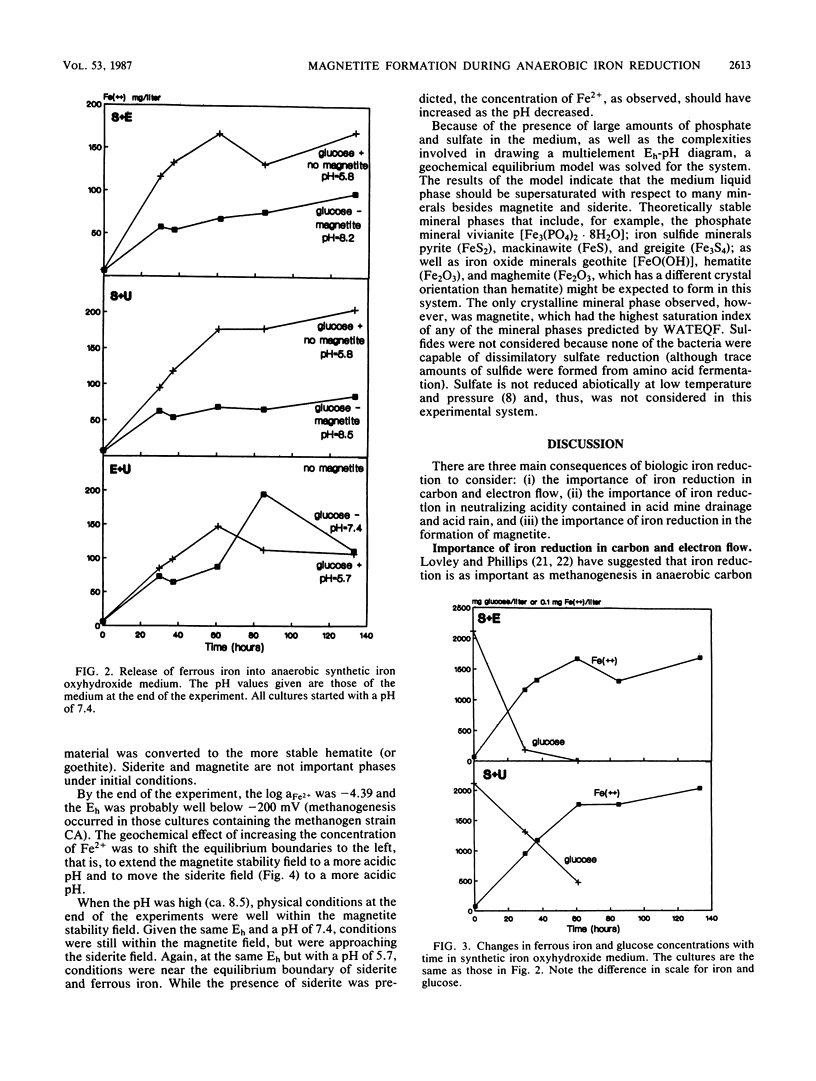
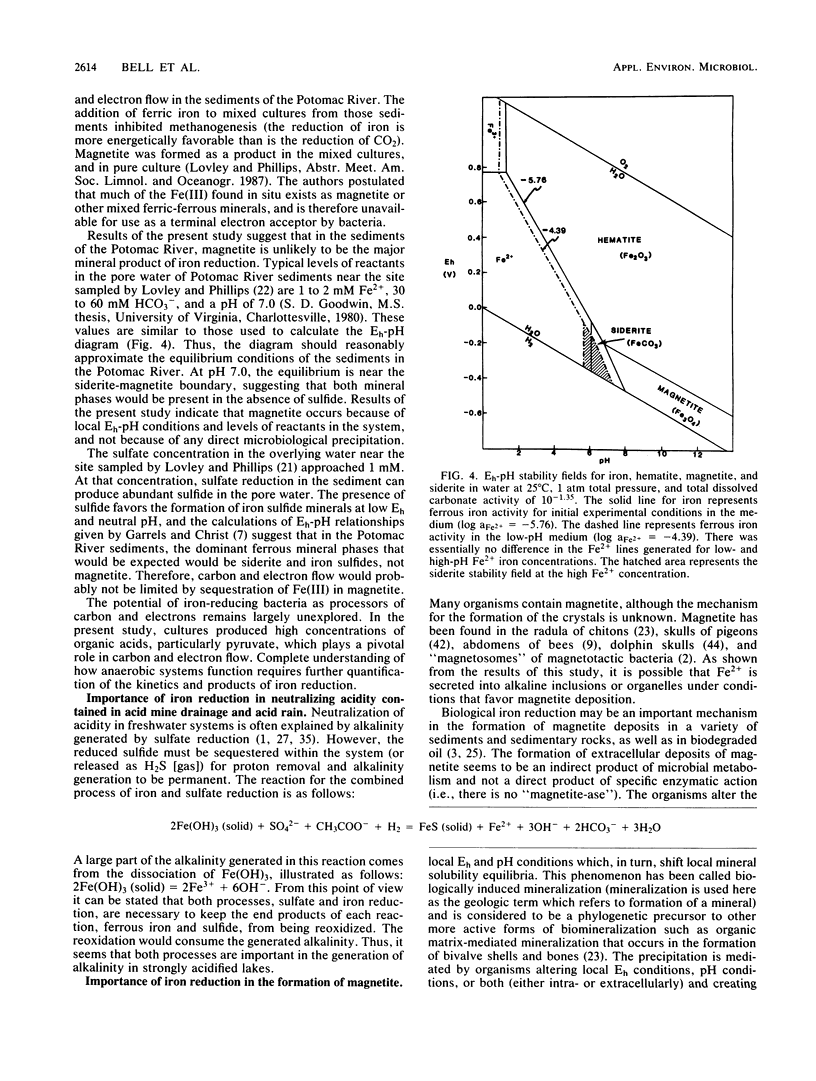
Several anaerobic bacteria isolated from the sediments of Contrary Creek, an iron-rich environment, produced magnetite when cultured in combinations but not when cultured alone in synthetic iron oxyhydroxide medium. When glucose was added as a carbon source, the pH of the medium decreased (to 5.5) and no magnetite was formed. When the same growth medium without glucose was used, the pH increased (to 8.5) and magnetite was formed. In both cases, Fe2+ was released into the growth medium. Geochemical equilibrium equations with Eh and pH as master variables were solved for the concentrations of iron and inorganic carbon that were observed in the system. Magnetite was predicted to be the dominant iron oxide formed at high pHs, while free Fe2+ or siderite were the dominant forms of iron expected at low pHs. Thus, magnetite formation occurs because of microbial alteration of the local Eh and pH conditions, along with concurrent reduction of ferric iron (direct biological reduction or abiological oxidation-reduction reactions).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore R. Magnetotactic bacteria. Science. 1975 Oct 24;190(4212):377–379. doi: 10.1126/science.170679. [DOI] [PubMed] [Google Scholar]
- Fulghum R. S., Worthington J. M. Butyl rubber stoppers increase the shelf life of prereduced, anaerobically sterilized media. Appl Environ Microbiol. 1977 May;33(5):1220–1221. doi: 10.1128/aem.33.5.1220-1221.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J. L., Kirschvink J. L., Deffeyes K. S. Bees have magnetic remanence. Science. 1978 Sep 15;201(4360):1026–1028. doi: 10.1126/science.201.4360.1026. [DOI] [PubMed] [Google Scholar]
- Herlihy A. T., Mills A. L. Sulfate reduction in freshwater sediments receiving Acid mine drainage. Appl Environ Microbiol. 1985 Jan;49(1):179–186. doi: 10.1128/aem.49.1.179-186.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal potomac river. Appl Environ Microbiol. 1986 Oct;52(4):751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986 Apr;51(4):683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstam H. A. Minerals formed by organisms. Science. 1981 Mar 13;211(4487):1126–1131. doi: 10.1126/science.7008198. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974 May;27(5):985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Appl Environ Microbiol. 1982 Feb;43(2):319–324. doi: 10.1128/aem.43.2.319-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott C., Gould J. L., Kirschvink J. L. Pigeons have magnets. Science. 1979 Sep 7;205(4410):1027–1029. doi: 10.1126/science.472725. [DOI] [PubMed] [Google Scholar]
- Zoeger J., Dunn J. R., Fuller M. Magnetic material in the head of the common Pacific dolphin. Science. 1981 Aug 21;213(4510):892–894. doi: 10.1126/science.7256282. [DOI] [PubMed] [Google Scholar]



