Abstract
Difficulty managing the stress of conflict in close relationships can lead to mental and physical health problems, possibly through dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, the neuroendocrine stress response system. Temperament, an individual characteristic, and attachment, a dyadic characteristic, have both been implicated in emotion regulation processes and physiological reactivity, yet there is no clear consensus on how the two work together to influence the stress response, especially after childhood. The present study investigated the ways in which temperament and attachment together predict HPA response in emerging adult couples. Analyses using multilevel modeling (HLM) found that partners' dyadic fit on attachment avoidance impacted females' cortisol response patterns, and attachment avoidance further moderated the effect of males' emotionality on both their own and their partners' cortisol. Results are discussed in terms of emotional coregulation processes in romantic attachment.
Although the experience and regulation of emotion has generally been considered a personal phenomenon, there is growing recognition of the importance of interpersonal relationships for the amplification and repair of emotional states (Diamond & Aspinwall, 2003). More attention has been focused on psychobiological regulation processes in infant-caregiver dyads (e.g., Schore, 1996), yet intimate partnerships throughout life provide a context for the regulation of emotional and physical well-being (Cacioppo, 1994; Hofer, 1984), making biobehavioral coregulation in adult romantic dyads a key target for further study. The way in which a person or couple responds to stressful situations has important implications for mental and physical health; an inability to cope with the stresses that inevitably arise within relationships sets the stage for distressed relationships, which in turn increase the risk for internalizing disorders (Coyne et al., 2002; Davila et al., 2003) and morbidity/mortality (e.g., Helgeson, 1991; Hibbard & Pope, 1993). Physiological stress response, as measured by the output of the hypothalamic-pituitary-adrenal (HPA) axis, offers a promising route by which relationship stress might lead to such disorders, given that dysregulation of this system consistently characterizes depressive and/or anxiety disorders (e.g., Butler & Nemeroff, 1990; Young et al., 2004), as well as impaired cardiovascular and immune function (Glaser & Kiecolt-Glaser, 1994; Kuhn, 1989). To understand differences in couples' responses to conflict and the health implications of these responses, we propose a biopsychosocial model of emotion regulation, in which a combination of intra- and interpersonal characteristics shapes one's response to stressors across various psychobiological systems. In this paper, we seek to illuminate a central part of the pathway from interpersonal stress to health outcomes by investigating how components of romantic partners' emotion regulation systems work together within and across partners to predict their neuroendocrine response to conflict.
Two variables that should be important for regulating emotion in relationship conflict are temperament, an individual characteristic that dictates innate emotionality, and attachment, a dyadic characteristic that describes how a person uses close relationship partners to attenuate distress in the face of threat. Although these two variables have often been linked, both conceptually and empirically, there is no clear consensus on how the two factors uniquely contribute to stress response, especially on the physiological level. Additionally, most of the research on temperament and attachment focuses on infants or children, rather than on adolescents or adults, even though these factors are assumed to remain relatively stable and influential across development. The current study was designed to address this gap in the emotion regulation literature by testing effects of emerging adult partners' temperament and attachment on their HPA activity during conflict, considering both main effects and interactions within and across partners.
HPA Response and Emotion Regulation in Couples
The HPA axis releases the adrenocortical steroid hormone cortisol in response to stress, particularly situations that involve social threat and an element of uncontrollability (Dickerson & Kemeny, 2004). The relevance of this system as a marker of emotion regulation is suggested by its association with subjective distress (Miller et al., 2007). In addition, dysregulation of the HPA system has commonly been found in depression and/or anxiety disorders (e.g., Gold et al., 1988; Young et al., 2004), which entail a breakdown of the capacity to regulate negative emotion. In particular, increased cortisol reactivity to stressful situations and/or slower recovery to baseline levels, as well as chronically elevated cortisol levels, have been implicated in internalizing disorders (e.g., Heim et al., 1998; Miller et al., 2007). These features, suggestive of stress hyperactivation, also mark risk for multiple physical ailments, such as diabetes, hypertension, cancer, and cardiovascular disease (McEwen, 1998).
Romantic relationships provide a context for the occurrence and consequences of psychosocial stress that activates the HPA system, and ineffective physiological regulation may be both driven by and a contributor to distressed relationships. A series of studies by Kiecolt-Glaser and colleagues (Heffner et al., 2004; Kiecolt-Glaser et al., 2003; see also Kiecolt-Glaser & Newton, 2001, for a review) have found that partners' HPA reactivity/recovery patterns relate to their behavior in conflict interactions and relationship quality, with differences in newlyweds' HPA reactivity prospectively predicting troubled marriages 10 years later. Given these links among exaggerated physiological stress response, disturbed relationships, and poor mental/physical health, we must begin to explore specific pathways of risk for HPA dysregulation and associated negative outcomes in young couples. Evidence from the above studies points to a role for both individual partners' emotional characteristics and their ability to use one another for support in explaining HPA differences during conflict, suggesting that temperament and attachment may each contribute to couples' stress response.
Temperament and Emotion Regulation
Temperament refers to a set of biologically based traits that appear early in life and show at least moderate consistency throughout life (Vaughn & Bost, 1999). As a psychobiological variable, temperament both influences and is influenced by physiological responses to events (Gunnar & Mangelsdorf, 1989). Measures of temperament often include some measure of emotionality (e.g., Buss & Plomin, 1984; Goldsmith & Campos, 1986; Rothbart, 1989; Thomas & Chess, 1977), with a focus on the tendency toward negative emotional experience and/or expression. The emotionality dimension taps a weakness in the regulation of negative affect that may involve more extreme and/or extended negative reactivity to internal or external stimuli. At the physiological level, emotionality has been found to relate to higher cortisol reactivity to stress (Gunnar et al., 1989; van Bakel & Riksen-Walraven, 2004; Zobel et al., 2004). This relationship may be buffered by social context, though, with children high in negative emotionality more likely to show cortisol elevations under conditions of less than optimal care (Gunnar & Donzella, 2002). The role of emotionality in adult stress reactivity/recovery remains largely unexplored, though the definition of temperament suggests that it remains an important factor in emotion regulation throughout life. Study in older samples is needed to clarify what emotionality means for mature psychobiological regulation, as well as how relationship factors may buffer or exacerbate its effects.
Attachment and Emotion Regulation
The way in which an individual uses relationships with others in stressful situations may also be conceptualized as a factor in emotion regulation, and attachment is sometimes discussed as an affect regulation strategy. Bowlby's (1973) definition of attachment as an evolutionarily adaptive bond between infant and caregiver has been extended to other close relationships, including romantic relationships (Hazan & Shaver, 1987), in which the attachment figure provides the sense of “felt security” important for the successful regulation of negative affect (Sroufe & Waters, 1977). Although early approaches to attachment quality distinguished people in terms of categories, more recent approaches find that individual differences in attachment can best be captured by the two dimensions of anxiety, which refers to desire for closeness coupled with anxiety about abandonment, and avoidance, which refers to discomfort with closeness and dependency (Fraley et al., 2000). These attachment styles are associated with distinct emotion regulation strategies; whereas secure individuals are able to acknowledge negative feelings and cope with them with the help of others, avoidant individuals attempt to deactivate and deny negative emotion, and anxious-ambivalent individuals show a hyperactivation of distress (Shaver & Mikulincer, 2002)1.
In the context of relationships, secure partners are better able to seek and provide support in anxiety-producing situations (Simpson et al., 1992), and they behave in more constructive ways to resolve conflict (Kobak & Hazan, 1991; Senchak & Leonard, 1992). Not only do secure individuals report less discomfort and more satisfaction in conflict situations, they are also rated by outsiders as showing less negative affect and more constructive conflict management behaviors (Feeney, 1998). Anxious-ambivalent partners, on the other hand, report and display greater distress during conflict, and avoidant partners have lower-quality interactions (Simpson et al., 1996). These differences appear to translate into differences in psychobiological regulation; both anxiety and avoidance have been found to predict heightened physiological stress reactivity in adults (Carpenter & Kirkpatrick, 1996; Feeney & Kirkpatrick, 1996). Effects on the HPA system, in particular, have been most clearly documented in children, with security predicting lower baseline cortisol levels (Gunnar et al., 1996) and lower adrenocortical stress reactivity (Spangler & Schieche, 1998). Only one study, conducted with a large subsample of the current study, investigated attachment-related differences in adult's HPA reactivity; main effects of partners' anxiety and avoidance on cortisol response to conflict tended to confirm the role of anxiety in hyperactivating the stress response and avoidance in deactivating it (Powers et al., 2006). Although this work provides a starting point for understanding the biological sequelae of romantic partners' attachment styles, significant questions remain regarding the fit between temperament and attachment and between partners' temperament/attachment characteristics, as determinants of HPA reactivity and recovery.
Moderating Effects
Recent work in emotion regulation has suggested the importance of not only main effects of individual difference variables, but also constellations of such variables within persons and across persons in a dyadic context (Diamond & Aspinwall, 2003). Several studies have found that attachment security moderates relations between infant temperament and HPA reactivity, with cortisol elevations evident only in the temperamentally vulnerable children who are also insecurely attached (Nachmias et al., 1996; Schieche & Spangler, 2005; Spangler & Schieche, 1998). These indications suggest that attachment may serve as a buffering or exacerbating factor in emotionality, a hypothesis that has yet to be tested in older samples.
Beyond within-partner moderating influences of attachment on temperament, there may be cross-partner moderating effects, or “goodness of fit” on temperament and attachment characteristics that are meaningful for each partner's HPA reactivity. Both the temperament and attachment literatures show evidence of partner-partner interactive effects on adjustment, and researchers in developmental psychobiology have framed the human attachment dyad as a mutually regulating unit (e.g., Gunnar & Donzella, 2002; Schore, 2000). Particularly in the context of a conflict, partners must be able to transition together between self-regulation and interactive regulation to achieve a mutually rewarding “dyadic state of consciousness” (Tronick et al., 1998), making the contribution of each partner's regulatory capacities important for the couple's coregulation of emotion.
The Current Study
The present study was designed to test an integrated model of emotion regulation within developing romantic relationships by investigating links among temperament, attachment, and HPA response to a conflict discussion in emerging adult couples. Based on the evidence reviewed above concerning the role of emotionality and attachment in emotion regulation, we hypothesized that attachment anxiety would strengthen associations (hyperactivating) between partners' emotionality and HPA activation, whereas attachment avoidance would weaken such associations (deactivating). In addition to temperament-attachment interactions, partner-partner interaction effects were expected. Specifically, dysregulation (in the sense of high negative emotionality and/or attachment insecurity) of one partner was hypothesized to exacerbate the stress-promoting effects of such characteristics in the other partner; conversely, a more regulated style in one partner could buffer the effects of the other partner's dysregulation.
Research Design and Method
Participants
Participants in this study were a part of a larger longitudinal study investigating a biopsychosocial model of factors hypothesized to contribute to the development of depression. Data collection began in October, 2000 and was completed in December, 2003. Participants were 199 emerging adult heterosexual couples (total of 398 individuals) who were involved in a relationship for at least 2 months. Ages ranged from 18 to 21, and the ethnic distribution was similar to that of the New England community from which the sample was drawn. See Table 1 for further description of the sample. Participants were recruited from the western Massachusetts area through flyers, poster, and presentations in University of Massachusetts undergraduate courses (almost all were students at the university). Each participant was paid $80 for completing this portion of the study, and students enrolled in psychology courses had the additional option of receiving research credit points toward their final grade.
Table 1.
Demographic Characteristics of the Sample
| Couple | |||
|---|---|---|---|
| Male Partner | Female Partner | ||
| Age M (SD) | 19.12 (.78) | 19.36 (.77) | |
| Ethnicity | |||
| Asian/Pacific Island | 6% | 4% | |
| African American | 2% | .5% | |
| Latino | 4.5% | 4% | |
| Caucasian | 84% | 90% | |
| Other | 3.5% | 1.5% | |
| Parents' Education (mother, father) | |||
| High school | 24%, 24% | 20%, 22% | |
| College | 52%, 46% | 50%, 41% | |
| Graduate School | 24%, 30% | 30%, 37% | |
| Parents' Work (mother, father) | |||
| Unemployed | 12%, 6% | 9%, 5% | |
| Part-time | 11%, 1% | 13%, 5% | |
| Full-time | 59%, 71% | 53%, 70% | |
| Retired/other | 18%, 22%% | 25%, 20% | |
| Participant Education | |||
| High school | .5% | 1% | |
| Part college | 96% | 96% | |
| 2-Year college degree | 3.5% | 3% | |
| Relationship Status | |||
| Dating, nonexclusive | 4.5% | ||
| Dating, exclusive | 48.3% | ||
| Long-term, committed relationship | 46.7% | ||
| Engaged | .5% | ||
| Relationship Length | |||
| 2-6 months | 26% | ||
| 7-12 months | 19.5% | ||
| 1-2 years | 36% | ||
| 2-3 years | 9.5% | ||
| > 3 years | 9% |
Procedure
All data used in the current study were obtained during the first of three data-collection sessions conducted for a larger study. For this first session, participants were asked to come to the lab with their romantic partners, and in the interests of obtaining accurate hormone measurements, they were instructed not to drink alcohol, use illegal drugs, or visit the dentist within the 24 hours prior to the study, or to exercise, eat, drink (except water), smoke cigarettes, or brush their teeth up to 2 hours prior. If participants violated any of these conditions or had an elevated temperature, they were scheduled to return at a later date. Participants also rinsed their mouths thoroughly with water 10 minutes before giving the first saliva sample to minimize the potential for contamination.
During data collection, each participant completed a series of questionnaires and participated in a discussion with his or her romantic partner that required negotiating an area of conflict. Each partner was asked to identify a topic that had been a source of heated and unresolved discussions in the past month. A researcher randomly selected one of the topics by flipping a coin, and the couple was asked to spend 15 minutes describing the issue and attempting to come to a resolution to the problem. Sixty-eight percent of the topics chosen in this way were nominated by the male or female partner only; of these, 50% were from the male and 50% from the female partner. The topics for the remaining 32% of couples were nominated by both partners. Each discussion was videotaped without the presence of a researcher in the room.
Seven salivary cortisol samples were collected over the course of the session to measure participants' HPA reactivity to the conflict negotiation task (see Table 2). Saliva samples were collected according to procedures suggested by Salimetrics, LLC. Participants passively drooled down a straw into a small plastic vial with their heads tilted forward until the required amount of saliva was collected. The vial was then sealed and immediately placed in frozen storage (−20 degrees C) until shipment to Salimetrics, LLC (on dry ice) for analysis. It takes cortisol 15-20 minutes to travel from the adrenal cortex to saliva, so the entry sample collected at the beginning of the session measures participants' hormone levels about 15 minutes prior to entering the laboratory and reflects their stress response to the general task of participating in a psychological study. The second sample, collected 15 minutes after a detailed description of the conflict negotiation task, assesses stress reactivity associated with explicitly anticipating conflict with a romantic partner. The third sample, collected 10 minutes after completing the conflict task, represents stress levels during the conflict discussion itself, and the remaining post-task samples collected 20, 30, 45, and 60 minutes afterwards depict stress recovery. Preliminary analyses of a subsample of the study have indicated that despite the potential for increased cortisol reactivity to the act of entering the laboratory, there is a significant change in cortisol levels when partners are in the conflict discussion. The decision to collect the final cortisol sample 60 minutes after the conflict discussion was made based on consultation with an expert in the assessment of HPA reactivity (Douglas Granger, Director of Salimetrics, Pennsylvania State University), and published studies have supported the premise that this is an adequate time for cortisol recovery (Kemeny, 2003).
Table 2.
Time Key for Cortisol Sampling
| Sample | Time (hours centered on discussion sample) | Code |
|---|---|---|
| 1 | −.88 | Entry |
| 2 | −.53 | Anticipation |
| 3 | 0 | Discussion |
| 4 | .17 | Completion |
| 5 | .34 | Recovery |
| 6 | .59 | Recovery |
| 7 | .84 | Recovery |
Measures
Cortisol Levels as Assessed by Saliva Samples
Saliva samples were assayed for cortisol using an enzyme immunoassay (EIA) specifically designed for use with saliva according to the manufacturer's recommended protocol (Salimetrics, State College, PA). The test has a lower limit of sensitivity of .007 μg/dl, range of sensitivity from .007 to 1.12 μg/dl, and average intra- and inter-assay coefficients of variation of 4.13% and 8.89% respectively. All saliva samples were assayed in duplicate. Since blood contamination can falsely elevate salivary analyte levels, samples were tested for blood contamination by Salimetrics before assay for cortisol. An examination of the distributions of participants' salivary blood levels and cortisol values confirmed a linear positive relationship between the two; therefore, a continuous blood score was included as a covariate to control for its influence.
Control Variables
A questionnaire was developed for this project to assess variables that might affect HPA reactivity, including medications, cigarette smoking, season, and sleep/wake times. In addition, characteristics of the relationship such as length of time together and commitment status, as well as whose topic (his, hers, or both) was under discussion, were tested as possible covariates. For females, antibiotic medication2 and birth control pills were found to relate to cortisol; for males, allergy medication and time they went to sleep the previous night related to cortisol values. These variables were included as controls in all analyses. Because all data collection sessions began at the same time (4:00pm), daily cortisol fluctuations did not need to be controlled for.
Experiences in Close Relationships (ECR)
The ECR is a 36-item self-report measure used to assess attachment in romantic relationships (Brennan, Clark, & Shaver, 1998). Items are rated on a 7-point Likert scale, ranging from 1 (Disagree strongly) to 7 (Agree strongly). Two dimensions of attachment are measured: Avoidance (avoidance of intimacy and dependence on one's partner) and Anxiety (anxiety about rejection and abandonment). Reliability for each of the scales was found to be acceptable in this sample (Cronbach's alpha = .86 for Avoidance and .91 for Anxiety).
Emotionality, Activity, Sociability (EAS)
The EAS is a 20-item self-report measure of temperament conceptualized as emergent personality developed by Buss and Plomin (1986). Items are rated on a 5-point Likert scale, ranging from 1 (not at all characteristic of me) to 5 (very characteristic of me). The content scale used in the current study is Emotionality, which includes subscales for Distress, Fearfulness, and Anger. Reliability for Emotionality was found to be adequate in this sample (Cronbach's alpha = .83).
Analytic Strategy
Multilevel modeling was chosen as the appropriate analytic technique, given the dependency of dyadic data (his and her outcome scores within a couple are correlated with one another) and the temporal trajectory of cortisol levels. Hierarchical linear modeling using the HLM 6.0 program, as described by Raudenbush and Bryk (2002), was used to explore predictive relationships in this dataset. Because cortisol values were positively skewed, natural log-transformed values were used as the outcomes in model testing. In an HLM model such as the one used here, the couple is approached as the unit of analysis, with a female outcome score and a male outcome score nested within the couple, and information about the association between the scores in the couple is used to generate a more precise standard error in testing regression coefficients. A further advantage to this technique is that it takes into account measurement error, splitting each outcome into a true score and an error term, allowing researchers to look at the true score relationships.
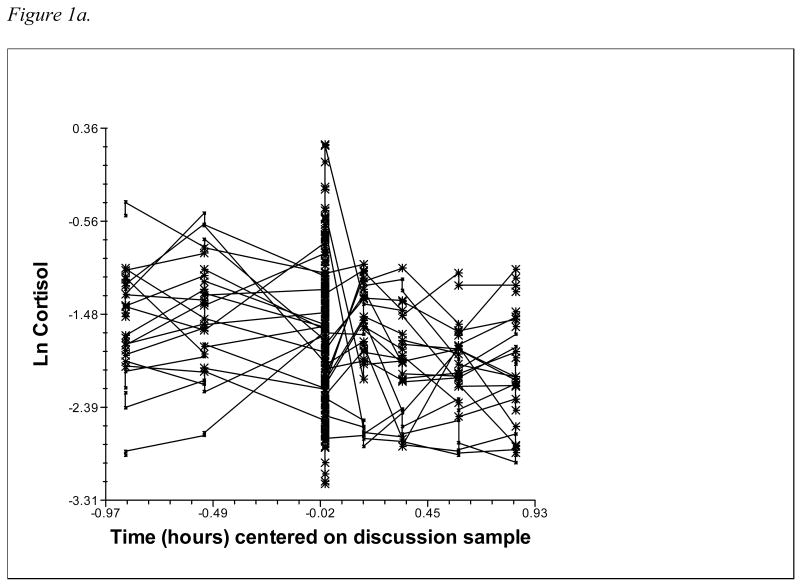
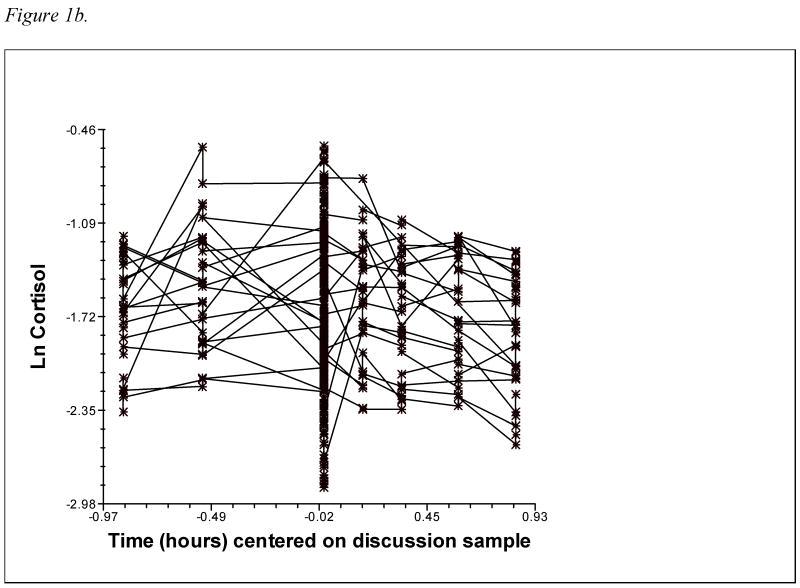
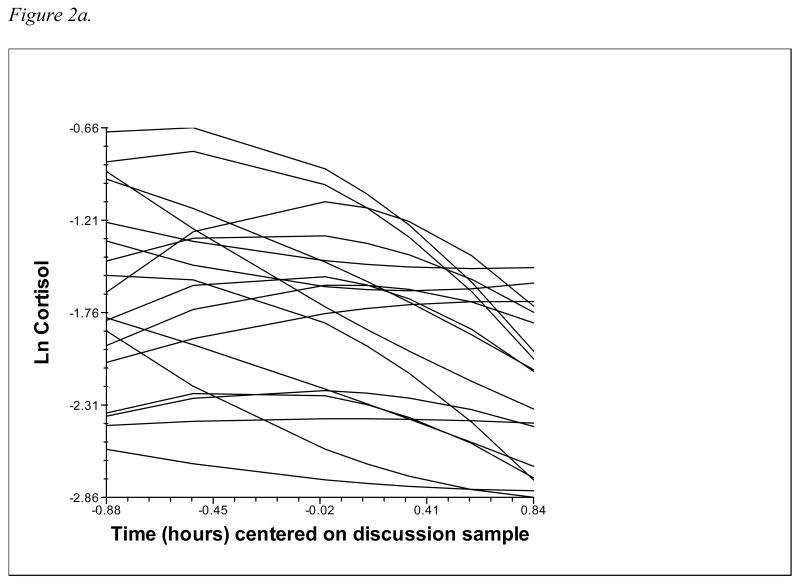
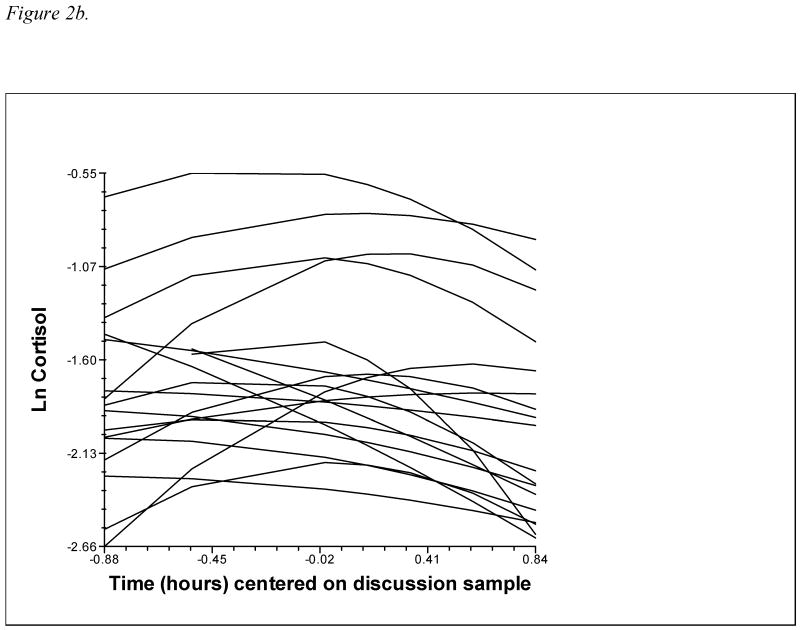
In analyses relating temperament and attachment to cortisol reactivity/recovery, individual cortisol trajectories are modeled at level 1, and then predictors are added at level 2 to explain the variability in the level 1 parameters. This study modeled cortisol trajectories of male and female partners nested within couples at level 1, with variables unique to each member of the couple (emotionality, avoidance, and anxious-ambivalence, as well as interaction terms) added at level 2. Partners' cortisol trajectories were modeled with intercept, slope, and quadratic terms to reflect the curvilinear pattern of rising and falling cortisol associated with reactivity to and recovery from the stressor. This quadratic model was selected based on both theoretical considerations – HPA response to a discrete stressor should involve cortisol levels rising to a peak and then declining back to baseline – and statistical evidence that the addition of a quadratic term resulted in a significant improvement in model fit (see Figures 1a and 1b for a depiction of male and female raw cortisol trajectories; Figures 2a and 2b show level 1 fitted quadratic curves). The two-level equation for partners' cortisol trajectories is shown below:
Figure 1.
1a. Male partners' raw cortisol values over time (10% random sample).
1b. Female partners' raw cortisol values over time (10% random sample).
Figure 2.
2a. Male partners' level 1 fitted cortisol trajectories (10% random sample).
2b. Female partners' level 1 fitted cortisol trajectories (10% random sample).
Level 1 (within-couple)
Level 2 (between-couple)
(predictors are control variables, emotionality, attachment, and emotionality × attachment or male × female emotionality/attachment terms; similar explanatory equations for πm/w1-2i)
In HLM, a centering point is selected at which time is considered to be zero, and the intercept and slope terms represent the level and instantaneous rate of change in the outcome at that time point. For the current study, partners' stress response patterns during the conflict discussion itself were of greatest interest, so models were centered at the time 3 saliva sample. This means that male and female cortisol intercepts represent their level of HPA activation (based on both baseline activity and stress-related reactivity), and their slopes represent the degree of ongoing reactivity or recovery (also a function of the timing of peak reactivity) during the stressor. The quadratic terms reflect the overall pace of participant's reactivity/recovery curves. Each of these parameters – level of activation during stress, timing and speed of reactivity/recovery – could be important for health outcomes.
Results
To address the research questions, a series of HLM models were estimated. First, a baseline model with no predictors at level 2 was fit to the data to illustrate average male and female outcome scores, the correlation between partners' scores, and the proportion of variability to be explained at level 2. Next, sets of predictors were added to build a full model that included all hypothesized main effects and interactions. Because a large number of predictors were entered and tested simultaneously, increasing the possibility of a type I error, a more conservative alpha level of p ≤ .01 was used to determine which effects should be considered further.
To create a baseline cortisol model against which other explanatory models could be tested, variables that could affect cortisol measurements (e.g., medications, blood contamination, etc.) were entered as predictors of male and female intercept, linear, and quadratic terms. Based on this model, male and female partners' cortisol levels during the discussion were significantly correlated (tau = .31), with nonsignificant associations between partners' instantaneous rates of change during the discussion (tau = −.01) and their deceleration rates across the sampling period (tau = .002). Beyond these average trajectories, though, there was significant variability across couples in all male and female cortisol parameters, which could be explained by adding predictors at level 2.
Self × Partner Model
The full model, which included main effects and self × partner interactions on emotionality, anxiety, and avoidance, yielded a significant improvement in fit compared to baseline, χ2(54) = −78.82, p < .05. This model explained 8.3% of the variance in female and 10.7% of the variance in male cortisol levels (intercept), 4.4% of female and 11.3% of male instantaneous rates of change (linear slope), and 3.4% of female and 9.6% of male deceleration rates in cortisol trajectories (quadratic terms).
As seen in Table 3, attachment anxiety exerted a main effect on cortisol trajectories, particularly for males; higher anxiety was associated with higher cortisol levels during the discussion for both partners, and for males it also related to less recovery during the discussion/later cortisol peak, as well as a steeper reactivity/recovery curve overall. The female partner's emotionality also predicted less recovery during the discussion/later peak for the male. One self × partner effect reached our significance criterion; the interaction of his and her attachment avoidance predicted female cortisol level during the discussion. An examination of predicted cortisol trajectories plotted at high (75th percentile) and low (25th percentile) values of his and her avoidance indicated that female cortisol was highest if partners were concordant for higher (or lower) avoidance (see Figure 3). Viewed from another angle, the female's own avoidance related most strongly to differences in her cortisol (higher) if her partner was highly avoidant.
Table 3.
Self × Partner Temperament, Attachment Predicting Cortisol Trajectories
| Cortisol Parameter | T3 Level | T3 Slope | Quadratic | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coeff | SE | p | Coeff | SE | p | Coeff | SE | p | |
| Female Partner | |||||||||
| Intercept | −1.693 | .04 | < .001 | −.121 | .02 | < .001 | −.188 | .03 | < .001 |
| Blood | .415 | .12 | .001 | ||||||
| Antibiotic | .448 | .18 | .01 | −.332 | .13 | .01 | |||
| Birth Control | .151 | .07 | .04 | ||||||
| Male Avoidance | −.041 | .06 | .47 | −.004 | .03 | .88 | .045 | .04 | .24 |
| Female Avoidance | .051 | .06 | .36 | −.045 | .03 | .10 | −.047 | .04 | .23 |
| Male Anxiety | −.009 | .05 | .85 | .026 | .02 | .25 | .038 | .03 | .23 |
| Female Anxiety | .134 | .05 | .005 | .023 | .02 | .33 | −.020 | .03 | .54 |
| Male Emotionality | .024 | .03 | .36 | .008 | .01 | .55 | .002 | .02 | .89 |
| Female Emotionality | −.022 | .02 | .31 | .002 | .01 | .87 | −.002 | .02 | .88 |
| M × F Avoidance | .163 | .07 | .01 | −.001 | .006 | .85 | −.049 | .04 | .28 |
| M × F Anxiety | −.042 | .04 | .31 | −.012 | .03 | .72 | .004 | .03 | .88 |
| M × F Emotionality | .004 | .01 | .72 | .003 | .02 | .90 | −.0004 | .01 | .96 |
| Male Partner | |||||||||
| Intercept | −1.676 | .04 | < .001 | −.289 | .02 | < .001 | −.206 | .03 | < .001 |
| Blood | .445 | .14 | .002 | ||||||
| Time Asleep | .062 | .02 | .01 | ||||||
| Allergy Medication | .329 | .12 | .008 | ||||||
| Male Avoidance | .014 | .06 | .80 | .008 | .03 | .80 | −.035 | .04 | .42 |
| Female Avoidance | .060 | .06 | .28 | −.008 | .03 | .79 | −.006 | .04 | .89 |
| Male Anxiety | .129 | .04 | .006 | .063 | .02 | .01 | −.104 | .04 | .005 |
| Female Anxiety | .020 | .05 | .68 | −.030 | .03 | .25 | .051 | .04 | .17 |
| Male Emotionality | −.002 | .03 | .96 | −.032 | .01 | .03 | .043 | .02 | .04 |
| Female Emotionality | −.005 | .02 | .83 | .034 | .01 | .006 | −.019 | .02 | .27 |
| M × F Avoidance | .068 | .06 | .30 | .008 | .04 | .82 | −.063 | .05 | .22 |
| M × F Anxiety | −.034 | .04 | .42 | .048 | .02 | .04 | .032 | .03 | .33 |
| M × F Emotionality | .023 | .01 | .05 | −.011 | .01 | .10 | −.007 | .01 | .44 |
Figure 3.
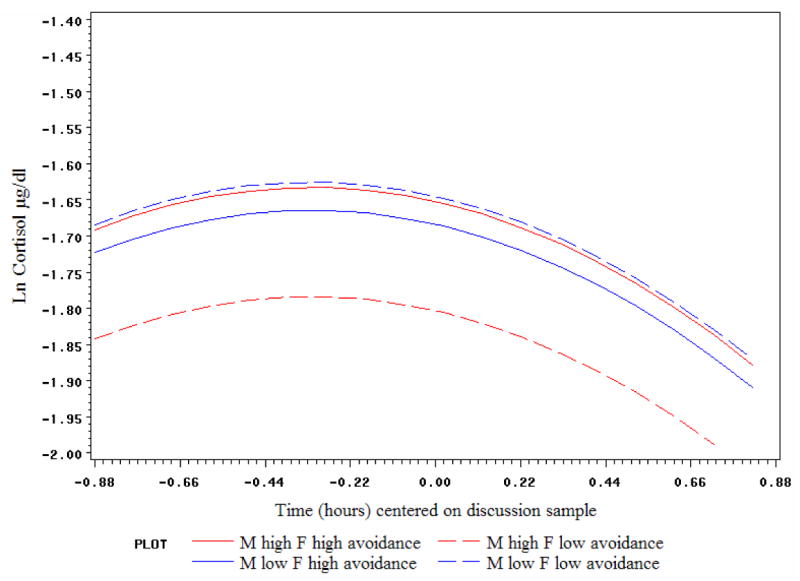
Male and female partner avoidance interact to predict females' cortisol levels during conflict: Predicted trajectories shown at high (75th percentile) and low (25th percentile) values of male and female avoidance.
Temperament × Attachment Model
The full model including main effects of partners' emotionality, anxiety, and avoidance, as well as emotionality × anxiety/avoidance interactions, provided a significant improvement in fit compared to baseline, χ2(60) = −96.98, p < .01. This model explained 10.5% of the variance in female and 11.3% of the variance in male cortisol levels, 8.8% of female and 8.2% of male instantaneous rates of change, and 10.9% of female and 11.8% of male deceleration rates in cortisol trajectories.
Not surprisingly, the same main effects of attachment anxiety (on both partners' cortisol) and of female emotionality (on male cortisol) were found as above. A significant interaction of male emotionality × attachment avoidance impacted both his own and his partner's cortisol level during the discussion, as well as her deceleration rate. As depicted in Figure 4, male avoidance tended to lower his cortisol only if he was high in emotionality; at lower levels of emotionality, avoidance was associated with higher cortisol. The effect of male negative emotionality × avoidance on female partner cortisol was in the same direction but even stronger, with the highest female cortisol levels expected when her partner was high in emotionality and low in avoidance (see Figure 5). In addition, male avoidance appeared to relate to slower female deceleration rates if he was high, but not low, in emotionality.
Figure 4.
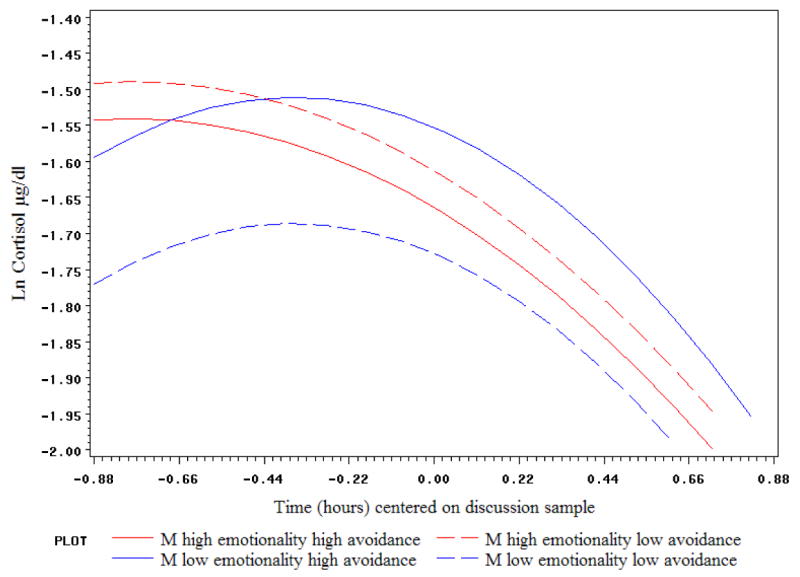
Male emotionality and avoidance interact to predict their own cortisol levels during conflict: Predicted trajectories shown at high (75th percentile) and low (25th percentile) values of male emotionality and avoidance.
Figure 5.
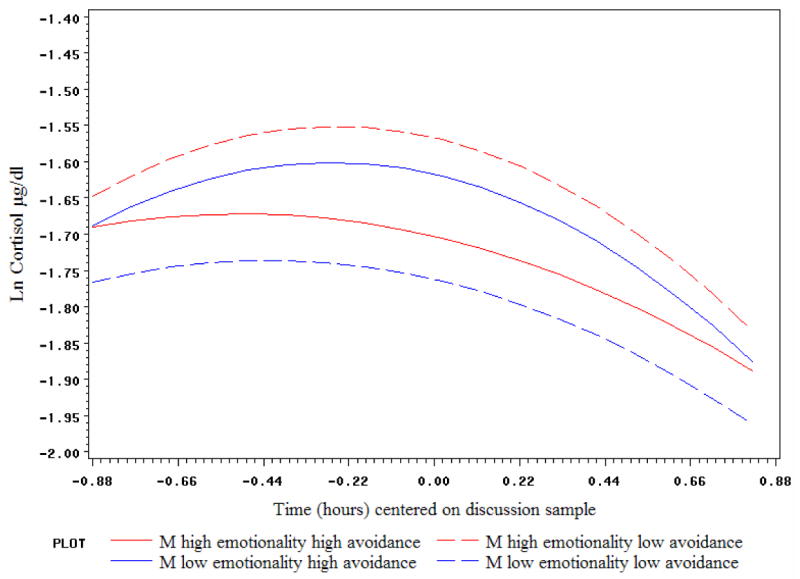
Male emotionality and avoidance interact to predict female partners' cortisol levels and deceleration rates during conflict: Predicted trajectories shown at high (75th percentile) and low (25th percentile) values of male emotionality and avoidance.
Discussion
The overarching purpose of the current study was to build a multifaceted model of emotion regulation that illustrates how intrapersonal (temperament) and interpersonal (attachment) regulation resources within an intimate dyad work together to impact a physiological marker of stress reactivity and recovery (HPA response). The current findings do support a role for both temperament and attachment in explaining couples' stress response, with distinct processes for men and women that often depend on the combination of characteristics within and across partners.
In contrast to studies with children, we found no main effects of partners' emotionality on their own stress response; rather, this sample showed cross-partner and interactive effects suggesting a more indirect or conditional influence of temperament on adult emotion regulation processes. The one main effect indicated that men who were with high emotionality women maintained their stress response during the conflict rather than showing the normative pattern of recovery from an earlier (pre-conflict) peak. Women who are prone to high negative affect may be less able to provide the support that has been found to mitigate men's cortisol response to stress (Kirschbaum et al., 1995). The lack of a parallel effect of men's emotionality on their women partners would fit with this explanation, given that women in the Kirschbaum study did not benefit from partner support. In this way, women's temperament characteristics may aid in or detract from coregulation processes in romantic relationships.
As found previously (Powers et al., 2006), attachment anxiety related to higher levels of HPA activation during relationship conflict for males. In addition, the current analyses clarify that when the individual element of temperament and the interaction of all variables between partners are included in the equations, the importance of attachment anxiety for women is revealed. This finding is remarkably congruent with attachment theory (e.g., Shaver & Mikulincer, 2002), which states that an anxious style of emotion regulation hyperactivates the negative emotional state (and, in this case, the psychophysiological stress system). On the other hand, the deactivating influence of attachment avoidance was not as evident, emerging only under certain conditions; in other conditions, avoidance actually predicted increased HPA activity. This latter association is incongruent with avoidant individuals' self-reported experience of stress, but it may speak to the greater dissociations between behavioral and hormonal stress responses in insecure attachment (Gunnar et al., 1996). This expands earlier work (Powers et al., 2006) that had revealed main effects for women's avoidance on their HPA response but had not investigated effects of interactions between partners' avoidance attachment styles. The elusive and sometimes contradictory nature of avoidance effects found in previous studies may have to do with unmeasured moderating factors, including those addressed here and others as yet untested.
The heart of this investigation lies in its attention to moderating effects within the couple, and these proved essential to understanding the impact of attachment avoidance on HPA function. On the one hand, partners' dyadic fit on avoidance predicted the degree of women's HPA activation during conflict. If her partner was highly avoidant, her own avoidance failed to reduce her stress response and instead seemed to increase it in a manner not evident at low levels of partner avoidance. It would appear that partner avoidance only interferes with coregulation processes if the woman is also avoidant, perhaps resulting in a mutually disengaged standoff. If she is not avoidant, she may be able to compensate for the behavioral deficits of her partner in the conflict interaction, with a corresponding benefit for her neuroendocrine response. Gender differences in attunement to the quality of the relationship, with women more likely to function as relationship “barometers” (Floyd & Markman, 1983), may help to understand why the combination of two avoidant partners would increase women's stress specifically during the conflict. When mutual avoidance makes it difficult for partners to do the “work” of negotiating relationship conflict, the woman's sense of responsibility for doing so might generate a stress response seen in her cortisol level even if she appears disengaged. Her own avoidance, when set against the obstacles of her partner's avoidance, may contribute to the uncontrollability element found to elicit exaggerated cortisol response. At the other extreme, two partners extremely low in avoidance may engage in a more emotional or engaged interaction that also mobilizes her cortisol response to a greater degree; in this context, higher cortisol may signal a constructive engagement with the interaction and not a harmful response, as long as she is also able to recover. Further examination of how cortisol relates to the quality of conflict interactions in high/low avoidant partner pairings would help to clarify the implications of this effect for couples' adjustment.
Not only did the effect of avoidance depend on partner avoidance, it also depended on (men's) emotionality. For men who were high emotionality only, avoidance appeared to exert a deactivating effect on cortisol levels, which extended to the woman partner's cortisol. For men who were lower on this temperament dimension, conversely, the most favorable (least activated) profiles were associated with low avoidance. Better resources for managing negative emotion conferred by both low emotionality and low avoidance/greater security make it unsurprising that this combination would give rise to the least stress. These men should be able to engage in conflict resolution in a way that poses little threat to either themselves or their partners, aiding in emotional coregulation during conflict. In men who are prone to negative emotion, on the other hand, the emotional cutoff associated with avoidance may inhibit hostile or dysphoric conflict-escalating behavior that exacerbates the harmful aspects of conflict for both partners. To understand why men's, but not women's, emotionality would show this effect, we consider the fact that boys are less socialized in emotion than girls by parents and peers (e.g., Garside & Klimes-Dougan, 2002). With less awareness of how to navigate emotion and fewer tools for modifying its expression, the gap between men who suppress negative emotion and those who express it may be greater than that for women. At the same time, the effect on women's deceleration rates suggests that even though they were more stressed during the conflict with high emotionality-low avoidance partners, they were reacting/recovering more rapidly and so perhaps showed fewer long-term, extended stress effects compared to women with high emotionality-high avoidance partners. Again, further exploration of cortisol and behavioral patterns in couples with high/low male emotionality and avoidance would help to illuminate when and how avoidance serves an adaptive function, and what alternatives could be introduced to increase the overall quality of the relationship.
As with any study, there are limitations in methodology and interpretation that prevent us from making sweeping claims about what drives the human condition. Although we found a number of effects that were statistically significant and theoretically relevant, the size of these effects and their ability to explain variance in cortisol parameters were limited. This is likely due, at least in part, to our inability to control for all the factors extraneous to our investigation that could impact cortisol values. For example, body mass index and abdominal fat, both associated with cortisol function (e.g., Rebuffe et al., 1992), were not measured in this study. In addition, our sample was quite limited in demographic characteristics, representing a population of relatively healthy, high-functioning and educated young adults. The range of both predictor variables and outcomes may thus have been restricted, making it harder to detect effects. This sample specificity also limits our ability to make broader conclusions about mechanisms driving health outcomes in the general population. At the same time, the fact that we did find effects of temperament and attachment on HPA function within a somewhat restricted sample suggests that these relationships could and should be addressed in other demographic groups (i.e., older adults, lower SES, ethnic minorities).
Another area that calls for further expansion is the behavioral backdrop for paths from temperament/attachment to HPA activity during couples conflict. Much of the explanation for what drives these effects and what they mean for adjustment is based on surmise that would require further research to support or refute. In particular, we have suggested that partners' behavioral patterns (i.e., escalation, disengagement, support) in the conflict interactions act as mechanisms for partner emotion regulation characteristics to impact each other's HPA response. A sensible next step would be to relate partner behaviors (and perceptions of own and partner behavior) to both temperament/attachment variables and to HPA trajectories to test these ideas.
On the other side of the equation, we have treated HPA response as the final outcome and made inferences about adaptive/maladaptive temperament/attachment combinations based on the assumption that higher and/or more extended cortisol activation means dysregulation. This is a reasonable assumption based on previous research, but to make any strong statements about relatively healthy or unhealthy neuroendocrine patterns, we must relate cortisol trajectories to the more distal outcomes introduced at the beginning of this paper – relationship quality, mental and physical health. Our interpretation of effects is also limited by the aspects of cortisol trajectories examined in this paper – levels, instantaneous rates of change, and deceleration rates during the conflict discussion. Further tests at different points in the trajectory (i.e., anticipation of conflict, recovery following conflict), as well as different analytic techniques (e.g., area under the curve), would allow us to more fully characterize HPA reactivity and recovery patterns in these couples.
To turn to the overall contributions of this study and implications for emotion regulation, we can confirm the role of both intrapersonal (temperament) and interpersonal (attachment) factors in psychophysiological stress response. In general, greater dysregulation at one level predicts dysregulation at the next, but these effects are often conditional on other variables – gender, characteristics within oneself, and characteristics of one's partner. Emotional experience and regulation does not seem to be merely an individual process, but rather an interpersonal one that unfolds throughout life in different relationships, both influencing and influenced by the biosocial matrix in which we exist.
Table 4.
Self, Partner Temperament × Attachment Predicting Cortisol Trajectories
| Cortisol Parameter | T3 Level | T3 Slope | Quadratic | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coeff | SE | p | Coeff | SE | p | Coeff | SE | p | |
| Female Partner | |||||||||
| Intercept | −1.658 | .05 | <.001 | −.124 | .02 | <.001 | −.187 | .03 | <.001 |
| Blood | .418 | .12 | .001 | ||||||
| Antibiotic | .391 | .18 | .03 | −.314 | .13 | .01 | |||
| Birth Control | .122 | .07 | .08 | ||||||
| Male Avoidance | −.007 | .05 | .90 | −.004 | .03 | .89 | .024 | .04 | .52 |
| Female Avoidance | .046 | .05 | .40 | −.042 | .03 | .11 | −.040 | .04 | .29 |
| Male Anxiety | −.007 | .04 | .87 | .021 | .02 | .34 | .040 | .03 | .19 |
| Female Anxiety | .125 | .04 | .008 | .029 | .02 | .19 | −.029 | .03 | .34 |
| Male Emotionality | .024 | .02 | .35 | .011 | .01 | .38 | .001 | .02 | .94 |
| Female Emotionality | −.019 | .02 | .38 | .001 | .010 | .90 | −.002 | .01 | .89 |
| M Emotionality × Avoidance | −.104 | .03 | .003 | −.017 | .02 | .32 | .077 | .02 | .001 |
| F Emotionality × Avoidance | −.018 | .02 | .44 | .009 | .01 | .45 | −.003 | .02 | .86 |
| M Emotionality × Anxiety | −.014 | .02 | .48 | −.015 | .01 | .11 | −.012 | .01 | .36 |
| F Emotionality × Anxiety | .015 | .02 | .42 | .017 | .01 | .06 | −.016 | .01 | .20 |
| Male Partner | |||||||||
| Intercept | −1.64 | .05 | <.001 | −.277 | .03 | <.001 | −.255 | .04 | <.001 |
| Blood | .422 | .14 | .004 | ||||||
| Time Asleep | .062 | .02 | .01 | ||||||
| Allergy Medication | .315 | .12 | .01 | ||||||
| Male Avoidance | .047 | .06 | .40 | .008 | .03 | .79 | −.052 | .04 | .24 |
| Female Avoidance | .050 | .06 | .37 | −.017 | .03 | .59 | −.0004 | .04 | .99 |
| Male Anxiety | .131 | .04 | .005 | .055 | .02 | .03 | −.110 | .04 | .003 |
| Female Anxiety | .001 | .046 | .98 | −.021 | .03 | .43 | .070 | .04 | .06 |
| Male Emotionality | .002 | .03 | .94 | −.036 | .01 | .02 | .046 | .02 | .03 |
| Female Emotionality | .005 | .02 | .82 | .030 | .01 | .01 | −.024 | .02 | .16 |
| M Emotionality × Avoidance | −.084 | .03 | .01 | −.008 | .02 | .68 | .041 | .03 | .13 |
| F Emotionality × Avoidance | .006 | .02 | .80 | −.001 | .01 | .97 | .002 | .02 | .92 |
| M Emotionality × Anxiety | .002 | .02 | .92 | −.014 | .01 | .20 | .014 | .02 | .36 |
| F Emotionality × Anxiety | −.011 | .02 | .56 | .002 | .01 | .86 | .027 | .02 | .07 |
Acknowledgments
This study was supported in part by grant R01 MH60228-01A1 from the National Institute of Mental Health to Sally Powers.
Footnotes
These patterns are thought to result from the internalization of caregiver response to negative emotion, making attachment style to some extent an individual characteristic. At the same time, these strategies always imply a response (adequate or inadequate) from the attachment partner and are most clearly realized in relationship interactions, which is why we consider it a dyadic aspect of emotion regulation.
Because antibiotic medication may indicate an underlying infectious process that impacts various aspects of HPA function, a sensitivity analysis was conducted to compare models tested on the full sample with models tested only on participants who were not on antibiotic medication. None of the model parameters changed by more than 20%, so the full sample was retained for analyses.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bowlby J. Attachment and loss: Vol 2 Separation: Anxiety and anger. New York: Basic; 1973. [Google Scholar]
- Brennan KA, Clark CL, Shaver PR. Self-report measurement of adult attachment: An integrative overview. In: Simpson JA, Rholes WS, editors. Attachment theory and close relationships. New York: Guilford; 1998. pp. 46–76. [Google Scholar]
- Buss AH, Plomin R. A temperament theory of personality. New York: Wiley-Interscience; 1975. [Google Scholar]
- Buss AH, Plomin R. Temperament: Early developing personality traits. Hillsdale, NJ: Erlbaum; 1984. [Google Scholar]
- Buss AH, Plomin R. The EAS approach to temperament. In: Plomin R, Dunn J, editors. The study of temperament: Changes, continuities, and challenges. Hillsdale, NJ: Erlbaum; 1986. pp. 67–79. [Google Scholar]
- Butler PD, Nemeroff CB. Corticotropin releasing factor as a possible cause of comorbidity in anxiety and depressive disorders. In: Maser JD, Uoninger CR, editors. Comorbidity of Mood and Anxiety Disorders. Washington, DC: American Psychiatric Press; 1990. pp. 413–435. [Google Scholar]
- Cacioppo JT. Social neuroscience: Autonomic, neuroendocrine, and immune response to stress. Psychophysiology. 1994;31:113–128. doi: 10.1111/j.1469-8986.1994.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Carpenter EM, Kirkpatrick LA. Attachment style and presence of a romantic partner as moderators of psychophysiological responses to a stressful laboratory situation. Personal Relationships. 1996;3:351–367. [Google Scholar]
- Coyne JC, Thompson R, Palmer SC. Marital quality, coping with conflict, marital complaints, and affection in couples with a depressed wife. Journal of Family Psychology. 2002;16:26–37. doi: 10.1037//0893-3200.16.1.26. [DOI] [PubMed] [Google Scholar]
- Davila J, Karney BR, Hall TW, Bradbury TN. Depressive symptoms and marital satisfaction: Within-subject associations and moderating effects of gender and neuroticism. Journal of Family Psychology. 2003;17:557–570. doi: 10.1037/0893-3200.17.4.557. [DOI] [PubMed] [Google Scholar]
- Diamond LM, Aspinwall LG. Emotion regulation across the life span: An integrative perspective emphasizing self-regulation, positive affect, and dyadic processes. Motivation and Emotion. 2003;27:125–156. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Feeney JA. Adult attachment and relationship-centered anxiety: Response to physical and emotional distancing. In: Simpson JA, Rholes WS, editors. Attachment theory and close relationships. New York: Guilford; 1998. pp. 189–218. [Google Scholar]
- Feeney BC, Kirkpatrick LA. Effects of adult attachment and presence of romantic partners on physiological responses to stress. Journal of Personality & Social Psychology. 1996;70:255–270. doi: 10.1037//0022-3514.70.2.255. [DOI] [PubMed] [Google Scholar]
- Floyd FJ, Markman HJ. Observational biases in spousal observations: Toward a cognitive behavioral model of marriage. Journal of Consulting & Clinical Psychology. 1983;51:450–457. doi: 10.1037//0022-006x.51.3.450. [DOI] [PubMed] [Google Scholar]
- Fraley RC, Waller NG, Brennan K. An item response theory analysis of self-report measures of adult attachment. Journal of Personality & Social Psychology. 2000;78:350–365. doi: 10.1037//0022-3514.78.2.350. [DOI] [PubMed] [Google Scholar]
- Garside RB, Klimes-Dougan B. Socialization of discrete negative emotions: Gender differences and links with psychological distress. Sex Roles. 2002;47:115–128. [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, editors. Handbook of human stress and immunity. San Diego, CA: Academic Press; 1994. [Google Scholar]
- Gold PW, Goodwin FK, Chrousos GP. Clinical and biochemical manifestations of depression: Relations to the neurobiology of stress. New England Journal of Medicine. 1988;319:348–353. doi: 10.1056/NEJM198808113190606. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Campos JJ. Fundamental issues in the study of early temperament: The Denver Twin Temperament Study. In: Lamb M, Brown A, Rogoff B, editors. Advances in developmental psychology. Vol. 4. Hillsdale, NJ: Erlbaum; 1986. pp. 7–37. [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M. Stress reactivity and attachment security. Developmental Psychobiology. 1996;29:191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Mangelsdorf S. The dynamics of temperament-physiology relations: A comment on biological processes in temperament. In: Kohnstamm GA, Bates JE, editors. Temperament in childhood. Oxford, England: John Wiley & Sons; 1989. pp. 145–152. [Google Scholar]
- Gunnar MR, Mangelsdorf S, Larson M, Hertsgaard L. Attachment, temperament, and adrenocortical activity in infancy: A study of psychoendocrine regulation. Developmental Psychology. 1989;25:355–363. [Google Scholar]
- Hazan C, Shaver PR. Romantic love conceptualized as an attachment process. Journal of Personality and Social Psychology. 1987;52:511–524. doi: 10.1037//0022-3514.52.3.511. [DOI] [PubMed] [Google Scholar]
- Heffner KL, Kiecolt-Glaser JK, Loving TJ, Glaser R, Malarkey WB. Spousal support satisfaction as a modifier of physiological responses to marital conflict in younger and older couples. Journal of Behavioral Medicine. 2004;27:233–254. doi: 10.1023/b:jobm.0000028497.79129.ad. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Bane C, Glaser R, Malarkey WB. Love, marriage, and divorce: Newlyweds' stress hormones foreshadow relationship changes. Journal of Consulting and Clinical Psychology. 2003;71:176–188. doi: 10.1037//0022-006x.71.1.176. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: His and hers. Psychological Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kobak RR, Hazan C. Attachment in marriage: Effects of security and accuracy of working models. Journal of Personality & Social Psychology. 1991;60:861–869. doi: 10.1037//0022-3514.60.6.861. [DOI] [PubMed] [Google Scholar]
- Heim CGY, Heit SC, Bonsall R, Miller AH, Nemeroff CB. Increased sensitivity of the hypothalamic-pituitary-adrenal axis to psychosocial stress in adult survivors of childhood abuse. In: Abst SN, editor. Annual Meeting Society of Neuroscience. 1998. pp. 201–212. [Google Scholar]
- Helgeson VS. The effects of masculinity and social support on recovery from myocardial infarction. Psychosomatic Medicine. 1991;53:621–633. doi: 10.1097/00006842-199111000-00004. [DOI] [PubMed] [Google Scholar]
- Hibbard JH, Pope CR. The quality of social roles as predictors of morbidity and mortality. Social Science and Medicine. 1993;36:217–225. doi: 10.1016/0277-9536(93)90005-o. [DOI] [PubMed] [Google Scholar]
- Hofer M. Relationships as regulators: A psychobiologic perspective on bereavement. Psychosomatic Medicine. 1984;46:183–197. doi: 10.1097/00006842-198405000-00001. [DOI] [PubMed] [Google Scholar]
- Kemeny ME. The psychobiology of stress. Current Directions in Psychological Science. 2003;12:124–129. [Google Scholar]
- Kirschbaum C, Klauer T, Filipp S, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kuhn CM. Adrenocortical and gonadal steroids in behavioral cardiovascular medicine. In: Schneiderman N, Weiss SM, Kaufman PG, editors. Handbook of research methods in cardiovascular behavioral medicine. New York: Plenum; 1989. pp. 185–204. [Google Scholar]
- McEwen BS. The neurobiology of stress: From serendipity to clinical relevance. Brain Research. 1998;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Development. 1996;67:508–522. [PubMed] [Google Scholar]
- Powers SI, Pietromonaco PR, Gunlicks M, Sayer A. Dating couples' attachment styles and patterns of cortisol reactivity and recovery in response to a relationship conflict. Journal of Personality and Social Psychology. 2006;90:613–628. doi: 10.1037/0022-3514.90.4.613. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Second. Newbury Park, CA: Sage; 2002. [Google Scholar]
- Rebuffe-Scrive M, Walsh UA, McEwen B, Rodin J. Effect of chronic stress and exogenous glucocorticoids on regional fat distribution and metabolism. Physiology & Behavior. 1992;52:583–590. doi: 10.1016/0031-9384(92)90351-2. [DOI] [PubMed] [Google Scholar]
- Rothbart MK. Temperament in childhood: A framework. In: Kohnstamm GA, Bates JE, Rothbart MK, editors. Temperament in childhood. New York: Wiley; 1989. pp. 59–73. [Google Scholar]
- Schieche M, Spangler G. Individual differences in biobehavioral organization during problem-solving in toddlers: The influence of maternal behavior, infant-mother attachment, and behavioral inhibition on the attachment-exploration balance. Developmental Psychobiology. 2005;46:293–306. doi: 10.1002/dev.20065. [DOI] [PubMed] [Google Scholar]
- Schore AN. Effects of a secure attachment relationship on right brain development, affect regulation, and infant mental health. Infant Mental Health Journal. 1996;22:269–276. [Google Scholar]
- Schore AN. Attachment and the regulation of the right brain. Attachment and Human Development. 2000;2:23–47. doi: 10.1080/146167300361309. [DOI] [PubMed] [Google Scholar]
- Senchak M, Leonard KE. Attachment styles and marital adjustment among newlywed couples. Journal of Social and Personal Relationships. 1992;9:51–64. [Google Scholar]
- Simpson JA, Rholes WS, Nelligan JS. Support seeking and support giving within couples in an anxiety-provoking situation: The role of attachment styles. Journal of Personality & Social Psychology. 1992;62:434–446. [Google Scholar]
- Simpson JA, Rholes WS, Phillips D. Conflict in close relationships: An attachment perspective. Journal of Personality & Social Psychology. 1996;71:899–914. doi: 10.1037//0022-3514.71.5.899. [DOI] [PubMed] [Google Scholar]
- Spangler G, Schieche M. Emotional and adrenocortical responses of infants to the strange situation: The differential function of emotional expression. International Journal of Behavioral Development. 1998;22:681–706. [Google Scholar]
- Sroufe LA, Waters E. Attachment as an organizational construct. Child Development. 1977;48:1184–1199. [Google Scholar]
- Thomas A, Chess S. Temperament and development. New York: Brunner/Mazel; 1977. [Google Scholar]
- Tronick EZ, Brushweiller-Stern N, Harrison AM, Lyons-Ruth K, Morgan AC, Nahum JP, et al. Dyadically expanded states of consciousness and the process of therapeutic change. Infant Mental Health Journal. 1998;19:290–299. [Google Scholar]
- van Bakel HJA, Riksen-Walraven JM. Stress reactivity in 15-month-old infants: Links with infant temperament, cognitive competence, and attachment security. Developmental Psychobiology. 2004;44:157–167. doi: 10.1002/dev.20001. [DOI] [PubMed] [Google Scholar]
- Vaughn BE, Bost KK. Attachment and temperament: Redundant, independent, or interacting influences on interpersonal adaptation and personality development. In: Cassidy J, Shaver PR, editors. Handbook of attachment: Theory, research, and clinical applications. New York: Guilford; 1999. pp. 198–225. [Google Scholar]
- Young EA, Abelson JL, Cameron OG. Effect of comorbid anxiety disorders on the hypothalamic-pituitary-adrenal axis response to a social stressor in major depression. Biological Psychiatry. 2004;56:113–120. doi: 10.1016/j.biopsych.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Zobel A, Barkow K, Schulze-Rauschenbach S. High neuroticism and depressive temperament are associated with dysfunctional regulation of the hypothalamic-pituitary-adrenocortical system in healthy volunteers. Acta Psychiatrica Scandinavica. 2004;109:392–399. doi: 10.1111/j.1600-0447.2004.00313.x. [DOI] [PubMed] [Google Scholar]