Abstract
A novel derivatization method employing 1,2-dimethylimidazole-4-sulfonyl chloride (DMISC) to improve the mass spectrometric response for phenolic compounds in liquid chromatography electrospray ionization mass spectrometry (LC-ESI-MS) and tandem mass spectrometry (LC-ESI-MS/MS) is described. Several environmentally relevant compounds, including chloro-, aryl- and alkylphenols, steroidal estrogens, and hydroxy-polycyclic aromatic hydrocarbons (OHPAHs), were selected to evaluate this technique. A facile derivatization procedure employing DMISC results in dimethylimidazolesulfonyl (DMIS) derivatives that are stable in aqueous solution. These DMIS derivatives produced intense [M+H]+ ions in positive-ion LC-ESI-MS. The product ion spectra of the [M+H]+ ions of simple phenols were dominated by ions representing the DMIS and dimethylimidazole moieties, whereas product ion spectra of the DMIS derivatives of OHPAHs with three or more fused aromatic rings showed prominent ArO+ ions, the relative intensity of which increased with the number of rings. The DMIS derivatives of the selected phenolic compounds showed excellent chromatographic properties. To substantiate the utility of derivatization with DMISC, an analytical method employing enzyme hydrolysis, solid phase extraction, derivatization with DMISC, and analysis by LC-ESI-MS/MS with multiple reaction monitoring for determination in human urine of 1-hydroxypyrene, a widely used biomarker for the assessment of human exposure to PAHs, was developed and validated.
Keywords: liquid chromatography electrospray ionization tandem mass spectrometry, chemical derivatization of phenols, dimethylimidazolesulfonyl derivatives, 1-hydroxypyrene
I. Introduction
Numerous drugs and other xenobiotics, their metabolites, and compounds of environmental concern are substituted phenols. Chloro-, aryl- and alkylphenols and steroidal estrogens are among the ubiquitous environmental pollutants introduced by agricultural, medicinal, and industrial activities. Numerous phenols have been identified as priority pollutants in numerous countries, and some are suspected to be endocrine disruptors [1,2]. Analysis of natural and synthetic estrogens is required for environmental as well as clinical purposes, since estrogens from human sources are widespread in the environment and are believed to cause adverse effects in wildlife [3]. Polycyclic aromatic hydrocarbon (PAH) metabolites are important biomarkers for determining human exposure to PAHs [4]. Development of sensitive, reliable and robust approaches for the analysis of these and other phenolic compounds in human (e.g., serum and urine) and environmental samples (e.g., water and sediment) continues to be a research goal.
The major challenge in the analysis of phenolic compounds is to attain the high sensitivity required for determination of these compounds at the trace levels present in many human and environmental samples. Compounds with phenolic groups have been determined by a variety of methods including gas chromatography/mass spectrometry (GC/MS) after solid phase extraction (SPE) and derivatization by trimethylsilation [5-7] and high-performance liquid chromatography (HPLC) with fluorescence detection [8]. Recently, liquid chromatography electrospray ionization mass spectrometry (LC-ESI-MS) and tandem mass spectrometry (LC-ESI-MS/MS) in the negative ion mode have been employed to analyze these compounds [9-12]. However, many phenols are not strong acids and therefore show low ionization efficiency in ESI-MS, leading to higher detection limits compared with the limits that are obtained for many highly ionizable compounds. Efforts have been focused on chemical derivatization as a method for enhancing the detection of phenolic compounds by HPLC with fluorescence [13] and mass spectrometric detection [14]. For example, derivatization of phenols with dansyl chloride was originally developed to incorporate a fluorogenic label into the analytes to improve fluorescence detection in HPLC [15,16]. Recently, derivatization with dansyl chloride has also been used to enhance the sensitivity of LC-ESI-MS/MS analysis of phenolic compounds [17-21].
In this paper, we present a novel derivatization scheme employing 1,2-dimethylimidazole-4-sulfonyl chloride (DMISC) to improve the sensitivity of LC-ESI-MS/MS for the determination of phenolic compounds. Several environmentally relevant phenolic compounds were selected for evaluation of the derivatization technique. We then developed and validated an analytical method incorporating derivatization with DMSIC and analysis by LC-ESI-MS/MS for the determination of 1-hydroxypyrene, a widely used biomarker of PAH exposure, in human urine.
2. Experimental
2.1. Reagents and Chemicals
2-Chlorophenol, 4-phenylphenol, 4-tert-octylphenol, 1-naphthol, 2-naphthol, 9-phenanthrol, 1-hydroxypyrene, estrone (E1), estradiol (E2), and ethinylestradiol (EE2), and type H-2 β-glucuronidase were purchased from Sigma-Aldrich (St. Louis, MO). 3-Hydroxybenzo[k]fluoranthene and 3-phenanthrol were purchased from the NCI Chemical Carcinogen Reference Standard Repository, Midwest Research Institute (Kansas City, MO). 1-Hydroxypyrene-d9 (98%) was purchased from Toronto Research Chemicals (Ontario, Canada). DMISC was purchased from Oakwood Products (West Columbia, SC). Artificial urine was obtained from Ward’s Natural Science (Rochester, NY). SPEC C18columns (3 mL) were purchased from Varian (Palo Alto, CA). Phenol was purchased from Invitrogen (Carlsbad, CA). HPLC-grade acetonitrile was purchased from J.T. Baker (Phillipsburg, NJ). Analytical-grade acetone, hexane, and methanol were purchased from Mallinckrodt Baker (Paris, KY). Water was purified with a MilliQ system (Millipore, Billerica, MA). A human urine pool obtained from healthy, non-smoking volunteers was used for validation of the analytical method for 1-hydroxypyrene. Aliquots of this urine pool were stored at -80 °C.
2.2. Derivatization
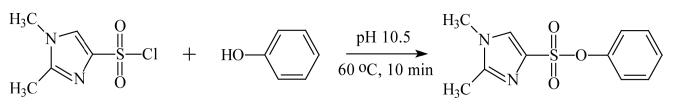
Formation of DMISC derivatives was performed in an analogous manner to that described for dansylation (17), but employing DMISC rather than dansyl chloride. An aliquot of analytical standard in methanolic solution or purified urine extract was added to a reaction vial and evaporated to dryness under N2. The residue was re-dissolved in 100 μL of 0.1 M sodium bicarbonate buffer, pH 10.5, followed by the addition of 100 μL of 1.0 mg/mL DMISC in acetone. For the derivatization of phenol and 2-chlorophenol, the evaporation step was omitted due to the volatility of these analytes. Sodium bicarbonate buffer and DMISC were added directly to solutions of these phenols. After addition of DMISC, vials were vortexed, placed in a heater block preset at 60 °C, and allowed to react for 10 min (Fig. 1). The reaction mixtures were then cooled to room temperature and extracted twice with 1-mL portions of n-hexane. The combined organic extracts were evaporated to dryness under N2 and re-dissolved in 100 μL portions of acetonitrile/water (50/50, v/v) for analysis by LC-ESI-MS/MS.
Fig. 1.
Derivatization of phenol by reaction with DMISC.
2.3. Analysis of 1-Hydroxypyrene in Human Urine
2.3.1. Sample Preparation
Sample preparation was carried out as described by Smith et.al. [7], but with a slight modification of the SPE procedure. Aliquots (10 μL) of the internal standard, 1-hydroxypyrene-d9 (0.2 μg/mL in methanol), were added to 1-mL samples of human urine. These samples were then mixed with 1.5-mL portions of sodium acetate buffer (0.1 M, pH 5.0), and 5 μL aliquots of β-glucuronidase/sulfatase (100,000 units/mL of β-glucuronidase and 7,500 units/mL of sulftase) were added. The samples were then incubated at 37 °C for 2 h, after which they were loaded onto C18 SPE columns that had been preconditioned with 250 μL of methanol and 250 μL of water. The columns were washed with 250 μL of 90:10 (v/v) water:methanol, and 1-hydroxypyrene was eluted from the columns with 300 μL portions of methanol. The methanol eluates were evaporated under N2, and the 1-hydroxypyrene was derivatized following the procedure described above for analysis by LC-ESI-MS/MS.
2.3.2. Analysis by LC-ESI-MS/MS
The analytical instrumentation consisted of an Agilent 1100 series liquid chromatograph interfaced with an Applied Biosystems/MDS Sciex API 2000 triple quadrupole mass spectrometer equipped with a turbo ion spray source. A Luna 3μ Phenyl-Hexyl column (2.0 × 150 mm, 3μm particle size; Phenomenex, Torrance, CA) was used for LC-ESI-MS/MS analysis. The injection volume was 20 μL. The mobile phase program consisted of an initial hold at 70% solvent A (0.1% acetic acid in H2O for positive ion LC-ESI-MS/MS, or 10 mM ammonium acetate for negative ion LC-ESI-MS/MS)/30% solvent B (acetonitrile) for 1 min, followed by a linear gradient to 50% A/50% B over 4 min, a linear gradient to 30% A/70 % B in 15 min, a hold at 70% B for 2 min, a linear gradient returning to 70% A over 3 min, and a re-equilibration hold at 70% A/30% B for 8 min prior to the next sample injection. The flow rate was 0.2 mL/min, and the column was at ambient temperature. LC-ESI-MS and LC-ESI-MS/MS were performed in the positive ion mode. Full-scan (Q1) LC-ESI-MS was performed over the range of m/z 200 to 500 with 1.5-s scans. In LC-ESI-MS/MS product ion scanning, [M+H]+ ions identified in previous LC-ESI-MS analyses were isolated by Q1, and were subjected to collision-induced dissociation (CID) in Q2; Q3 was operated in the scanning mode from m/z 80 to at least 20 m/z above the value of the [M+H]+ under analysis with 1.5-s scans. For the analysis of 1-hydroxypyrene as its DMIS derivative by LC-ESI-MS/MS with multiple reaction monitoring (MRM), the ion source and instrumental parameters were optimized for 1-hydroxypyrene-DMIS, monitoring the m/z 377 → 217 MS/MS transition, and 1-hydroxypyrene-d9-DMIS, monitoring the m/z 386 → 226 MS/MS transition, using flow injection. The dwell times were 150 ms, and both Q1 and Q3 were operated with unit mass resolution. Nitrogen was used as the curtain gas (setting 30), gas 1 (setting 60), gas 2 (setting 30), and the collision gas (setting 12). The declustering potential, entrance potential, collision energy, and collision exit potential were 60, 6, 30 and 10 V, respectively. The electrospray voltage was set at 5000 V, and the turbo gas temperature was set at 500 °C. For comparative analyses of underivatized 1-hydroxypyrene by LC-ESI-MS/MS using negative ion detection, conditions were similarly optimized for detection of the MS/MS transition of m/z 217 → 189. Mass spectral data were analyzed using Analyst 1.4 software (Applied Biosystems MDS Sciex, Concord, ON, Canada).
2.3.3 Preparation of 1-hydroxypyrene standard solutions and calibration curves
The stock solution of 0.2 mg/mL 1-hydroxypyrene was obtained by dissolving 2.0 mg of 1-hydroxypyrene in 10 mL of methanol. Working standards of 1, 10, 100, and 10,000 ng/mL were prepared by serial dilution of the stock solution into methanol, and were stored in amber vials at -80 °C under N2. The stock solution of 1-hydroxypyrene-d9 was prepared as above and diluted to 0.2 μg/mL. Because humans are continually exposed to PAHs, samples of human urine invariably contain some level of 1-hydroxypyrene. Therefore, artificial urine was used as the matrix to prepare calibration standards. These standards were prepared by adding the appropriate amount of working standards to 1-mL aliquots of artificial urine to give the final concentrations of 10, 20, 50, 100, 200, 500, and 1000 ng/L. These calibration standards were analyzed by following the sample preparation procedure described above.
2.3.4 Limit of Detection (LOD) and Limit of Quantitation (LOQ)
The LOD and LOQ for determination of 1-hydroxypyrene by this method were calculated as 3.3S0 and 10S0 respectively [22], where S0 is the standard deviation from the analysis of eight artificial urine samples spiked at a concentration at 20 ng/L.
2.3.5 Method validation
To validate this method for 1-hydroxypyrene analysis, aliquots (1 mL) of artificial urine were spiked with appropriate amounts of working standards of 1-hydroxypyrene, in quadruplicate, to achieve concentrations of 50 ng/L (low level), 200 ng/L (medium level), and 800 ng/L (high level). To each of these samples, 10 μL aliquots of 0.2 μg/mL 1-hydroxypyrene-d9 were added as the internal standard. The samples were prepared as described above prior to analysis by LC-ESI-MS/MS. Three validation batches were prepared to assess the precision and accuracy of this method, and each batch was analyzed on a different day. Each batch included one set of calibration standards and four replicates of the low level, medium level, and high level spiked samples prepared above.
2.3.5 Analysis of 1-hydroxyprene in human urine
Aliquots (1 mL) of the human urine pool, unspiked or spiked with final concentrations of 0, 50, 100, and 200 ng/L (n = 4 at each level), were analyzed as described above. The total 1-hydroxypyrene concentrations in the unspiked and spiked samples were calculated based on the calibration curve. The accuracy of the method was evaluated by comparing the measured concentrations with the sum of the calculated endogenous and spiked concentrations.
3. Results and Discussion
3.1. LC-ESI-MS/MS of the DMIS derivatives of phenol and 2-chlorophenol
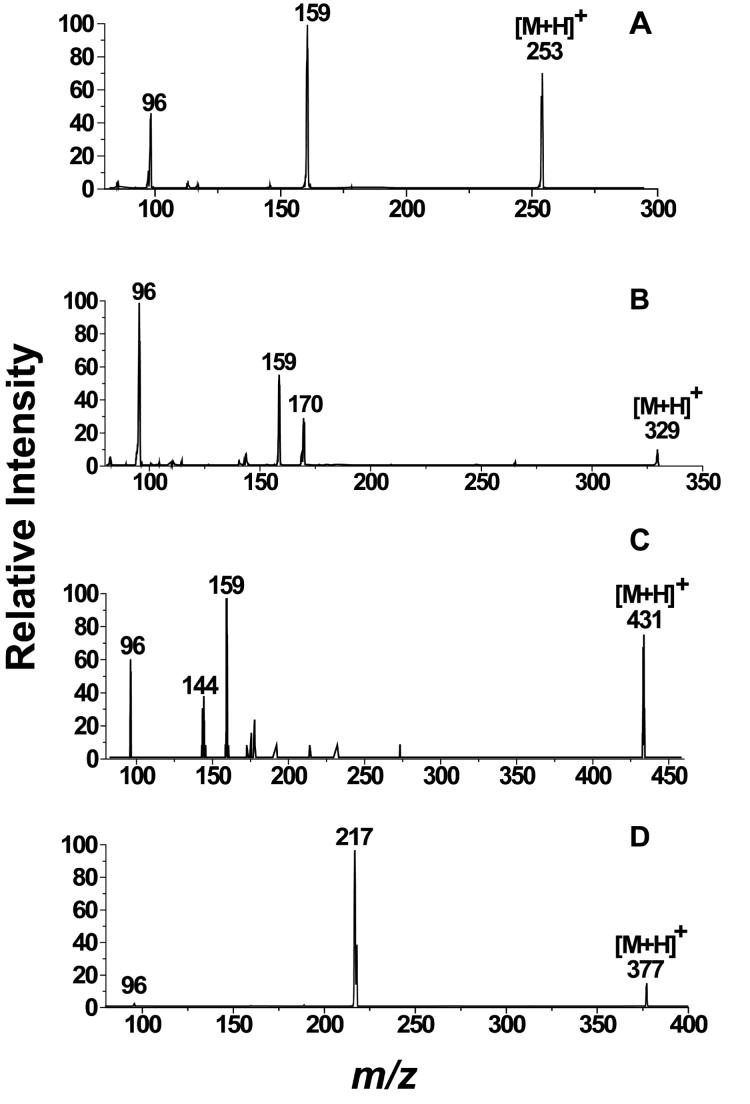
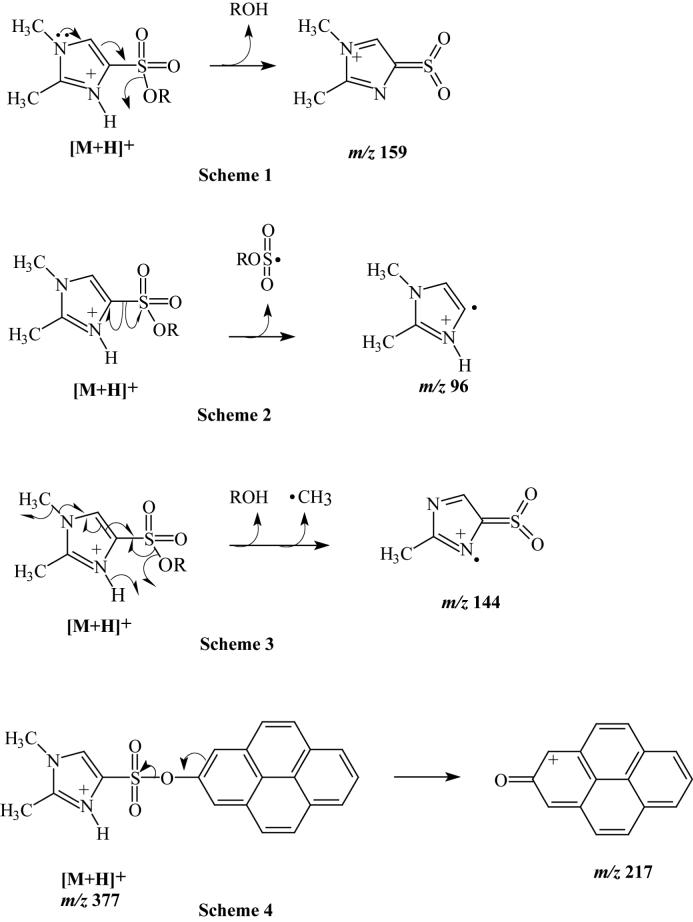
The full-scan mass spectrum of the DMIS derivative of phenol showed an intense [M+H]+ ion at m/z 253, and upon CID, ions of m/z 159 and 96 were the only ones observed in the product ion spectrum (Fig. 2A). The relative intensities of the product ions are given in Table 1. We propose that these ions of m/z 159 and 96, which were observed in most of the MS/MS spectra of DMIS derivatives that we recorded, are formed by heterolytic cleavage of the S-O bond between the DMIS and phenol groups, and homolytic cleavage of the C-S bond between the imidazole ring and the sulfonyl group, respectively (Fig. 3, Schemes 1 and 2). The ESI product ion spectrum of the DMIS derivative of 2-chlorophenol showed a similar fragmentation pattern. The 35Cl-containing [M+H]+ isotopic peak of 2-chlorophenol-DMIS at m/z 287 was selected in the first quadrupole and, upon CID, the product ion mass spectrum, like that of derivatized phenol, showed product ions at m/z 159 and 96.
Fig. 2.
LC-ESI-MS/MS product ion spectra of the [M+H]+ ions of DMIS derivatives. Shown are product ion spectra of (A) DMIS-phenol at 25 eV collision energy; (B) DMIS-phenylphenol at 30 eV collision energy; (C) DMIS-E2 at 40 eV energy; and (D) DMIS-1-hydroxypyrene at 25 eV collision energy.
Table 1.
LC-ESI-MS/MS product ion spectra of DMIS derivatives.
| Analyte | DMIS derivative, m/z of [M+H]+ | Collision Energy, eV | Product ions, m/z (% Relative intensity) |
|---|---|---|---|
| Phenol | 253 | 25 | 159 (100), 96 (46) |
| 2-Chlorophenol | 287 | 25 | 159 (100), 96 (68) |
| 4-Phenylphenol | 329 | 30 | 96 (100), 159 (56), 170 (31) |
| 4-tert-Octylphenol | 365 | 35 | 159 (100), 96 (60), 88 (51), 253 (24), 144 (18), 135 (15) |
| E1 | 429 | 40 | 159 (100), 96 (38), 144 (18) |
| E2 | 431 | 40 | 159 (100), 96 (64), 144 (42) |
| EE2 | 455 | 40 | 159 (100), 96 (31), 144 (29), 133 (17), 213 (15) |
| 1-Naphthol | 303 | 30 | 96 (100), 144 (39), 159 (26), 143 (18), 115(6) |
| 2-Naphthol | 303 | 30 | 159 (100), 96 (90), 144 (48) |
| 3-Phenanthrol | 353 | 30 | 194 (100), 96 (45), 159 (26), 193 (21), 165 (15), 144 (4) |
| 9-Phenanthrol | 353 | 30 | 96 (100), 165 (66), 144 (59), 194 (59), 159 (45), 101 (42), 143 (40), 145 (40), 193 (28), 127 (23) |
| 1-Hydroxypyrene | 377 | 25 | 217 (100), 218 (43) |
| 8-Hydroxybenzo(k)fluoranthene | 427 | 30 | 267 (100), 268 (70), 96 (12) |
Fig. 3.
Proposed fragmentation pathways for CID of the DMIS derivatives of phenolic compounds based on LC-ESI-MS/MS product ion spectra. Schemes 1 through 3 are proposed for the formation of ions of m/z 159, 96, and 144 from the dimethylimidazole moieties of the protonated DMIS derivatives of phenolic compounds that are commonly observed in the product ion spectra. Scheme 4 is proposed for the formation of a highly resonance-stabilized ion derived from the 1-hydroxypyrene moiety after heterolytic cleavage of the protonated 1-hydroxypyrene DMIS derivative.
3.2. LC-ESI-MS/MS of the DMIS derivatives of 4-phenylphenol and 4-tert-octylphenol
Similar to the product ion spectra of the DMIS derivatives of phenol and 2-chlorophenol, ions at m/z 159 and 96 were the main peaks in the product ion spectra of derivatized 4-phenylphenol and 4-tert-octylphenol. The product ion at m/z 144 is thought to represent a radical cation formed by homolytic cleavages of the S-O bond between the phenol moiety and the DMIS group, and the N-C bond between the methyl group, and the imidazole nitrogen, with loss of ROH (the original phenol) and a methyl radical (Fig. 3, Scheme 3). We propose that the ion of m/z 170 present in the product ion spectrum of the [M+H]+ ion of 4-phenylphenol derivative (Fig. 2B) represents an ArOH+. ion formed by homolytic cleavage of the S-O bond between phenolic oxygen and the DMIS moiety with proton transfer. A product ion at m/z 253 was observed in the product ion spectrum of the [M+H]+ ion of the 4-tert-octylphenol derivative (Table 1). This ion could be produced from the loss of the alkyl moiety (C8H16, 112 Da) with hydrogen rearrangement.
3.3. LC-ESI-MS/MS of the DMIS derivatives of estrogens
One characteristic of chemical derivatization using DMISC is that reaction occurs with phenolic hydroxyl groups, but not with aliphatic hydroxyl groups. Steroidal estrogens generally have an A ring phenolic hydroxyl at C-3, which reacts with DMISC. The potent estrogens E2 and EE2 also have a D-ring aliphatic hydroxyl at C-17β, which does not react with DMISC. The product ion spectrum from CID of the [M+H]+ ion of the DMIS derivative of E2 is shown in Figure 2C. The product ion spectra of the [M+H]+ ions of the DMISC derivatives of the estrogens, E2, E1 and EE2 each showed m/z 159 as the base peak (Fig. 3, Scheme 1; Table 1), the dimethylimidazole ion at m/z 96 (Figure 3, Scheme 2), and the peak at m/z 144 representing the loss of ROH and a methyl radical from the [M+H]+ ion (Fig. 3, Scheme 3).
3.4. LC-ESI-MS/MS of the DMIS derivatives of hydroxy-polycyclic aromatic hydrocarbons (OHPAHs)
Full-scan mass spectra showed intense [M+H]+ ions for the DMIS derivatives of OHPAHs. Upon CID, ions of m/z 159, 96, and 144 (Fig. 3, Schemes 1-3), and ions representing ArOH+., ArO+ (Fig. 3, Scheme 4), and the loss of CO from ArO+ ([ArO-CO]+), were the major components of the product ion spectra of [M+H]+. These peaks had varying intensities, indicating the predominance of differing fragmentation mechanisms for OHPAH derivatives dependent on the number of aromatic rings (Table 1). The intensity of the peaks for ArO+ ions in the product ion spectra increased with the increasing number of fused aromatic rings in these compounds. The product ion spectra of derivatized 1- and 2-naphthol showed weak signals for the ArO+ ions at m/z 143 and for the [ArO-CO]+ ions at m/z 115. For derivatized 3-phenanthrol, the ArOH+. ion at m/z 194 was the base peak in the product ion spectrum; however, peaks at m/z 193 representing ArO+ and at m/z 165 representing [ArO-CO]+ were also of significant intensity. In the product ion spectra of [M+H]+ ions of derivatized OHPAH metabolites with four and five aromatic rings, represented by 1-hydroxypyrene and 3-hydroxybenzo(k)fluoranthene, respectively, peaks for the ArO+ ions were the most intense; no peaks indicative of [ArO-CO]+ ions were observed in the product ion spectra under these conditions (Fig. 2D, Table 1).
Enhancement of the sensitivity of 1-hydroxypyrene analysis by DMISC derivatization
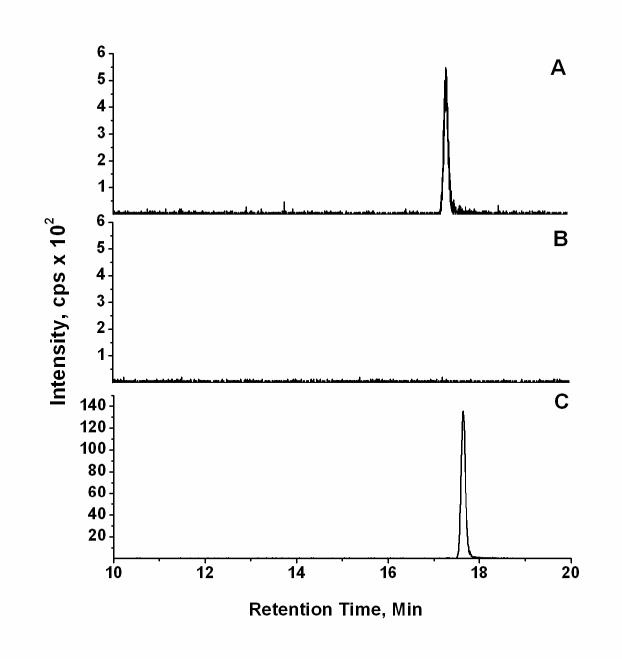
A comparison of the analysis of identical amounts of underivatized 1-hyroxypyrene by LC-ESI-MS/MS with negative ion detection of the m/z 217 → 189 MS/MS transition with that of the DMIS derivative of 1-hydroxypyrene with positive ion detection of the m/z 377 → 217 transition is shown in Fig. 4. The peak for underivatized 1-hydroxypyrene (500 pg on column) at retention time 17.3 min in Fig. 4A was not observed after reaction with DMISC (Fig 4B), indicating the derivatization reaction proceeded to >90% completion. Additional analyses using HPLC with fluorescence and UV detection indicated that the derivatization reactions for each of the compounds listed in Table 1 also occurred to >90% completion (data not shown). Analysis of 1-hydroxyprene as its DMIS derivative by LC-ESI-MS/MS with positive ion detection (Fig 4C) compared with analysis of an equivalent amount of underivatized 1-hydroxypyrene by negative ion LC-ESI-MS/MS (Fig. 4A) showed a 28-fold enhancement in sensitivity for analysis of the DMIS derivative based on peak areas.
Fig. 4.
Comparative analysis of underivatized 1-hydroxypyrene and the 1-hydroxypyrene DMIS derivative. The analysis of (A) underivatized 1-hydroxypyrene (500 pg on column) by negative ion LC-ESI-MS/MS, monitoring the MS/MS transition of m/z 217 → 189, is shown. An identical sample of 1-hydroxypyrene was subjected to derivatization with DMISC, and the final extract was analyzed for (B) unreacted 1-hydroxyprene by negative ion LC-ESI-MS/MS, monitoring the MS/MS transition of m/z 217 → 189, and (C) for the 1-hydroxypyrene DMIS derivative by positive ion LC-ESI-MS/MS, monitoring the MS/MS transition of m/z 377 → 217. Chromatographic conditions were the same in each case, with the exception that the mobile phase solvent A was 10 mM ammonium acetate in (A) and (B), and 0.1% acetic acid in (C).
3.5. Linearity, sensitivity, accuracy, and precision for 1-hydroxypyrene analysis
A set of calibration standards was prepared, and a calibration curve was repeated for each validation batch and LOD/LOQ experiment. The seven-point calibration curves showed excellent linearity (r2 ≥ 0.998) of the ratios of the analyte to internal standard responses with increasing 1-hydroxypyrene over the 10 to 1000 pg range and a constant amount (2 ng) of 1-hydroxypyrene-d9. These curves were used to determine the levels of 1-hydroxypyrene in the artificial urine samples that had been spiked at the levels of 50, 200, and 800 ng/L, and human urine samples that had been spiked at the levels of 0, 50, 100, and 200 ng/L. The LOD and LOQ of the method were determined to be 9.7 and 27 ng/L respectively. This calculated LOQ indicates that the method is sufficiently sensitive for determining 1-hydroxypyrene in urine of human exposure to environmental PAHs, as this value is well below reported mean background levels of 1-hydroxypyrene [23]. The analytical results for the determination of 1-hydroxypyrene at the three spike levels into artificial urine samples are presented in Table 2. The accuracy of the method was evaluated by the relative error (RE) and the precision by the relative standard deviation (RSD). The accuracy of each of three assays for 1-hydroxypyrene in artificial urine at three spiked levels was within 5.1%, and the precision ranged from 0.7 to 8.2%.
Table 2.
Interday analysis of 1-hydroxypyrene in spiked artificial urine.
| Addition of |
||||
|---|---|---|---|---|
| 50 pg/ml | 200 pg/ml | 800 pg/ml | ||
| Day 1 (n=4) | Mean | 50.1 | 204.2 | 806 |
| RSD% | 4.5 | 2.2 | 1.6 | |
| RE% | 0.2 | 2.1 | 0.8 | |
| Day 2 (n=4) | Mean | 47.4 | 202 | 791 |
| RSD% | 6.6 | 1.6 | 2.8 | |
| RE% | -5.1 | 1.0 | -1.1 | |
| Day 3 (n=4) | Mean | 48.7 | 208.2 | 802.8 |
| RSD% | 8.2 | 4.3 | 0.7 | |
| RE% | -2.6 | 4.1 | 0.3 | |
| Total interday (n=12) | Mean | 48.7 | 204.8 | 800.1 |
| RSD% | 6.4 | 3.0 | 1.9 | |
| RE% | -2.6 | 2.4 | 0.01 | |
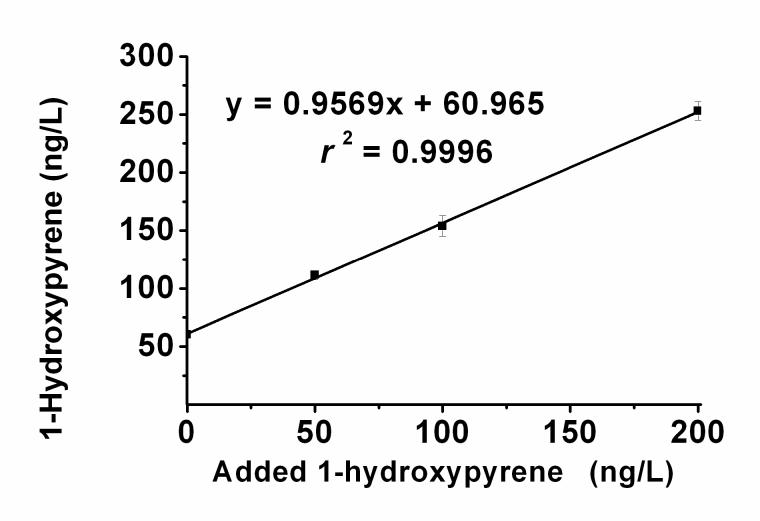
The method was also successfully applied to the analysis of 1-hydroxypyrene in a urine pool form non-smoking human subjects. Typical selected reaction ion chromatograms from the analysis of artificial urine as the blank and of samples of human urine pool, unspiked and spiked with 50, 100, and 200 ng/L 1-hydroxypyrene, are shown in Figure 5. The selected reaction of m/z 377 → 217 used in our method was found to be highly specific. There were no significant interferences from human urine constituents affecting our analysis of 1-hydroxypyrene in the selected reaction ion chromatograms. The accuracy and precision of the method for the determination of 1-hydroxypyrene in human urine is summarized in Table 3. The accuracy of the method, calculated by comparing measured concentrations in spiked human urine with the sum of the measured endogenous and spiked concentrations, were within 4.0% of the expected values, and precision ranged from 3.2% to 5.7%. Figure 6 shows the excellent linearity (r2 = 0.9996) obtained when the measured concentrations were plotted against the spiked levels of 1-hydroxypyrine. The theoretical value for the endogenous 1-hydroxypyrine concentration (the y intercept) was in close agreement with the measured concentration for the human urine pool (Fig. 6, Table 3).
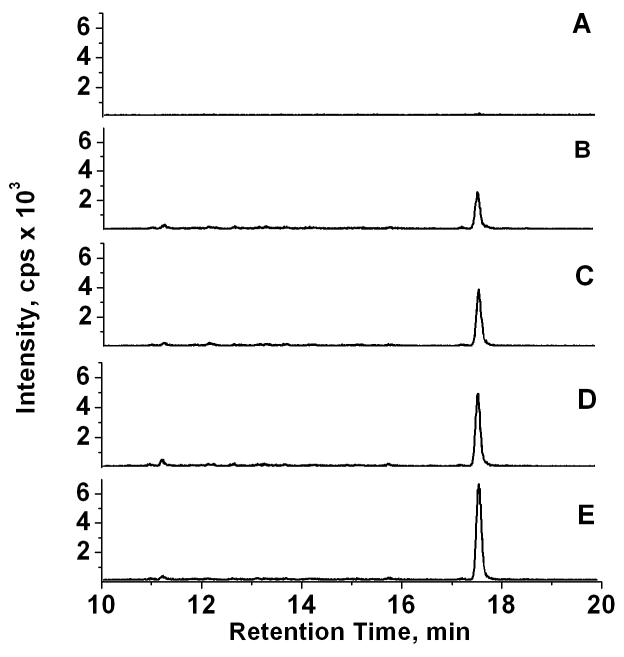
Fig. 5.
LC-ESI-MS/MS analysis of urinary 1-hydroxypyrene as its DMIS derivative. Samples (1 mL) were processed as described in the Experimental section. Shown are selected reaction ion chromatograms from the analysis of 1-hydroxypyrene as its DMIS derivative, monitoring the positive ion MS/MS transition of m/z 377 → 217, in (A) artificial urine as the blank, (B) the unspiked human urine pool, and the urine pool spiked with (C) 50 pg, (D) 100 pg, and (E) 200 pg of 1-hydroxypyrene.
Table 3.
Accuracy and precision of 1-hydroxypyrene analysis with human urine samples.
| Addition of |
||||
|---|---|---|---|---|
| Endogenous | 50 pg/ml | 100 pg/ml | 200 pg/ml | |
| Mean (n = 4) | 60.3 | 111.5 | 154 | 253 |
| RSD (%) | 3.5 | 3.6 | 5.7 | 3.2 |
| RE (%) | NA | 1.1 | -3.9 | -2.8 |
Fig. 6.
Analytical recovery of 1-hydroxypyrene spiked into human urine. Samples of the unspiked urine pool and the pool spiked with 50, 100, or 200 pg of 1-hydroxypyrene (n = 4 for each group) were analyzed as in Figure 5. The measured 1-hydroxypyrene concentrations were plotted versus those added, and the data were subjected to linear regression analysis.
3.6. Internal standard recovery
Internal standard recovery for the method was calculated through comparison of the peak areas for the internal standard MS/MS transition of m/z 386 → 226 from the analysis of spiked artificial urine samples subjected to an extraction/derivatization procedure - including C18 SPE, derivatization with DMISC, and liquid-liquid extraction with hexane - with those directly analyzed after DMISC derivatization without extraction. The mean internal standard recovery from this procedure was 97.5 %. For the analysis of human urine, the internal standard was added prior to the incubation with β-glucuronidase/sulfatase for conjugate hydrolysis. Mean internal standard recovery for this procedure was 83.9 %, which is comparable to those of other analytical procedures for 1-hydroxypyrene in which the enzymatic hydrolysis of conjugates is incorporated into the procedure [7,24].
4. Conclusions
A novel derivatization scheme employing DMISC to improve the sensitivity of LC/ESI-MS/MS for the determination of phenolic compounds has been developed. The reactive functional group of DMISC is sulfonyl chloride, which is also the reactive group in dansyl chloride, a reagent used to derivatize phenolic compounds to enhance LC-ESI-MS/MS sensitivity in several recent applications [17-21]. Compared with dansyl chloride, DMISC has two advantages for LC-ESI-MS/MS analysis. Firstly, compared with the calculated pKa of 3.3 for the acid form of the dansyl group [16], the pKa of dimethylimidazole acid form is 8.0 [25]. Thus, at many values of pH typically used in LC-ESI-MS/MS analyses, a DMIS derivative will show a higher degree of ionization than will the comparable dansyl derivative, and thus higher ionization efficiency in ESI [26], possibly leading to lower detection limits. A second advantage of derivatization with DMISC was observed in the MS/MS spectra of OHPAH derivatives. For OHPAH with more than three conjugated aromatic rings, such as 1-hydroxypyrene, the mass spectra of DMIS derivatives show high relative intensities of specific ArO+ ions, whereas reported product ion spectra of dansyl derivatives of 1-hydroxypyrene have predominantly shown ions representing the dansyl moiety [18]. The results reported here demonstrate the utility of derivatization with DMISC in analyses by LC-ESI-MS/MS, and indicate that this procedure represents a viable alternative or complementary technique to the use of dansyl chloride for the derivatization of phenolic compounds and metabolites. To substantiate the utility of derivatization with DMISC, we developed and validated an accurate, precise, and sensitive analytical method with analysis by LC-ESI-MS/MS for the determination of 1-hydroxypyrene, a widely used biomarker of PAH exposure, in human urine.
Acknowledgements
This work was supported by U.S. Center for Disease Control and Prevention Cooperative Agreement Number U1Q/CCU221159-04-2 and U.S. National Institutes of Health-National Cancer Institute Grant CA81243. The authors gratefully acknowledge use of the Wadsworth Center Biochemistry Core Facility.
References
- [1].Chlorophenols . Environmental Health Criteria Monographs (EHCs) 93; International Programme on Chemistry Safety (IPCS) World Health Organization; Geneva: 1989. [Google Scholar]
- [2].Kwack SJ, Kwon O, Kim HS, Kim SS, Kim SH, Sohn KH, Lee RD, Park CH. J. Toxicol. Environ. Health, Part A. 2002;65:419. doi: 10.1080/15287390252808082. [DOI] [PubMed] [Google Scholar]
- [3].Sumpter JP. Toxicol. Lett. 1998;102-103:337. doi: 10.1016/s0378-4274(98)00328-2. [DOI] [PubMed] [Google Scholar]
- [4].Brandt HCA, Watson WP. Ann. Occup. Hyg. 2003;47:349. doi: 10.1093/annhyg/meg052. [DOI] [PubMed] [Google Scholar]
- [5].Spink DC, Lincoln DW, II, Dickerman HW, Gierthy JF. Proc. Natl. Acad. Sci. USA. 1990;87:6917. doi: 10.1073/pnas.87.17.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kuklenyik Z, Ekong J, Cutchins CD, Needham LL, Calafat AM. Anal. Chem. 2003;75:6820. doi: 10.1021/ac0303158. [DOI] [PubMed] [Google Scholar]
- [7].Smith CJ, Walcott CJ, Huang W, Maggio V, Crainger J, Patterson DG., Jr. J. Chromatogr. B. 2002;778:157. doi: 10.1016/s0378-4347(01)00456-x. [DOI] [PubMed] [Google Scholar]
- [8].Hollender J, Koch B, Dott W. J. Chromatogr. B. 2000;739:225–229. doi: 10.1016/s0378-4347(99)00470-3. [DOI] [PubMed] [Google Scholar]
- [9].Ye X, Kuklenyik Z, Needham LL, Calafat AM. Anal. Chem. 2005;77:5407. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- [10].Sarrion MN, Santos FJ, Moyano E, Galceran MT. Rapid Commun. Mass Spectrom. 2003;17:39. doi: 10.1002/rcm.873. [DOI] [PubMed] [Google Scholar]
- [11].Xu X, Zhang J, Zhang L, Liu W, Weisel CP. Rapid Commun. Mass Spectrom. 2004;18:2299. doi: 10.1002/rcm.1625. [DOI] [PubMed] [Google Scholar]
- [12].Fan R, Dong Y, Zhang W, Wang Y, Yu Z, Sheng G, Fu J. J. Chromatogr. B. 2006;836:92. doi: 10.1016/j.jchromb.2006.03.044. [DOI] [PubMed] [Google Scholar]
- [13].Suliman FEO, Al-Kindi SS, Al-Kindy SMZ, Al-Lawati HAJ. J. Chromatogr. A. 2006;1101:179. doi: 10.1016/j.chroma.2005.09.094. [DOI] [PubMed] [Google Scholar]
- [14].Quirke JM, Adams CL, Berkel GJV. Anal. Chem. 1994;66:1302. [Google Scholar]
- [15].Feri-Hausler M, Feri RW. J. Chromatogr. 1973;79:209. doi: 10.1016/s0021-9673(01)85290-0. [DOI] [PubMed] [Google Scholar]
- [16].Krol GJ, Mannan CA, Pickering RE, Amato DV, Kho BT, Sonnenschein A. Anal. Chem. 1977;49:1836. doi: 10.1021/ac50020a049. [DOI] [PubMed] [Google Scholar]
- [17].Anari MR, Bakhtiar R, Zhu B, Husky S, Franklin RB, Evans DC. Anal. Chem. 2002;74:4136. doi: 10.1021/ac025712h. [DOI] [PubMed] [Google Scholar]
- [18].Li Y, Li AC, Shi H, Zhou S, Shou WZ, Jiang X, Naidong W, Lauterbach JH. Rapid Commun. Mass Spectrom. 2005;19:3331. doi: 10.1002/rcm.2196. [DOI] [PubMed] [Google Scholar]
- [19].Zhang F, Bartels MJ, Brodeur JC, McClymont EL, Woodburn KB. Rapid Commun. Mass Spectrom. 2004;18:2739. doi: 10.1002/rcm.1690. [DOI] [PubMed] [Google Scholar]
- [20].Nelson RE, Grebe SK, O’Kane DJ, Singh RJ. Clin. Chem. 2004;50:373. doi: 10.1373/clinchem.2003.025478. [DOI] [PubMed] [Google Scholar]
- [21].Tai SSC, Welch MJ. Anal. Chem. 2005;77:6359. doi: 10.1021/ac050837i. [DOI] [PubMed] [Google Scholar]
- [22].Taylor JK. Quality Assurance of Chemical Measurements. Lewis Publishers; Chelsea, Michigan: p. 79. [Google Scholar]
- [23].Huang W, Caudill SP, Grainger J, Needham LL, Patterson DG., Jr. Toxicol. Lett. 2006;163:10. doi: 10.1016/j.toxlet.2005.08.003. [DOI] [PubMed] [Google Scholar]
- [24].Li Z, Roanloff LC, Trinidad DA, hussain N, Jones RS, Porter EN, Patterson DG, Jr., Sjödin A. Anal. Chem. 2006;78:5744. doi: 10.1021/ac0606094. [DOI] [PubMed] [Google Scholar]
- [25].Newmyer SL, Ortiz de Montellano PR. J. Biol. Chem. 1996;271:14891. doi: 10.1074/jbc.271.25.14891. [DOI] [PubMed] [Google Scholar]
- [26].Enke CG. Anal. Chem. 1997;69:4885. doi: 10.1021/ac970095w. [DOI] [PubMed] [Google Scholar]