Abstract
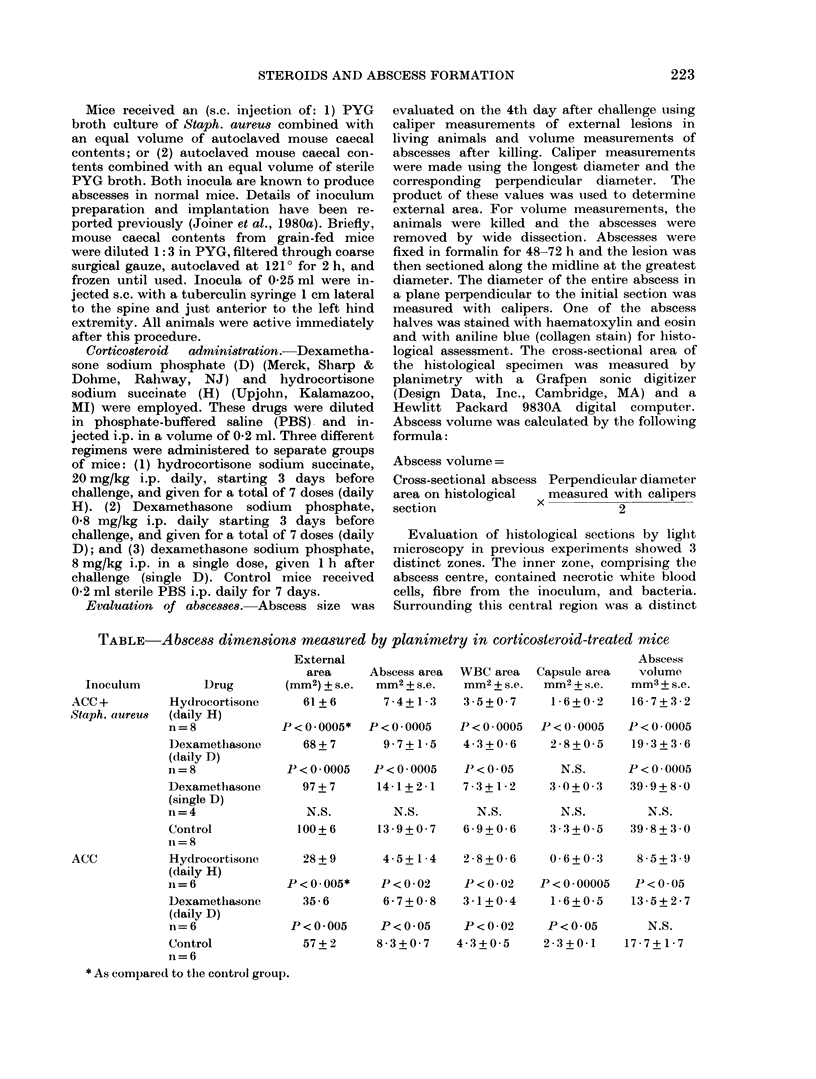
We have used a recently developed model for s.c. abscess formation to study the effect of corticosteroids on abscess formation in mice. Mice were given daily i.p. injections of either hydrocortisone, 20 mg/kg/day or dexamethasone, 0.8 mg/kg/day, starting 3 days before inoculation with Staph, aureus and continuing for the duration of the experiment. Another group of mice was given a single injection of dexamethasone, 8 mg/kg, 1 h after inoculation with Staph. aureus. Encapsulated abscesses developed in all animals by Day 4, and there was no mortality. Abscess volume +/- s.l. mean at 4 days was reduced (p less than 0.0005) from 39.9 +/- 3.0 mm3 in controls to 16.7 +/- 3.6 mm3 in the daily dexamethasone group. Abscess volume at 4 days after a single dose of dexamethasone was 39.9 +/- 8.0 mm3. Bacterial concentrations per ml of pus were equivalent in all groups (10(10,6)-10(10.9). The effect of steroids on formation of sterile abscesses was also studied. Abscess volumes were smaller in animals given daily hydrocortisone or dexamethasone when compared to controls, but the difference was significant only for mice receiving daily hydrocortisone. These results suggest that prolonged high-dose steroid administration decreased the magnitude of the acute inflammatory reaction responsible for abscess formation in the soft tissue but did not interfere significantly with the process of containment and encapsulation of s.c. abscesses. A single massive dose of steroid did not influence abscess formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON F., Jr, ADCOCK M. H. FAILURE OF PRETREATMENT WITH GLUCOCORTICOIDS TO MODIFY THE PHAGOCYTIC AND BACTERICIDAL CAPACITY OF HUMAN LEUKOCYTES FOR ENCAPSULATED TYPE I PNEUMOCOCCUS. J Bacteriol. 1965 May;89:1256–1261. doi: 10.1128/jb.89.5.1256-1261.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON F., Jr, SMITH M. R., WOOD W. B., Jr Studies on the pathogenesis of acute inflammation. II. The action of cortisone on the inflammatory response to thermal injury. J Exp Med. 1955 Dec 1;102(6):669–676. doi: 10.1084/jem.102.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal D. S. Subcutaneous staphylococcal infection in mice. 3. Effect of active and passive immunization and anti-inflammatory drugs. Br J Exp Pathol. 1967 Oct;48(5):483–500. [PMC free article] [PubMed] [Google Scholar]
- BOGGS D. R., ATHENS J. W., CARTWRIGHT G. E., WINTROBE M. M. THE EFFECT OF ADRENAL GLUCOCORTICOSTEROIDS UPON THE CELLULAR COMPOSITION OF INFLAMMATORY EXUDATES. Am J Pathol. 1964 May;44:763–773. [PMC free article] [PubMed] [Google Scholar]
- Claman H. N. Corticosteroids and lymphoid cells. N Engl J Med. 1972 Aug 24;287(8):388–397. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- Cluff L. E., Reynolds R. C., Page D. L., Breckenridge J. L. Staphylococcal bacteremia and altered host resistance. Ann Intern Med. 1968 Nov;69(5):859–873. doi: 10.7326/0003-4819-69-5-859. [DOI] [PubMed] [Google Scholar]
- Dale D. C., Fauci A. S., Wolff S. M. Alternate-day prednisone. Leukocyte kinetics and susceptibility to infections. N Engl J Med. 1974 Nov 28;291(22):1154–1158. doi: 10.1056/NEJM197411282912203. [DOI] [PubMed] [Google Scholar]
- Dale D. C., Petersdorf R. G. Corticosteroids and infectious diseases. Med Clin North Am. 1973 Sep;57(5):1277–1287. doi: 10.1016/s0025-7125(16)32228-3. [DOI] [PubMed] [Google Scholar]
- EBERT R. H., BARCLAY W. R. [Changes in connective tissue reaction induced by cortisone]. Ann Intern Med. 1952 Sep;37(3):506–518. doi: 10.7326/0003-4819-37-3-506. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C., Balow J. E. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976 Mar;84(3):304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- Glasser L., Huestis D. W., Jones J. F. Functional capabilities of steroid-recruited neutrophils harvested for clinical transfusion. N Engl J Med. 1977 Nov 10;297(19):1033–1036. doi: 10.1056/NEJM197711102971904. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G., CHURCH A. B. Adrenal steroids and infection: the effect of cortisone administration on polymorphonuclear leukocytic functions and on serum opsonins and bactericidins. J Clin Invest. 1961 May;40:794–798. doi: 10.1172/JCI104312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., Gelfand J. A., Onderdonk A. B., Bartlett J. G., Gorbach S. L. Host factors in the formation of abscesses. J Infect Dis. 1980 Jul;142(1):40–49. doi: 10.1093/infdis/142.1.40. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Onderdonk A. B., Gelfand J. A., Bartlett J. G., Gorbach S. L. A quantitative model for subcutaneous abscess formation in mice. Br J Exp Pathol. 1980 Feb;61(1):97–107. [PMC free article] [PubMed] [Google Scholar]
- Kivirikko K. I., Laitinen O., Aer J., Halme J. Studies with 14C-proline on the action of cortisone on the metabolism of collagen in the rat. Biochem Pharmacol. 1965 Oct;14(10):1445–1451. doi: 10.1016/0006-2952(65)90178-4. [DOI] [PubMed] [Google Scholar]
- Kruse N. J., Rowe D. W., Fujimoto W. Y., Bornstein P. Inhibitory effects of glucocorticoids on collagen synthesis by mouse sponge granulomas and granuloma fibroblasts in culture. Biochim Biophys Acta. 1978 Apr 19;540(1):101–116. doi: 10.1016/0304-4165(78)90439-7. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Spagnuolo P. J., Lentnek A. L. Inhibition of granulocyte adherence by ethanol, prednisone, and aspirin, measured with an assay system. N Engl J Med. 1974 Sep 26;291(13):642–646. doi: 10.1056/NEJM197409262911302. [DOI] [PubMed] [Google Scholar]
- Mandell G. L., Rubin W., Hook E. W. The effect of an NADH oxidase inhibitor (hydrocortisone) on polymorphonuclear leukocyte bactericidal activity. J Clin Invest. 1970 Jul;49(7):1381–1388. doi: 10.1172/JCI106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman R. A., Cutroneo K. R. Glucocorticoids selectively decrease the synthesis of hydroxylated collagen peptides. Mol Pharmacol. 1978 Jan;14(1):185–198. [PubMed] [Google Scholar]
- Perper R. J., Sanda M., Chinea G., Oronsky A. L. Leukocyte chemotaxis in vivo. II. Analysis of the selective inhibition of neutrophil or mononuclear cell accumulation. J Lab Clin Med. 1974 Sep;84(3):394–406. [PubMed] [Google Scholar]
- Schumer W. Steroids in the treatment of clinical septic shock. Ann Surg. 1976 Sep;184(3):333–341. doi: 10.1097/00000658-197609000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Q. T. Radioactivity of hydroxyproline from urine and collagens of normal and cortisone-treated rats. Biochem Pharmacol. 1967 Nov;16(11):2171–2179. doi: 10.1016/0006-2952(67)90016-0. [DOI] [PubMed] [Google Scholar]


