Abstract
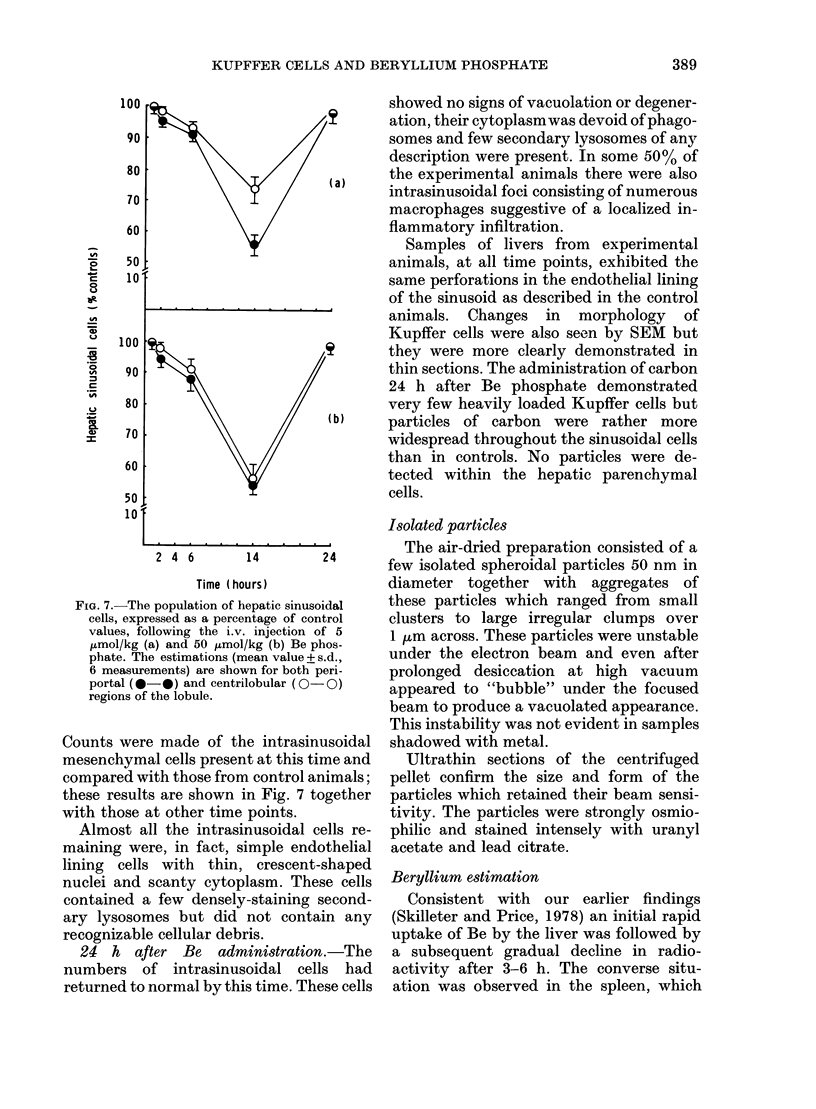
The i.v. administration of suspensions of beryllium phosphate (5-50 mumol/kg) to rats resulted in the vacuolation of hepatic Kupffer cells within 3 h. After 6 h necrotic Kupffer cells were common throughout the sinusoids of the liver but no changes were detected in the hepatic parenchymal cells during this period. A significant reduction in the numbers of intrasinusoidal cells was observed 14 h after treatment but this population had reverted to normal within 24 h. The administration of colloidal carbon to treated animals at this time did, however, demonstrate a reduction in the complement of functional endocytic cells. These results demonstrate a selective destruction of endocytic cells in the liver by this particulate toxin and the limited response by the organ to this injury. These observations are the most probable explanation for the reticuloendothelial blockade known to be caused in vivo by beryllium phosphate.
Full text
PDF


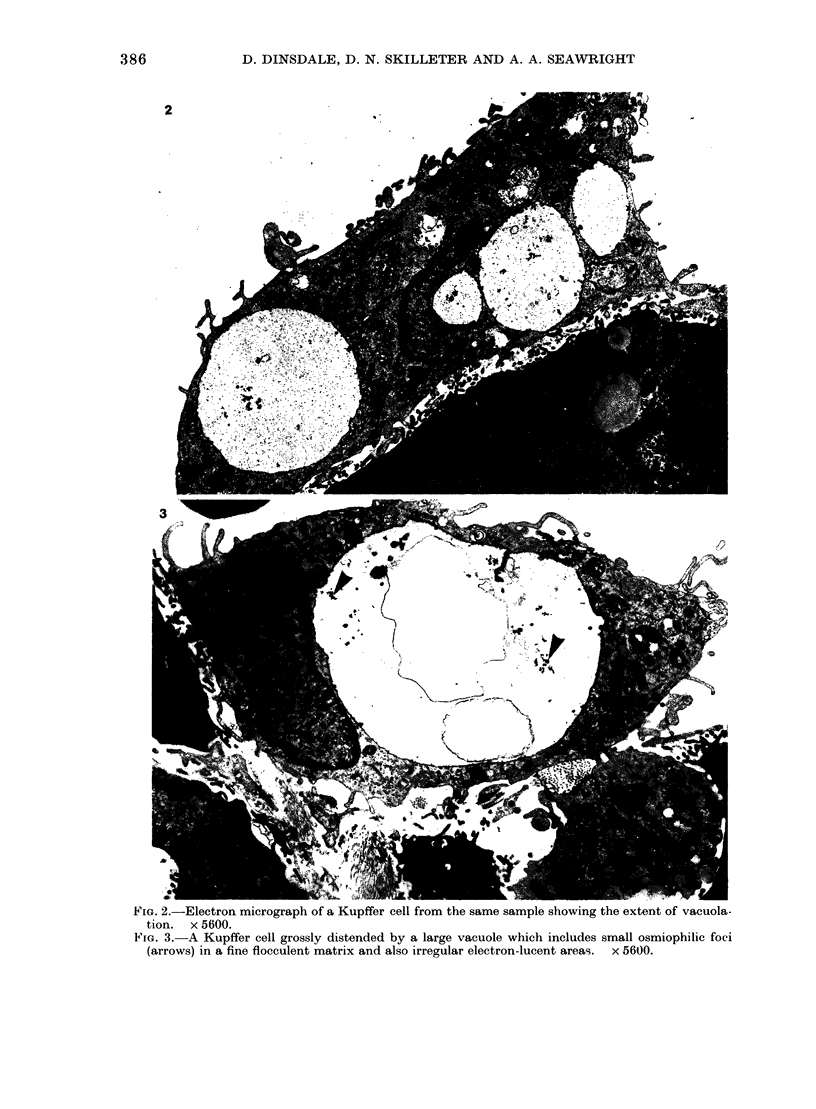





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N., BARNES J. M., DENZ F. A. Experimental beryllium poisoning. Br J Exp Pathol. 1949 Oct;30(5):375–389. [PMC free article] [PubMed] [Google Scholar]
- Aldridge W. N. The toxicity of beryllium. Lab Invest. 1966 Jan;15(1 Pt 1):176–180. [PubMed] [Google Scholar]
- CHENG K. K. Experimental studies on the mechanism of the zonal distribution of beryllium liver necrosis. J Pathol Bacteriol. 1956 Apr;71(2):265–276. doi: 10.1002/path.1700710202. [DOI] [PubMed] [Google Scholar]
- Hall D. J., Skerrett E. J., Thomas W. D. Critical point drying for scanning electron microscopy: a semi-automatic method of preparing biological specimens. J Microsc. 1978 Aug;113(3):277–290. doi: 10.1111/j.1365-2818.1978.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Hard G. C., Skilleter D. N., Reiner E. Correlation of pathology with distribution of Be following administration of beryllium sulfate and beryllium sulfosalicylate complexes to the rat. Exp Mol Pathol. 1977 Oct;27(2):197–212. doi: 10.1016/0014-4800(77)90030-2. [DOI] [PubMed] [Google Scholar]
- Kirn A., Steffan A. M., Anton M., Gendrault J. L., Bingen A. Phagocytic properties displayed by mouse hepatocytes after virus induced damage of the sinusoidal lining. Biomedicine. 1978 Feb;29(1):25–28. [PubMed] [Google Scholar]
- Locke M., Krishnan N., McMahon J. T. A routine method for obtaining high contrast without staining sections. J Cell Biol. 1971 Aug;50(2):540–544. doi: 10.1083/jcb.50.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto M. A scanning electron microscopic study on endothelial cells and Kupffer cells in rat liver sinusoids. Arch Histol Jpn. 1975 Mar;37(5):369–386. doi: 10.1679/aohc1950.37.369. [DOI] [PubMed] [Google Scholar]
- Skilleter D. N., Price R. J. Apparent two phase beryllium labelling of hepatic cell nuclei isolated after intravenous administration of beryllium compounds to rats. A possible explanation. Arch Toxicol. 1980 May;45(1):75–80. doi: 10.1007/BF00303298. [DOI] [PubMed] [Google Scholar]
- Skilleter D. N., Price R. J. Effects of beryllium compounds on rat liver Kupffer cells in culture. Toxicol Appl Pharmacol. 1981 Jun 30;59(2):279–286. doi: 10.1016/0041-008x(81)90199-x. [DOI] [PubMed] [Google Scholar]
- Skilleter D. N., Price R. J. The role of lysosomes in the hepatic accumulation and release of beryllium. Biochem Pharmacol. 1979 Dec 15;28(24):3595–3599. doi: 10.1016/0006-2952(79)90405-2. [DOI] [PubMed] [Google Scholar]
- Skilleter D. N., Price R. J. The uptake and subsequent loss of beryllium by rat liver parenchymal and non-parenchymal cells after the intravenous administration of particulate and soluble forms. Chem Biol Interact. 1978 Mar;20(3):383–396. doi: 10.1016/0009-2797(78)90116-3. [DOI] [PubMed] [Google Scholar]
- Vacher J., Deraedt R., Benzoni J. Compared effects of two beryllium salts (soluble and insoluble): toxicity and blockade of the reticuloendothelial system. Toxicol Appl Pharmacol. 1973 Mar;24(3):497–506. doi: 10.1016/0041-008x(73)90056-2. [DOI] [PubMed] [Google Scholar]
- Vacher J., Stoner H. B. The transport of beryllium in rat blood. Biochem Pharmacol. 1968 Jan;17(1):93–107. doi: 10.1016/0006-2952(68)90162-7. [DOI] [PubMed] [Google Scholar]
- Walton J. Lead aspartate, an en bloc contrast stain particularly useful for ultrastructural enzymology. J Histochem Cytochem. 1979 Oct;27(10):1337–1342. doi: 10.1177/27.10.512319. [DOI] [PubMed] [Google Scholar]
- Widmann J. J., Cotran R. S., Fahimi H. D. Mononuclear phagocytes (Kupffer cells) and endothelial cells. Identification of two functional cell types in rat liver sinusoids by endogenous peroxidase activity. J Cell Biol. 1972 Jan;52(1):159–170. doi: 10.1083/jcb.52.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970 Apr;31(1):125–150. doi: 10.1016/s0022-5320(70)90150-4. [DOI] [PubMed] [Google Scholar]
- Wisse E. Kupffer cell reactions in rat liver under various conditions as observed in the electron microscope. J Ultrastruct Res. 1974 Mar;46(3):499–520. doi: 10.1016/s0022-5320(74)90070-7. [DOI] [PubMed] [Google Scholar]
- Yensen J. Removal of epoxy resin from histological sections following halogenation. Stain Technol. 1968 Nov;43(6):344–346. [PubMed] [Google Scholar]