Abstract
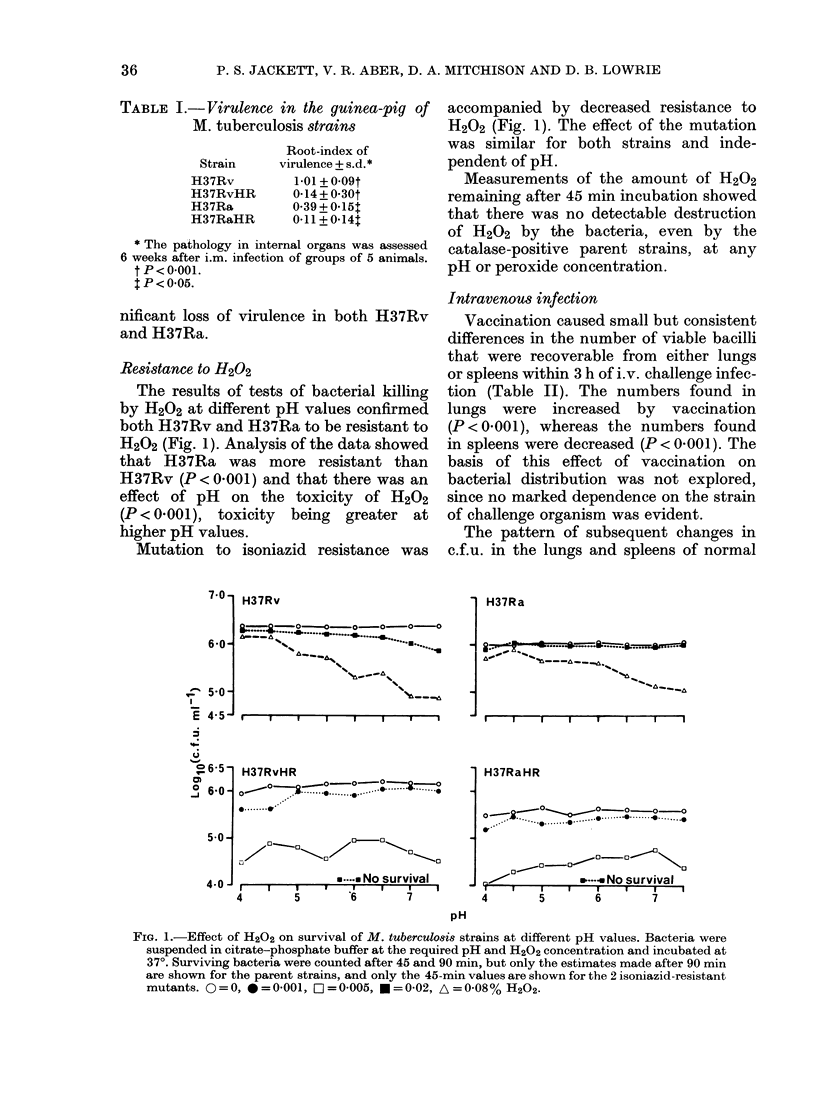
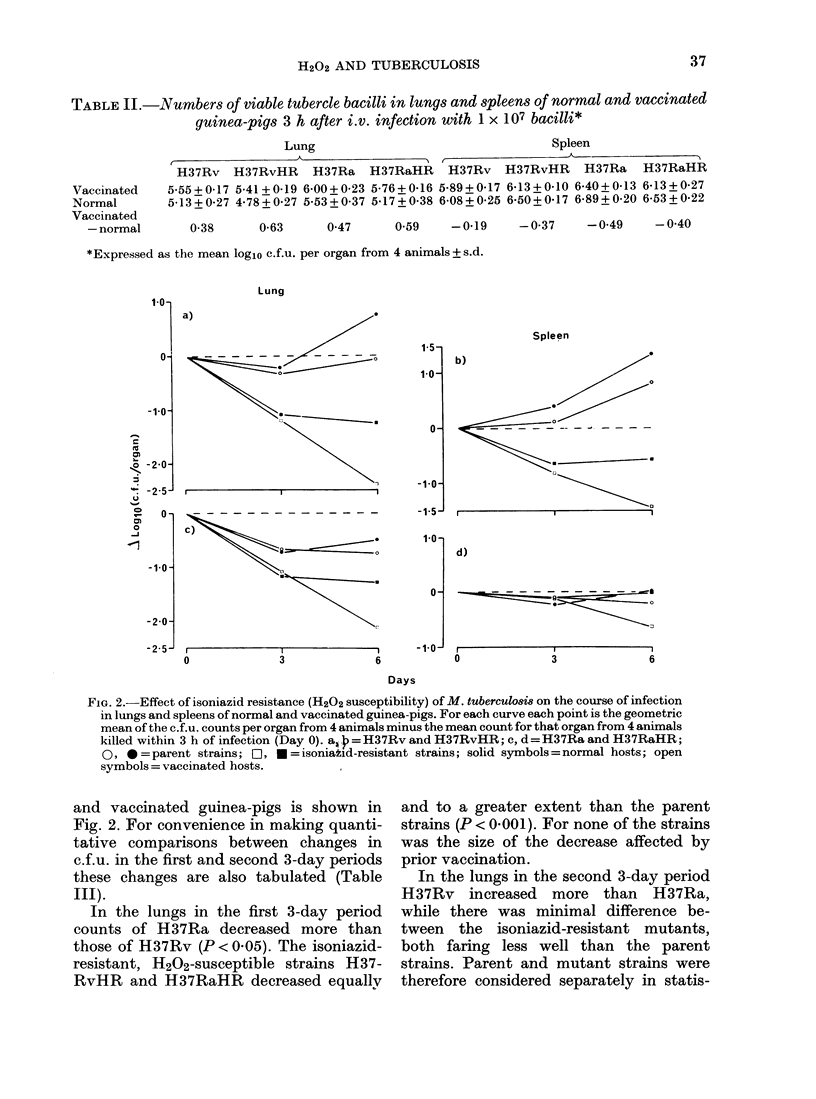
The course of infection with Mycobacterium tuberculosis strains H37Rv, H37Ra and their isoniazid-resistant, hydrogen peroxide-susceptible mutants in guinea-pig spleen and lung were assessed by measuring changes in number of viable bacteria during the first and second 3-day intervals after i.v. infection of normal and BCG-vaccinated animals. Vaccination had no effect on bacterial survival in the first 3 days of infection. The peroxide-susceptible mutants were killed or inhibited more than their parent strains; in normal animals this enhanced susceptibility was expressed equally during the first and second 3-day intervals while in vaccinated animals the effect was greater in the second 3-day interval. The results suggest that hydrogen peroxide is generated in significant amounts in the environment of tubercle bacilli lodged in normal tissues and in enhanced amounts when acquired immunity becomes expressed after a few days' lodgement in the tissues of vaccinated animals. Thus hydrogen peroxide resistance may contribute to virulence by protecting against both normal resident and immunologically activated macrophages.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen B. W. Mycobacterium tuberculosis strain H37Rv. J Med Lab Technol. 1969 Oct;26(4):389–390. [PubMed] [Google Scholar]
- Biggar W. D., Sturgess J. M. Hydrogen peroxide release by rat alveolar macrophages: comparison with blood neutrophils. Infect Immun. 1978 Feb;19(2):621–629. doi: 10.1128/iai.19.2.621-629.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Ismail G., Allen J. M., Baehner R. L. Oxidative metabolic responses of rabbit pulmonary alveolar macrophages. Blood. 1979 Mar;53(3):486–491. [PubMed] [Google Scholar]
- COLEMAN C. M., MIDDLEBROOK G. The effects of some sulfhydryl compounds on growth of catalase-positive and catalase-negative tubercle bacilli. Am Rev Tuberc. 1956 Jul;74(1):42–49. doi: 10.1164/artpd.1956.74.1.42. [DOI] [PubMed] [Google Scholar]
- Cohn M. L., Waggoner R. F., McClatchy J. K. The 7H11 medium for the cultivation of mycobacteria. Am Rev Respir Dis. 1968 Aug;98(2):295–296. doi: 10.1164/arrd.1968.98.2.295. [DOI] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbault G. G., Brignac P. J., Jr, Juneau M. New substrates for the fluorometric determination of oxidative enzymes. Anal Chem. 1968 Jul;40(8):1256–1263. doi: 10.1021/ac60264a027. [DOI] [PubMed] [Google Scholar]
- Ho R. S., Fok J. S., Harding G. E., Smith D. W. Host-parasite relationships in experimental airborne tuberculosis. VII. Fate of Mycobacterium tuberculosis in primary lung lesions and in primary lesion-free lung tissue infected as a result of bacillemia. J Infect Dis. 1978 Aug;138(2):237–241. doi: 10.1093/infdis/138.2.237. [DOI] [PubMed] [Google Scholar]
- Jackett P. S., Aber V. R., Lowrie D. B. The susceptibility of strains of Mycobacterium tuberculosis to catalase-mediated peroxidative killing. J Gen Microbiol. 1980 Dec;121(2):381–386. doi: 10.1099/00221287-121-2-381. [DOI] [PubMed] [Google Scholar]
- Jackett P. S., Aber V. R., Lowrie D. B. Virulence and resistance to superoxide, low pH and hydrogen peroxide among strains of Mycobacterium tuberculosis. J Gen Microbiol. 1978 Jan;104(1):37–45. doi: 10.1099/00221287-104-1-37. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHISON D. A., BHATIA A. L., RADHAKRISHNA S., SELKON J. B., SUBBAIAH T. V., WALLACE J. G. The virulence in the guinea-pig of tubercle bacilli isolated before treatment from South Indian patients with pulmonary tuberculosis. I. Homogeneity of the investigation and a critique of the virulence test. Bull World Health Organ. 1961;25:285–312. [PMC free article] [PubMed] [Google Scholar]
- MITCHISON D. A., SELKON J. B., LLOYD J. VIRULENCE IN THE GUINEA-PIG, SUSCEPTIBILITY TO HYDROGEN PEROXIDE, AND CATALASE ACTIVITY OF ISONIAZID-SENSITIVE TUBERCLE BACILLI FROM SOUTH INDIAN AND BRITISH PATIENTS. J Pathol Bacteriol. 1963 Oct;86:377–386. doi: 10.1002/path.1700860213. [DOI] [PubMed] [Google Scholar]
- McLeod R., Remington J. S. Influence of infection with Toxoplasma on macrophage function, and role of macrophages in resistance to Toxoplasma. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 2):170–186. doi: 10.4269/ajtmh.1977.26.170. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rister M., Baehner R. L. Effect of hyperoxia on superoxide anion and hydrogen peroxide production of polymorphonuclear leucocytes and alveolar macrophages. Br J Haematol. 1977 Jun;36(2):241–248. doi: 10.1111/j.1365-2141.1977.tb00645.x. [DOI] [PubMed] [Google Scholar]
- Schell R. F., Ealey W. F., Harding G. E., Smith D. W. The relationship between listericidal and mycobacteriostatic activity in BCG-vaccinated mice. J Reticuloendothel Soc. 1974 Sep;16(3):139–149. [PubMed] [Google Scholar]
- Stokes S. H., Davis W. B., Sorber W. A. Effect of phagocytosis on superoxide anion production and superoxide dismutase levels in BCG-activated and normal rabbit alveolar macrophages. J Reticuloendothel Soc. 1978 Aug;24(2):101–106. [PubMed] [Google Scholar]


