Abstract
Exposure of adult humans to manganese (Mn) has long been known to cause neurotoxicity. Recent evidence also suggests that exposure of children to Mn is associated with developmental neurotoxicity. Astrocytes are critical for the proper functioning of the nervous system, and they play active roles in neurogenesis, synaptogenesis and synaptic neurotransmission. In this report, to help elucidate the molecular events underlying Mn neurotoxicity, we systematically identified the molecular targets of Mn in primary human astrocytes at a genome-wide level, by using microarray gene expression profiling and computational data analysis algorithms. We found that Mn altered the expression of diverse genes ranging from those encoding cytokines and transporters to signal transducers and transcriptional regulators. Particularly, 28 genes encoding proinflammatory chemokines, cytokines and related functions were up-regulated whereas 15 genes encoding functions involved in DNA replication and repair and cell cycle checkpoint control were down-regulated. Consistent with the increased expression of proinflammatory factors, analysis of common regulators revealed that 16 targets known to be positively affected by the interferon-γ signaling pathway were up-regulated by Mn2+. In addition, 68 genes were found to be similarly up- or down-regulated by both Mn2+ and hypoxia. These results from genomic analysis are further supported by data from real-time RT-PCR, Western blotting, flow cytometric and toxicological analyses. Together, these analyses show that Mn2+ selectively affects cell cycle progression, the expression of hypoxia-responsive genes, and the expression of proinflammatory factors in primary human astrocytes. These results provide important insights into the molecular mechanisms underlying Mn neurotoxicity.
Keywords: Manganese, Inflammatory factors, Human Astrocytes, Microarray, Gene expression
Introduction
Manganese (Mn) is an essential metal required for many enzymatic reactions such as those catalyzed by arginase, glutamine synthetase and manganese-dependent superoxide dismutase. However, elevated Mn exposures can lead to its accumulation in the brain and causes serious neurotoxicity (Crossgrove and Zheng, 2004; Dobson et al., 2004; Erikson et al., 2004a; Hazell, 2002; Newland, 1999; Pal et al., 1999). In humans, Mn deficiency is rare whereas Mn neurotoxicity or manganism can occur due to occupational and environmental exposures (Crossgrove and Zheng, 2004; Pal et al., 1999). It is estimated that over 3700 tons of Mn are released into the atmosphere every year (2001), particularly from the gasoline additive, methylcyclopentadienyl manganese tricarbonyl (MMT). Signs of manganism resemble those of idiopathic Parkinson's disease, including dystonia, bradykinesia, rigidity, and tremor (Barbeau, 1984; Newland, 1999; Pal et al., 1999). In exposed humans and monkeys, Mn accumulates at the highest levels in the striatum, globus pallidus, and substantia nigra (Erikson et al., 2004a). In monkeys dosed for three months with MnO2, Mn concentrations reached 264 μM in the striatum and 334 μM in the globus pallidus (Suzuki et al., 1975). Mn exposure may also cause developmental neurotoxicity in children. A recent cross-sectional study of 201 ten year-old children living in Araihazar, Bangladesh showed that water Mn was significantly and adversely associated with Performance and Full Scale raw intelligence score (Wasserman et al., 2005).
Previous studies have suggested that Mn2+ can induce oxidative stress and affect iron metabolism and cellular energy metabolism (Dobson et al., 2004; Erikson et al., 2004a; Hazell, 2002; Li et al., 2005; Lu et al., 2005). Nonetheless, the understanding of the specific mechanisms underlying Mn neurotoxicity in humans remains far from clear. In mammals, Mn is absorbed and transported to the brain by the mechanisms responsible for the uptake of divalent metal ions, including the DMT-1-mediated and transferrin-mediated pathways (Erikson and Aschner, 2006; Erikson et al., 2004b). In the brain, astrocytes are a “sink” for Mn, with concentrations 10-50-fold higher than in neurons (Erikson and Aschner, 2006; Erikson et al., 2004b). Mn transport to astrocytes is significantly affected by Fe status. Also, evidence suggests that Mn transport involves multiple pathways that may be competitive or synergistic with iron transport (Crossgrove and Yokel, 2005; Erikson and Aschner, 2006; Erikson et al., 2004b).
In this report, we focus on the potential effects of Mn on human astrocytes. Astrocytes play active roles in many neuronal functions: maintaining ion and pH homeostasis, promoting the synthesis and removal of neurotransmitters, providing glucose supply and antioxidant defense, and regulating synaptic activity, synaptogenesis and neurogenesis (Auld and Robitaille, 2003; Magisretti and Ransom, 2002; Newman, 2003). Astrocytes are sensors of the brain environment, to which they immediately react on the genomic (gene expression) and non-genomic levels (Nedergaard and Dirnagl, 2005). Inflammatory activation of astrocytes or astrocyte dysfunctions are believed to be associated with several chronic neurological diseases, including prion, Alzheimer's and Parkinson's Diseases, and HIV-1 associated dementia (HAD) (Burwinkel et al., 2004; Choi et al., 2005; Meda et al., 2001; Wang et al., 2004). If Mn neurotoxicity is linked with inflammation, it is plausible that the initiating events may occur in astrocytes. One purpose of this study is to test the hypothesis that Mn may induce the expression of inflammatory mediators in astrocytes.
A new powerful way to begin identifying molecular targets of pathogenic insults to cells is to use microarray technology to obtain a general gene expression profile of affected versus control cells. Candidate affected genes and their functions can then be subjected to in-depth analysis to determine the mechanisms of their responses to a given stimulus. Here we applied this approach to identify candidate genes and gene families in human primary astrocytes that are affected by exposure of the cells to Mn2+. Furthermore, we applied biochemical and toxicological methods to confirm the prominent effects of Mn2+ in primary human astrocytes. Our studies suggest that Mn2+ selectively affects cell cycle progression and the expression of hypoxia-responsive genes and proinflammatory factors. These results provide new insights into the mechanisms underlying Mn neurotoxicity.
Materials and Methods
Primary human astrocytes and treatment
Astrocytes were isolated from second trimester (14–19 weeks of gestational age) human fetal brains obtained from elective abortions in full compliance with NIH guidelines, as previously described by Dr. Volsky and colleagues (Canki et al., 2001; Wang et al., 2004). Homogenous preparations of astrocytes were obtained using high-density culture conditions in the absence of growth factors in F12 Dulbecco's modified Eagles Medium (DMEM-F12) (GIBCO-Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS), penicillin, streptomycin, and gentamycin. Cells were maintained in this medium at 2–5 × 104 cells/cm2 and subcultured weekly up to six times. Cultures were regularly monitored by immunofluorescence staining for expression of the astrocytic marker glial fibrillary acidic protein (GFAP) and HAM-56 to identify cells of the monocyte lineage. Only cultures that contained ≥99% GFAP-positive astrocytes and no detectable HAM-56-positive cells were used in the experiments. Primary human astrocytes were treated with or without 200 μM MnCl2.
Cell growth and LDH assays
To measure the effect of MnCl2 on the growth rate of primary human astrocytes, 1.2×104 cells were plated on each well of 24-well plates. Cells were treated with the desired concentrations of MnCl2 for 7 days. Then, adherent, live cells were collected by trypsin treatment, and the number of cells was accounted by using a hemacytometer. At least three independent sets of data were collected in each experiment. To assess the effect of MnCl2 on cell viability of astrocytes, we measured the release of lactate dehydrogenase (LDH) into the medium. In this case, 7.5×104 cells were plated on each well of 24-well plates and treated with the desired concentrations of MnCl2 for 7 days. The medium was collected, and lactate dehydrogenase (LDH) activities were measured and calculated by using a kit purchased from Sigma (Saint Louis). The total cellular LDH activity was measured by detecting the activity in Triton X-100 lysed cells (Ehrich and Sharova, 2000).
RNA extraction and Affymetrix GeneChip expression analysis
Primary human astrocytes (passage 4) were treated with or without 200 μM MnCl2 for 7 days. Total RNA was extracted by using TRIzol reagent (GIBCOBRL Life Technologies). The quality of RNA was high as assessed by measuring absorbance at 260 and 280 nm, by gel electrophoresis, and by the quality of microarray data (see below). We isolated RNA from three independent batches of human astrocytes, which were from different subjects. No identifiable information from these subjects was available, in keeping with the guidelines on human subjects. Three independent batches of astrocytes from three different subjects, not the same subject, were used to ensure that our data and conclusions would not totally rely on one unidentified subject, who may have experienced influential environmental or genetic conditions. The synthesis of cDNAs and biotin-labeled cRNAs were carried out exactly as described in the Affymetrix GeneChip Expression Analysis Technical Manual (2000). The human genome U133 plus 2.0 arrays were purchased from Affymetrix, Inc. Probe hybridization and data collection were carried out by the Columbia University Affymetrix GeneChip processing center. Initial data analysis was performed by using the Affymetrix Microarray suite.
Computational analysis of microarray data
We analyzed microarray expression data by applying a series of quality control, statistical, filtering, gene ontology, and pathway analysis algorithms. First, by using GCOS1.2 with the advanced PLIER (probe logarithmic intensity error) algorithm, we calculated and examined the parameters reflecting the image quality of the arrays. Arrays with a high background level in any region were discarded and replaced. The average noise or background level was limited to less than 5%. The average intensity for those genes judged to be present was at least 10-fold higher than those judged to be absent. Also, arrays that deviated considerably in the percentage of present and absent genes from the majority of the arrays were replaced. Arrays with a β-actin 3′/5′ ratio greater than 2 were replaced. Next, microarray data were uploaded to GeneSpring 7.0 (Silicon Genetics) for further quality and statistical analysis. The data were normalized again by using the stringent per chip and per gene normalization algorithms; genes with low control signal (less than average) or not present in 1/3 of the samples were dropped out, before statistical analysis. The data were then analyzed by nonparametric two-way ANOVA (one parameter is cell, another parameter is treatment). The Benjamini & Hochberg false discovery rate multiple testing correction was applied with a false discovery rate of less than 5%. Two-way ANOVA was chosen for statistical analysis because the variations of astrocytes from different subject can be significant, and in certain cases, may exert stronger effects than the treatment of cells. Two-way ANOVA would take account of this and identify statistically significant genes whose transcript level was consistently altered by Mn. Second, to identify genes whose transcript level was significantly altered by Mn, we applied four consecutive filtering processes: The first three were applied to identify genes whose transcript level was altered by at least 1.5-fold (Mn-treated vs control) in every batch of human astrocytes. The last one was applied to identify genes whose overall transcript level calculated from three independent batches of astrocytes was altered by at least 2-fold. The folds of changes listed in the Tables are the overall changes calculated from data from the three batches of astrocytes by GeneSpring, which combines data from all three controls and three Mn2+-treated samples.
The identified Mn2+-altered genes were further analyzed and categorized by using various biological annotation programs including the DAVID/EASE program provided by NIAID (http://apps1.niaid.nih.gov/david/upload.asp) and the Gene Ontology (GO) algorithm in GeneSpring. Data from OMIM and the literature were also used to categorize the genes. Our analyses led to the identification of six notable functional groups of genes whose expression is altered by Mn2+ (see Results). Finally, we used another computational program called PathwayAssist (Stratagene Software) to analyze Mn2+-altered genes. This program uses information available in the current literature to identify common pathways, targets or regulators that are associated with the altered genes.
Quantitative real-time reverse-transcription-polymerase chain reaction (real-time RT-PCR)
Oligonucleotide primers for measuring transcript levels of genes were designed based on the sequences used to design microarray probes by using the primer 3 program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). β-actin was used as a control for the relative quantification of transcripts, because our microarray data showed that the β-actin transcript level was unaffected by Mn2+. RT-PCR reactions were performed by using a Roche LightCycler and the SYBR green kit according to specified protocols. Calculations were done by using the Roche LightCycler software (Bustin, 2002; Giulietti et al., 2001; Livak and Schmittgen, 2001). Primer sequences used for real-time PCR are listed in supplemental Table S1.
Flow cytometric analysis
Primary human astrocytes, seeded at 2×105 cells/cm2, were treated with no reagent (control) or 200 μM MnCl2 (Mn-treated) for 7 days. Then cells were collected by trypsinization and prepared for FACS analysis, as described (Darzynkiewicz et al., 1999). Briefly, cells were fixed in 70% ethanol and then stained with propidium iodide. 106 to 107 cells were used for each FACS analysis at the Columbia University Medical Center FACS analysis facility. Flow cytometric analysis was performed by using a Becton-Dickinson FACSCalibur instrument. Data were acquired by using the CellQuest PRO (Becton-Dickinson) software, and were analyzed by combining CellQuest PRO and FloJo (TreeStar) software.
Western blotting analysis
Astrocytes were washed twice in PBS, and whole-cell extracts were prepared by adding 10 packed cell volumes of 1x sample buffer (2% SDS, 100 mM dithiothreitol, 60 mM Tris pH 6.8, and 10% glycerol) and boiled for 5 minutes. Protein concentrations were determined by using the bicinchoninic acid (BCA) protein assay kit (Pierce). Approximately 20 μg protein was analyzed on 9% SDS-PAGE and transferred onto the Immuno-Blot PVDF Membrane (Bio-Rad). The membranes were probed with polyclonal antibodies, followed by detection with a chemiluminescence Western blotting kit (Boehringer Mannheim). Polyclonal antibodies against SOD2, COX2 and β-actin were purchased from Santa Cruz Biotechnology, Inc., Abcam Inc. and Cell Signaling Technology, respectively. The primary antibodies were used at 1:1000 dilution, while the secondary antibody was used at 1:2500 dilution.
Results
Mn2+ alters the transcript levels of a subset of genes with distinctive functional characteristics
Previous studies of Mn-exposed monkeys and rats showed that Mn concentration in the striatum and globus pallidus can reach concentrations higher than 200 μM (Erikson et al., 2004a; Suzuki et al., 1975). In addition, Mn concentration in astrocytes can be much higher than in neurons. Thus, to maximize the likelihood of identifying Mn targets that may be relevant to human exposures, we chose to examine the effect of 200 μM Mn on primary human astrocytes. We performed microarray gene expression analysis by using RNA samples from three independent batches of primary human astrocytes, treated with or without MnCl2 for 7 days. We used a series of quality control, normalization, statistical and filtering analysis algorithms to identify the differentially expressed genes in Mn2+-treated vs. untreated astrocytes, see Materials and Methods. We identified genes whose overall expression level in three independent batches of astrocytes was altered by > 2-fold and whose expression level in each batch of astrocytes was altered by >1.5-fold. We used these stringent statistical criteria to minimize the identification of false positive targets.
We identified 734 genes whose expression was selectively altered by Mn2+ (Table 1). 414 were up-regulated while 320 were down-regulated. Among them, about 316 are annotated to date (Table 1). The transcript levels of these altered genes in three different batches of astrocytes were displayed in Figure 1A (up-regulated) and 1B (down-regulated). By using the NIH program DAVID, PubMed, and the gene ontology algorithm GeneSpring, we identified several functionally distinctive classes of genes altered by Mn2+. These identified categories included genes encoding cytokines and inflammatory functions, regulators of cell cycle, DNA replication and repair, transporters, functions involved in development, transcriptional regulators and signal transducers (Table 1).
Table 1.
Numbers of Transcripts Altered by Mn in Primary Human Astrocytes*
| Tot (annotated) | Cyto | Cell | Transp | Dev | Sign Trans | Trans Reg |
|---|---|---|---|---|---|---|
The up-regulated and down-regulated genes in primary human astrocytes (Figure 1) were categorized by using the NIH DAVID annotation program and the Gene Ontology algorithm in GeneSpring. Listed here are the numbers of altered genes encoding cytokine and inflammatory functions (Cyto), regulators of cell cycle and DNA replication and repair (Cell), transporters (Transp), transcriptional regulators (Trans Reg) and signal transducers (Sign Trans), and those with developmental-relevant functions (Dev). The total (Tot) number of altered transcripts by Mn is also shown; the number of annotated(classified) genes is shown in parentheses. “+” indicates upregulated genes while “−” indicates down-regulated genes.
Figure 1.
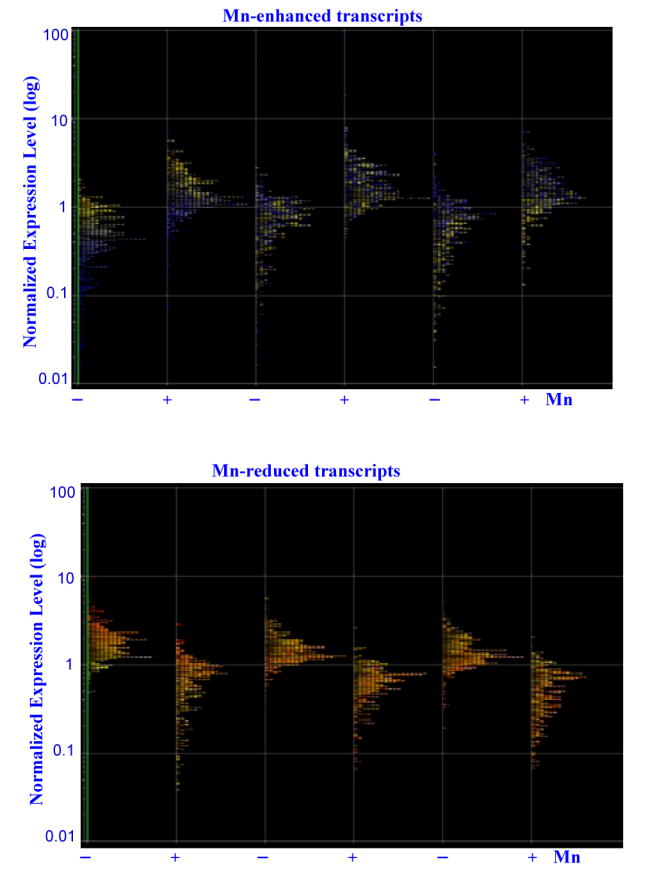
Graphic illustration of the normalized expression levels of Mn2+-altered genes in primary human astrocytes. Primary human astrocytes were treated with or without 200 μM Mn2+ for 7 days. RNA samples were extracted from these cells, and cRNA probes were generated and were hybridized to the Human Genome U133 Plus 2.0 arrays and scanned. Data were analyzed by using various algorithms in GeneSpring, including nonparametric two-way ANOVA, and with the Benjamini & Hochberg false discovery rate multiple testing correction (p-value less than 0.05). The differentially expressed genes in Mn2+-treated vs. untreated cells were subjected to four consecutive filtering processes to identify genes whose overall expression level in three independent batches of astrocytes was altered at least twofold and whose expression level in each batch of astrocytes was altered at least 1.5-fold. The expression levels of up-regulated genes (Top) and down-regulated genes (Bottom) by Mn2+ in three batches of independent astrocytes were plotted.
To confirm the results form microarray gene expression profiling, we used real-time RT-PCR analysis. We selectively examined the transcript levels of a series of Mn2+-altered genes, particularly those encoding inflammatory mediators, such as CD48, CR1, CXCL2, CD8B1, CXC3, and IL12A. Figure 2A shows that consistent with microarray data, Mn2+ significantly induced the expression of CD48, SOD2, CR1, CXCL2, CD8B1, IL12A, CNOT6L, GAL, HEPH (hephaestin), MYT1L, POU1F1, SULT1C1, and CXCL3.
Figure 2.
(A) Real-time RT-PCR analysis of the induction of the transcript levels of genes induced by Mn2+ in primary human astrocytes. Total RNA from three to six independent batches of human astrocytes was isolated, and real-time RT-PCR analysis was performed as described in Materials and Methods. β-actin was used as an internal standard. The folds of induction of the indicated transcript levels were calculated by using Roche software, as described in Materials and Methods. Abbreviations: CD48, CD48 antigen (B-cell membrane protein); SOD2, superoxide dismutase 2; CR1, complement component receptor 1; CXCL2, chemokine (C-X-C motif) ligand 2; CD8B1, CD8 antigen, beta polypeptide; IL12A, interleukin 12A; CNOT6L, CCR4-NOT transcription complex, subunit 6-like; GAL, galanin; HEPH, hephaestin; MYT1L, myelin transcription factor 1-like; POU1F1, POU domain, class 1, transcription factor 1; SULT1C1, sulfotransferase family, cytosolic, 1C, member; and CXCL3, chemokine (C-X-C motif) ligand 3. (B) Real-time RT-PCR analysis of the CXCL2 transcript level at different MnCl2 concentrations. Total RNA was isolated from human astrocytes treated with 0, 10, 50, or 200 μM MnCl2 for 7 days, and real-time RT-PCR analysis of CXCL2 transcript was performed as in A. (C) Real-time RT-PCR analysis of the SOD2 transcript level at different MnCl2 concentrations. Total RNA was isolated from human astrocytes treated with 0, 10, 50, or 200 μM MnCl2 for 7 days, and real-time RT-PCR analysis was performed as in A.
In addition, we performed dose-response analysis of SOD2 and CXCL2 transcript levels (Figure 2B and C). We found that 200 μM Mn2+ dramatically increased their transcript levels, whereas 10 μM and 50 μM Mn2+ did not (Figure 2B and 2C). This dose-response pattern is further confirmed by Western blotting analysis. As shown in Figure 3, 200 μM Mn2+ significantly increased SOD2 (Figure 3A) and COX2 (Figure 3B) protein levels (Figure 3), although 10 or 50 μM did not, after 7 days of treatment. Still, this effect of Mn2+ is likely relevant to human exposures, because Mn2+ concentration can reach higher than 200 μM in the brain and because Mn2+ can be elevated further in astrocytes (Erikson and Aschner, 2006; Fitsanakis et al., 2006; Suzuki et al., 1975). These results from real-time RT-PCR and Western blotting analyses support the validity of our microarray gene expression analysis.
Figure 3.

The effect of MnCl2 on the protein levels of SOD2 (A) and COX2 (B). Human astrocytes were treated with 0, 10, 50, or 200 μM MnCl2 for 7 days. Then protein extracts were prepared and subjected to Western blotting analysis. The PVDF membranes were probed with antibodies against SOD2, COX2 and β-actin, respectively. Equal amounts of cellular proteins were loaded in every lane.
A group of genes encoding functions involved in cell cycle control and DNA replication and repair was selectively down-regulated
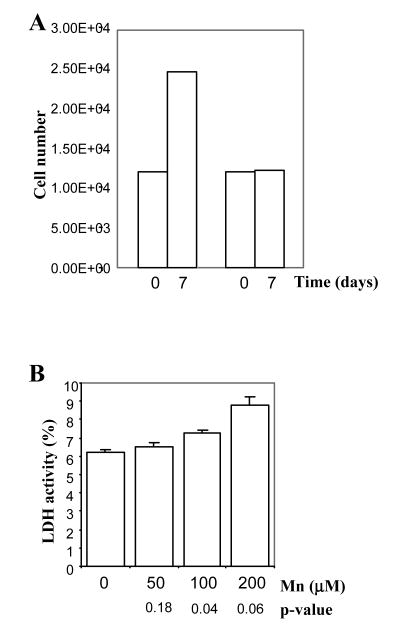
We found that 15 genes encoding functions involved in DNA replication and repair and cell cycle checkpoint control were down-regulated (Tables 1 and 2). Among these, of particular interest are ATM kinase (ataxia telangiectasia mutated), the checkpoint kinase BUB1, the Fanconi anemia FANCF protein, two subunits of DNA primase PRIM1 and PRIM2A, and the replication factor RFC5 (Abraham and Tibbetts, 2005; Margolis, 2004). Remarkably, by using flow cytometric analysis, we found that Mn2+ caused the accumulation of astrocytes in the S phase of the cell cycle (Table 3). This effect on cell cycle is entirely consistent with the effect of Mn2+ on the expression of genes involved in DNA replication and repair, as described above. Very likely, cells accumulated in the S phase cannot progress further and stop growing. Indeed, we found that the growth rate of Mn2+-treated astrocytes was significantly reduced (Figure 4A). However, no apoptotic cells were detected by TUNEL assays, even after prolonged incubation with 200 μM Mn2+ for 2 weeks (not shown). The lack of cell death is also supported by the measurement of LDH release to the medium. As shown in Figure 4B, LDH release to the medium in cells treated with 200 μM Mn2+ was barely increased by 2% (from 6.6% in untreated cells to 8.8%). This increase is only of marginal statistic significance, with a p-value of 0.06 (Figure 4B).
Table 2.
List of Mn-down-regulated Genes Controlling Cell Cycle and DNA Replication
| Gene Name | Fold Change | Common | Genbank | Description |
|---|---|---|---|---|
| 1553387_at | 0.5 | ATM | NM_138293 | ataxia telangiectasia mutated |
| 209642_at | 0.5 | BUB1 | AF043294 | BUB1 budding uninhibited by benzimidazoles 1 homolog |
| 204238_s_at | 0.5 | C6orf108 | NM_006443 | chromosome 6 ORF 108 |
| 202705_at | 0.4 | CCNB2 | NM_004701 | cyclin B2 |
| 204159_at | 0.3 | CDKN2C | NM_001262 | cyclin-dependent kinase inhibitor 2C |
| 1555758_a_at | 0.5 | CDKN3 | AF213040 | cyclin-dependent kinase inhibitor 3 |
| 204170_s_at | 0.5 | CKS2 | NM_001827 | CDC28 protein kinase regulatory subunit 2 |
| 218689_at | 0.4 | FANCF | NM_022725 | Fanconi anemia, complementation group F |
| 201539_s_at | 0.4 | FHL1 | U29538 | four and a half LIM domains 1 |
| 201755_at | 0.3 | MCM5 | NM_006739 | MCM5 minichromosome maintenance deficient 5 |
| 211450_s_at | 0.5 | MSH6 | D89646 | mutS homolog 6 |
| 205053_at | 0.3 | PRIM1 | NM_000946 | nascent-polypeptide-associated complex alpha polypeptide |
| 205628_at | 0.4 | PRIM2A | NM_000947 | primase, polypeptide 2A |
| 203209_at | 0.5 | RFC5 | BC001866 | replication factor C |
| 206067_s_at | 0.3 | WT1 | NM_024426 | Wilms tumor 1 |
Table 3.
The effect of Mn on cell cycle progression*
| Control | Mn-treated | p-value | |
|---|---|---|---|
| G1 | 55.8±0.9 | 36.7±1.3 | 0.0003 |
| S | 36.3±1.1 | 54.9±1.3 | 0.0003 |
| G2 | 3.8±0.7 | 4.5±0.2 | 0.57 |
Primary human astrocytes treated with no reagent (control) or 200 μM MnCl2 (Mn-treated) for 7 days were subjected to flow cytometric analysis as described in Materials and Methods. The shown data are averages from three experiments, and the two-tailed t-test p-value was calculated by using the SAS software.
Figure 4.
(A) The effect of MnCl2 on the growth of primary human astrocytes. Human astrocytes were treated with 0 or 200 μM MnCl2 for 7 days. Then, cells were counted as described in Materials and Methods. The data plotted here were calculated from six independent experiments. The error bars are plotted, but are too small to be discernable. (B) The effect of MnCl2 on LDH release by primary human astrocytes. Human astrocytes were treated with 0, 10, 50, or 200 μM MnCl2 for 7 days. Then, LDH released to medium was measured and calculated as percentage of total cellular LDH activity, as described in Materials and Methods. The data plotted here were calculated from six independent experiments. Two-tailed t-tests were performed to compare MnCl2-treated cells with untreated cells. P values were calculated by using the SAS software.
A group of genes encoding cytokines and immune functions was selectively up-regulated in Mn2+-treated human astrocytes
Mn2+ appears to induce the transcript levels of a group of genes that encode cytokines, chemokines and other immune functions in primary human astrocytes (Tables 1 and 4). Specifically, Mn2+ up-regulated a series of proinflammatory cytokines and chemokines, including CCL7 (MCP3), CXCL14, CXCL2 (MIP2A), CXCL3 (MIP2B), CXCL6 (GCP2), defensin DEFB103, epiregulin EREG, and interleukins IL12A and IL7 (Tables 1 and 4). Other up-regulated pro-inflammatory or immune functions include CD40 and CD8 antigen, the DR subunit of the class II major histocompatibility complex HLA-DRB4, cyclooxygenase COX2 (PTGS2), and pentaxin-related gene PTX3 (Parslow et al., 2001). Notably, the transcript levels of several angiogenic growth factors, including adrenomedullin (ADM), angiopoietin-like 4 (ANGPTL4), placental growth factor (PGF), and vascular endothelial growth factor (VEGF), were up-regulated (Tables 4 and 5). These factors are also induced by hypoxia in various cells (Iimuro et al., 2004; Manalo et al., 2005; Tait and Jones, 2004). In another study, we identified hypoxia-responsive genes in human astrocytes (Mense et al., 2006). Comparison of hypoxia-responsive genes with Mn2+-responsive genes identified 68 genes that were affected by hypoxia and Mn2+ in the same manner (Table 5). Fifty genes were up-regulated, and 18 genes were down-regulated by both Mn2+ and hypoxia in primary human astrocytes (Table 5).
Table 4.
List of Mn-up-regulated Genes Encoding Cytokine and Inflammatory Functions
| Gene Name | Fold Change | Common | Genbank | Description |
|---|---|---|---|---|
| 202912_at | 3.8 | ADM | NM_001124 | adrenomedullin |
| 221009_s_at | 10 | ANGPTL4 | NM_016109 | angiopoietin-like 4 |
| 223333_s_at | AF169312 | |||
| 208075_s_at | 3.1 | CCL7 | NM_006273 | chemokine (C-C motif) ligand 7 |
| 233137_at | 4.2 | CD48 | AF143887 | CD48 antigen |
| 1553562_at | 5.1 | CD8B1 | NM_172100 | CD8 antigen, beta polypeptide 1 (p37) |
| 208488_s_at | 4.2 | CR1 | NM_000651 | complement component (3b/4b) receptor 1 |
| 237038_at | 2.4 | CXCL14 | AI927990 | chemokine (C-X-C motif) ligand 14 |
| 218002_s_at | NM_004887 | |||
| 222484_s_at | AF144103 | |||
| 209774_x_at | 4.1 | CXCL2 | M57731 | chemokine (C-X-C motif) ligand 2 |
| 204470_at | NM_001511 | |||
| 207850_at | 4.7 | CXCL3 | NM_002090 | chemokine (C-X-C motif) ligand 3 |
| 206336_at | 2.4 | CXCL6 | NM_002993 | chemokine (C-X-C motif) ligand 6 |
| 224239_at | 3.2 | DEFB103 | AF301470 | defensin, beta 103 |
| 203717_at | 2.8 | DPP4 | NM_001935 | dipeptidylpeptidase 4 |
| 205767_at | 8.8 | EREG | NM_001432 | epiregulin |
| 221577_x_at | 2.7 | GDF15 | AF003934 | growth differentiation factor 15 |
| 209728_at | 3.5 | HLA-DRB4 | BC005312 | major histocompatibility complex, class II, DR beta 3 |
| 207160_at | 4.2 | IL12A | NM_000882 | interleukin 12A |
| 206693_at | 2.5 | IL7 | NM_000880 | interleukin 7 |
| 1554544_a_at | 2.4 | MBP | L18865 | CDNA clone MGC:70813 |
| 206560_s_at | 3.6 | MIA | NM_006533 | melanoma inhibitory activity |
| 209652_s_at | 2.9 | PGF | BC001422 | placental growth factor |
| 204748_at | 2.7 | PTGS2 | NM_000963 | prostaglandin-endoperoxide synthase 2 |
| 206157_at | 2.3 | PTX3 | NM_002852 | pentaxin-related gene |
| 212171_x_at | 2.0 | VEGF | H95344 | vascular endothelial growth factor |
| 211527_x_at | M27281 |
Table 5.
List of Genes That Were Affected Similarly by Mn2+ and Hypoxia
| Gene Name | Fold Change | Common | Genbank | Description |
|---|---|---|---|---|
| 216113_at | 4.3 | ABI2 | AF070566 | abl interactor 2 |
| 202912_at | 3.8 | ADM | NM_001124 | adrenomedullin |
| 223333_s_at | 11 | ANGPTL4 | AF169312 | angiopoietin-like 4 |
| 221009_s_at | 8.8 | ANGPTL4 | NM_016109 | angiopoietin-like 4 |
| 205678_at | 2.2 | AP3B2 | NM_004644 | adaptor-related protein complex 3, beta 2 subunit |
| 213606_s_at | 0.5 | ARHGDIA | AI571798 | Rho GDP dissociation inhibitor (GDI) alpha |
| 205109_s_at | 2.4 | ARHGEF4 | NM_015320 | Rho guanine nucleotide exchange factor (GEF) 4 |
| 243062_at | 6.9 | BHD | AV694665 | folliculin |
| 1553202_at | 0.2 | C10orf24 | NM_152709 | chromosome 10 ORF 24 |
| 232635_at | 0.4 | C14orf145 | AV703868 | chromosome 14 ORF 145 |
| 235616_at | 3.2 | C20orf17 | AI694059 | chromosome 20 ORF 17 |
| 218541_s_at | 2.6 | C8orf4 | NM_020130 | chromosome 8 ORF 4 |
| 228302_x_at | 2.5 | CaMKIINalpha | AW162846 | CAM-KII INHIBITORY PROTEIN |
| 1554411_at | 0.3 | CTNNB1 | AB062292 | catenin, beta 1, 88kDa |
| 208282_x_at | 0.1 | DAZ2 | NM_020363 | deleted in azoospermia 2 |
| 1570227_at | 2.8 | DKFZP434K046 | BC016065 | hypothetical protein |
| 239380_at | 17 | FIS | AI190413 | Homo sapiens cDNA clone |
| 219650_at | 0.4 | FLJ20105 | NM_017669 | |
| 238949_at | 2.1 | FLJ31951 | BF681162 | Homo sapiens cDNA clone |
| 1559652_at | 0.3 | FLJ35954 | BM722867 | Homo sapiens cDNA clone |
| 227902_at | 4.4 | FLJ38705 | AK024438 | hypothetical protein |
| 1554154_at | 4.6 | GDAP2 | BC013132 | ganglioside induced differentiation associated protein 2 |
| 230498_at | 3.8 | GPR24 | AI934819 | G protein-coupled receptor 24 |
| 216417_x_at | 0.3 | HOXB9 | X16172 | Human HOX-2.5 |
| 207160_at | 4.2 | IL12A | NM_000882 | interleukin 12A |
| 206693_at | 2.5 | IL7 | NM_000880 | interleukin 7 |
| 229261_at | 2.1 | ITGA3 | BF508819 | integrin, alpha 3 |
| 221841_s_at | 3.2 | KLF4 | BF514079 | Kruppel-like factor 4 (gut) |
| 227868_at | 2.3 | LOC154761 | AI928764 | Homo sapiens cDNA clone |
| 227016_at | 2.6 | LOC157697 | AA767385 | hypothetical protein |
| 222336_at | 3.0 | LOC201895 | AW974915 | hypothetical protein |
| 222037_at | 0.2 | MCM4 | AI859865 | Homo sapiens cDNA clone |
| 201755_at | 0.3 | MCM5 | NM_006739 | minichromosome maintenance deficient 5 |
| 1554540_at | 0.2 | MGC27277 | BC042869 | hypothetical protein |
| 238560_at | 2.7 | NDP52 | AI684710 | nuclear domain 10 protein |
| 206686_at | 3.6 | PDK1 | NM_002610 | pyruvate dehydrogenase kinase 1 |
| 226452_at | 3.2 | PDK1 | AU146532 | pyruvate dehydrogenase kinas 1 |
| 217705_at | 2.6 | PRKCM | AW085172 | protein kinase C, mu |
| 206157_at | 2.3 | PTX3 | NM_002852 | pentaxin-related gene |
| 214409_at | 3.4 | RFPL3S | AL021937 | |
| 217691_x_at | 2.2 | SLC16A3 | AA853175 | monocarboxylic acid transporter 3 |
| 215078_at | 12 | SOD2 | AL050388 | superoxide dismutase 2 |
| 243834_at | 3.0 | TNRC6 | BF507964 | trinucleotide repeat containing 6 |
| 205694_at | 0.3 | TYRP1 | NM_000550 | tyrosinase-related protein 1 |
| 231990_at | 0.2 | USP15 | AK023703 | ubiquitin specific protease 15 |
| 211527_x_at | 2.0 | VEGF | M27281 | vascular endothelial growth factor |
| 212171_x_at | 2.0 | VEGF | H95344 | vascular endothelial growth factor |
| 206067_s_at | 0.3 | WT1 | NM_024426 | Wilms tumor 1 |
| 232427_at | 3.1 | ZNF224 | BE464105 | zinc finger protein 224 |
| 233944_at | 4.8 | AU147118 | CDNA FLJ12079 fis | |
| 234021_at | 3.9 | AK024984 | CDNA: FLJ21331 fis, | |
| 1558783_at | 3.7 | AK024805 | CDNA: FLJ21152 fis | |
| 1559760_at | 3.6 | BC036933 | ||
| 244175_at | 3.5 | BF223582 | cDNA clone | |
| 1559053_at | 3.4 | BC027905 | cDNA clone | |
| 239567_at | 3.2 | AW974998 | CDNA clone | |
| 1565913_at | 2.5 | H59257 | cDNA clone | |
| 1558447_at | 2.4 | BC032415 | CDNA clone | |
| 232876_at | 2.3 | AK025534 | cDNA clone | |
| 213931_at | 2.3 | AI819238 | repressor Id-2 - human | |
| 213003_s_at | 2.2 | BF061054 | CEBP DELTA | |
| 240094_at | 2.2 | AL042660 | Transcribed sequences | |
| 235242_at | 2.1 | BE739287 | Homo sapiens cDNA clone I | |
| 1562529_s_at | 2.0 | BC040965 | cDNA clone | |
| 234957_at | 0.5 | AF017336 | ||
| 220859_at | 0.5 | NM_014136 | ||
| 223661_at | 0.4 | AF130080 | CDNA clone | |
| 231223_at | 0.2 | R41565 | Transcribed sequences |
Mn2+ caused changes in the transcript levels of a number of transporters
Our gene ontology analyses identified 18 genes encoding functions involved in the transport of proteins, small molecules and ions that were up-regulated while an equal number of genes were down-regulated (Table 1 and Supplemental Table S2). These transporters include ABC transporters (up-regulated, ABCA9; and down-regulated, ABCA5); cholesterol transporters APOL1 and APOL2; transporters of small molecules and ions, including SLC11A2 (DMT1), SLC16A3, SLC2A13, SLC22A15, SLC4A10; functions involved in Ca2+ entry, including the channel proteins TRPM1 and TRPM4, and the inhibitor of Ca2+ channel phospholamban PLN; functions involved in membrane and vesicle trafficking RAB21, RAB23, SEC15L1 and VPS13A; and the nuclear import receptor importin IPO11 (Harteneck, 2005; Plafker and Macara, 2000). Interestingly, a recent microarray analysis of gene expression in the prefrontal cortex of schizophrenia and control brains revealed the significant up-regulation of the APOL1 and APOL2 genes (Mimmack et al., 2002).
Mn2+ altered the transcript levels of a number of genes encoding developmentally relevant functions
Our analyses identified 38 (up-regulated, 25; down-regulated, 13) additional Mn2+-altered genes (besides those relevant ones shown in Tables 2, 4 and S2) encoding developmentally-relevant functions (Tables 1 and supplemental Table S3). Among the up-regulated 25 genes (supplemental Table S3), CELSR1, neuregulin NRG2, neuropilin NRP1, calcyclin S100A6 and transcriptional regulators POU1F1, NOTCH4, FOXP2 and TFAP2B (Mani et al., 2005) are known to play important roles in mammalian neural development or are expressed in neural cells. Notably, neuregulin NRG2 (Michailov et al., 2004) (Table S3), a member of the ERBB family of tyrosine kinase transmembrane receptors including the EGF receptor, and epiregulin (Park et al., 2004), a member of the epidermal growth factor family (Table 4), were both up-regulated. Likewise, neuropilin NRP1 (Castellani, 2002), a receptor for VEGF (Table S3), and VEGF (Table 4), were both up-regulated. Evidently, both the EGF and VEGF signaling pathways may be affected by Mn2+ in astrocytes.
The transcript levels of a number of signal transducers and transcriptional regulators were altered by Mn2+
The effects of Mn2+ on gene expression (Table 1) are presumably mediated by their effects (direct or indirect) on the levels or activities of transcriptional regulators and/or their upstream effectors, i.e., signal transducers. Thus, it is expected that the expression of certain transcriptional regulators and signal transducers would be altered by Mn2+. Indeed, our analyses identified a series of signal transducers and transcriptional regulators whose transcript level was altered by Mn2+. In total, we identified about 41 altered signal transducers and 30 altered transcriptional regulators (Table 1). Tables S4 and S5 list those additional 21 altered signal transducers and 19 altered transcriptional regulators, besides those already shown in Tables 2, 4, S2, and S3, such as IGB5, FOXP2 and POU1F1. The alteration of the transcript levels of some of these signal transducers and transcriptional regulators would likely contribute to the alteration of downstream genes shown in Tables 2, 4, S2, and S3, although too little is known to correlate these regulators with their downstream effects in most cases. Interestingly, the hypoxia-inducible factor HIF1A was up-regulated whereas its inhibitor HIF1AN (Mahon et al., 2001) was down-regulated. This opposing effect of Mn2+ on HIF1A and HIF1AN expression is in complete agreement with their perspective functions and with the result that 68 genes, such as ADM and VEGF (Table 5), that were induced/suppressed by Mn2+ were also induced/suppressed by hypoxia (Mense et al., 2006).
The IFN-γ and EGF signaling pathways might be perturbed by Mn2+ in primary human astrocytes
To identify common signaling pathways that may mediate the effects of Mn2+ on gene expression, we used a computational program called PathwayAssist (Stratagene Software). We found that Mn2+ altered the transcript levels of genes that are targets of the IFN-γ signaling pathway (Schroder et al., 2004) and the EGF signaling pathway (Schlessinger, 2004). Twenty-six altered genes are known to be the targets of the IFN-γ signaling pathway while 11 are known to be the targets of the EGF signaling pathway (Table 6). Particularly, among those targets of the IFN-γ signaling pathway, 20 of them were shown to be positively affected by the IFN-γ signaling pathway in certain human cells, and 16 out of 20 were also up-regulated by Mn2+ (see italicized genes in Table 6).
Table 6.
List of Mn-altered Genes That Are Regulated by the IFN-γ or EGF Signaling Pathway
| Gene Name | Fold Change | Common | Genbank | Description |
|---|---|---|---|---|
| IFNγ-regulated | ||||
| 202912_at | 3.8 | ADM* | NM_001124 | adrenomedullin |
| 208644_at | 0.5 | ADPRT | M32721 | ADP-ribosyltransferase |
| 202095_s_at | 0.4 | BIRC5 | NM_001168 | baculoviral IAP repeat-containing 5 |
| 208075_s_at | 3.1 | CCL7 | NM_006273 | chemokine (C-C motif) ligand 7 |
| 233137_at | 4.2 | CD48 | AF143887 | CD48 antigen (B-cell membrane protein) |
| 213003_s_at | 2.3 | CEBPD | BF061054 | CCAAT/ENHANCER BINDING PROTEIN DELTA |
| 209774_x_at | 4.1 | CXCL2 | M57731 | chemokine (C-X-C motif) ligand 2 |
| 204470_at | NM_001511 | |||
| 224239_at | 3.2 | DEFB103 | AF301470 | defensin, beta 103 |
| 203717_at | 2.8 | DPP4 | NM_001935 | dipeptidylpeptidase 4 |
| 207160_at | 4.2 | IL12A | NM_000882 | interleukin 12A |
| 215182_x_at | 2.5 | IL1R1 | AL050122 | |
| 221841_s_at | 3.2 | KLF4 | BF514079 | Kruppel-like factor 4 (gut) |
| 240338_at | 2.2 | LRAP | AI807341 | leukocyte-derived arginine aminopeptidase |
| 244704_at | 0.5 | NFYB | AW083948 | neuronal thread protein |
| 200790_at | 0.4 | ODC1 | NM_002539 | ornithine decarboxylase 1 |
| 1559400_s_at | 5.4 | PAPPA | BG620958 | |
| 211840_s_at | 0.4 | PDE4D | U50157 | phosphodiesterase 4D, cAMP-specific |
| 204748_at | 2.7 | PTGS2 | NM_000963 | prostaglandin-endoperoxide synthase 2 |
| 203110_at | 0.4 | PTK2B | U43522 | PTK2B protein tyrosine kinase 2 beta |
| 206157_at | 2.3 | PTX3 | NM_002852 | pentaxin-related gene, rapidly induced by IL-1 beta |
| 228037_at | 0.4 | RARA | AA404273 | retinoic acid receptor, alpha |
| 1568765_at | 2.3 | SERPINE1 | BC020765 | serine (or cysteine) proteinase inhibitor, clade E, member 1 |
| 237106_at | 2.2 | SLC11A2 | AI051244 | solute carrier family 11, member 2 |
| 215078_at | 12 | SOD2 | AL050388 | superoxide dismutase 2 |
| 212171_x_at | 2.0 | VEGF | H95344 | vascular endothelial growth factor |
| 211527_x_at | M27281 | |||
| EGF-regulated | ||||
| 221681_s_at | 2.0 | DSPP | AF094508 | Homo sapiens dentin phosphoryn mRNA, complete cds. |
| 236990_at | 0.2 | EIF2AK3 | AV699382 | cDNA clone |
| 205767_at | 8.7 | EREG | NM_001432 | epiregulin |
| 234240_at | 0.2 | GSN | AL049930 | Homo sapiens mRNA |
| 209957_s_at | 0.4 | NPPA | M30262 | natriuretic peptide precursor A |
| 233626_at | 4.3 | NRP1 | AK024580 | neuropilin 1 |
| 202730_s_at | 0.4 | PDCD4 | NM_014456 | programmed cell death 4 |
| 205934_at | 0.4 | PLCL1 | NM_006226 | phospholipase C-like 1 |
| 217705_at | 2.6 | PRKCM | AW085172 | protein kinase C, mu |
| 202388_at | 2.1 | RGS2 | NM_002923 | regulator of G-protein signalling 2 |
| 220983_s_at | 2.1 | SPRY4 | NM_030964 | sprouty homolog 4 |
The italicized genes are those known to be activated by INF-γ and were up-regulated by Mn
Discussion
In this report, we applied microarray gene expression profiling and computational algorithms to identify molecular targets of the common environmental toxicant Mn2+ in primary human astrocytes. Further, we used real-time RT-PCR, Western blotting, flow cytometric, and toxicological analyses to confirm the key results from microarray expression analysis. Our analyses provide several new insights into the molecular neurotoxic actions of Mn. Such insights may provide a molecular basis for further studies to elucidate the molecular events underlying Mn neurotoxicity in humans. First, data from microarray expression profiling (Table 2), flow cytometry (Table 3), and measurement of cell growth and LDH release (Figure 4) show that Mn2+ selectively affected astrocyte cell proliferation, while it did not significantly affect cell death.
Second, 68 induced/suppressed genes by Mn2+, such as angiogenic factors (e.g., ADM, ANGPTL4 and VEGF) and SOD2 (Table 5), were also induced/suppressed by hypoxia. Consistent with this, we found that 11 target genes of the EGF signaling pathway were also targeted by Mn2+ (Table 6). In a previous analysis (Mense et al., 2006), we found that hypoxia induced nearly 40 genes that are targets of the EGF signaling pathway. These results raise the possibility that Mn treatment and hypoxia act on a significant number of common target genes and that Mn treatment resembles certain aspects of brain hypoxia or ischemia.
Third, a series of genes encoding proinflammatory functions was up-regulated by Mn2+ (Table 4). Particularly, the transcript levels of several genes encoding proinflammatory chemokines, CCL7 (MCP3), CXCL14, CXCL2 (MIP2A), CXCL3 (MIP2B) and CXCL6 (GCP2), and interleukins IL12A and IL7, were all enhanced in Mn2+-treated astrocytes. Proinflammatory chemokines and cytokines are thought to play a critical role in the induction and perpetuation of inflammation in the brain (Burwinkel et al., 2004; Choi et al., 2005; Meda et al., 2001). Furthermore, 16 known targets of the IFN-γ signaling pathway that are activated by the pathway were also activated (Table 6), suggesting the IFN-γ signaling pathway was activated in Mn2+-treated astrocytes although the transcript level of IFN-γ per se was not detected to be increased. IFN-γ plays a major immunomodulatory role in astrocytes, and the activation of its signaling pathway leads to the expression of various inflammation-associated molecules (Choi et al., 2005; Lin et al., 2004), as those shown in Tables 4 and 6. Glial activation and the production of proinflammatory chemokines and cytokines have been shown to be associated with many central nervous system (CNS) diseases, such as bacterial or viral infection, multiple sclerosis, prion infection, Parkinson's disease, Alzheimer's disease and ischemia (Burwinkel et al., 2004; Choi et al., 2005; Meda et al., 2001).This association is in complete agreement with our findings that proinflammatory chemokines and cytokines are up-regulated and that the IFN-γ signaling pathway may be activated by Mn2+ in astrocytes.
Interestingly, several genes shown in Tables S4 and S5 are known to be expressed in the brain and/or to play important roles in the proper CNS functioning. These include the GABA A receptor GABRG2, galanin (GAL), the G protein-coupled receptor GPR24, protocadherins PCDH7 and PCDHA6, RGS2, catenins CTNNA1 and CTNNB1, poliovirus receptor PVR, the neurokinin 1 receptor TACR1, presenilin binding protein CSEN, and myelin transcription factor 1-like MYT1L. Particularly, galanin is a 29 amino acid neuropeptide that plays many important roles in CNS (Robinson, 2004). It can modulate gastrointestinal motility, pituitary hormone release, neurotrophic action, nociception, anxiety, feeding and cognition (Robinson, 2004; Wrenn et al., 2004). GPR24 (MCHR1) may be involved in the control of obesity, depression and/or anxiety (Georgescu et al., 2005). CSEN appears to play important roles in pain processing (Cheng et al., 2002) while MYT1L is expressed in neural cells of the developing mammalian CNS (Kim et al., 1997).
In summary, our studies using a combination of genomic, biochemical and toxicological approaches uncovered several note-worthy characteristics of the effects of Mn2+ on primary human astrocytes. The most striking characteristics include its effects on cell cycle progression, the expression of hypoxia-responsive genes and the expression of proinflammatory factors. The effects of Mn2+ on the expression of hypoxia-responsive genes and proinflammatory factors are consistent with the previous finding that the production of proinflammatory cytokines is increased in mouse astrocytes following ischemia (Lau and Yu, 2001). Our findings provide a molecular basis for further investigation of the mechanisms underlying Mn neurotoxicity and perhaps even other neurodegenerative diseases. With a better understanding of the functions of signal transducers and transcriptional regulators and mechanisms of gene regulation, our data may provide further insights into the molecular events underlying Mn neurotoxicity.
Supplementary Material
Acknowledgments
This work is supported in part by public health grants HL65568 (LZ) and NS31492 (GB and DJV).
- ANOVA
analysis of variance
- Mn
manganese
- IFN-γ
interferon gamma
- EGF
epidermal growth factor
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- International Manganese Institute brochure. In International Manganese Institute (Paris, France), 2001.
- Abraham RT, Tibbetts RS. Cell biology. Guiding ATM to broken DNA. Science. 2005;308:510–511. doi: 10.1126/science.1112069. [DOI] [PubMed] [Google Scholar]
- Auld DS, Robitaille R. Glial cells and neurotransmission: an inclusive view of synaptic function. Neuron. 2003;40:389–400. doi: 10.1016/s0896-6273(03)00607-x. [DOI] [PubMed] [Google Scholar]
- Barbeau A. Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias) Neurotoxicology. 1984;5:13–35. [PubMed] [Google Scholar]
- Burwinkel M, Riemer C, Schwarz A, Schultz J, Neidhold S, Bamme T, Baier M. Role of cytokines and chemokines in prion infections of the central nervous system. Int J Dev Neurosci. 2004;22:497–505. doi: 10.1016/j.ijdevneu.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- Canki M, Thai JN, Chao W, Ghorpade A, Potash MJ, Volsky DJ. Highly productive infection with pseudotyped human immunodeficiency virus type 1 (HIV-1) indicates no intracellular restrictions to HIV-1 replication in primary human astrocytes. J Virol. 2001;75:7925–7933. doi: 10.1128/JVI.75.17.7925-7933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani V. The function of neuropilin/L1 complex. Adv Exp Med Biol. 2002;515:91–102. doi: 10.1007/978-1-4615-0119-0_8. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, et al. DREAM is a critical transcriptional repressor for pain modulation. Cell. 2002;108:31–43. doi: 10.1016/s0092-8674(01)00629-8. [DOI] [PubMed] [Google Scholar]
- Choi WH, Ji KA, Jeon SB, Yang MS, Kim H, Min KJ, Shong M, Jou I, Joe EH. Anti-inflammatory roles of retinoic acid in rat brain astrocytes: Suppression of interferon-gamma-induced JAK/STAT phosphorylation. Biochem Biophys Res Commun. 2005;329:125–131. doi: 10.1016/j.bbrc.2005.01.110. [DOI] [PubMed] [Google Scholar]
- Crossgrove J, Zheng W. Manganese toxicity upon overexposure. NMR Biomed. 2004;17:544–553. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossgrove JS, Yokel RA. Manganese distribution across the blood-brain barrier. IV. Evidence for brain influx through store-operated calcium channels. Neurotoxicology. 2005;26:297–307. doi: 10.1016/j.neuro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Juan G, Bedner E. Determining cell cyle stages by flow cytometry. In: Bonifacino JS, Dasso M, Harford JB, Lippincott-Schwartz J, Yamada KM, editors. Current protocols in cell biology. New York: John Wiley & Sons, Inc.; 1999. pp. 8.4.1–8.4.18. [DOI] [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- Ehrich M, Sharova L. In vitro methods for detecting cytotoxicity. In: Maines MD, Costa LTG, Reed DJ, Sassa S, Sipes IG, editors. Current protocols in toxicology. New York: John Wiley & Sons, Inc; 2000. pp. 2.6.1–2.6.27. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Aschner M. Increased manganese uptake by primary astrocyte cultures with altered iron status is mediated primarily by divalent metal transporter. Neurotoxicology. 2006;27:125–130. doi: 10.1016/j.neuro.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Dobson AW, Dorman DC, Aschner M. Manganese exposure and induced oxidative stress in the rat brain. Sci Total Environ. 2004a;334-335:409–416. doi: 10.1016/j.scitotenv.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson KM, Syversen T, Steinnes E, Aschner M. Globus pallidus: a target brain region for divalent metal accumulation associated with dietary iron deficiency. J Nutr Biochem. 2004b;15:335–341. doi: 10.1016/j.jnutbio.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Fitsanakis VA, Zhang N, Avison MJ, Gore JC, Aschner JL, Aschner M. The use of magnetic resonance imaging (MRI) in the study of manganese neurotoxicity. Neurotoxicology. 2006;27:798–806. doi: 10.1016/j.neuro.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- Harteneck C. Function and pharmacology of TRPM cation channels. Naunyn Schmiedebergs Arch Pharmacol. 2005 doi: 10.1007/s00210-005-1034-x. [DOI] [PubMed] [Google Scholar]
- Hazell AS. Astrocytes and manganese neurotoxicity. Neurochem Int. 2002;41:271–277. doi: 10.1016/s0197-0186(02)00013-x. [DOI] [PubMed] [Google Scholar]
- Iimuro S, Shindo T, Moriyama N, Amaki T, Niu P, Takeda N, Iwata H, Zhang Y, Ebihara A, Nagai R. Angiogenic effects of adrenomedullin in ischemia and tumor growth. Circ Res. 2004;95:415–423. doi: 10.1161/01.RES.0000138018.61065.d1. [DOI] [PubMed] [Google Scholar]
- Kim JG, Armstrong RC, v Agoston D, Robinsky A, Wiese C, Nagle J, Hudson LD. Myelin transcription factor 1 (Myt1) of the oligodendrocyte lineage, along with a closely related CCHC zinc finger, is expressed in developing neurons in the mammalian central nervous system. J Neurosci Res. 1997;50:272–290. doi: 10.1002/(SICI)1097-4547(19971015)50:2<272::AID-JNR16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Lau LT, Yu AC. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J Neurotrauma. 2001;18:351–359. doi: 10.1089/08977150151071035. [DOI] [PubMed] [Google Scholar]
- Li GJ, Zhao Q, Zheng W. Alteration at translational but not transcriptional level of transferrin receptor expression following manganese exposure at the blood-CSF barrier in vitro. Toxicol Appl Pharmacol. 2005;205:188–200. doi: 10.1016/j.taap.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Kemper A, McCarthy KD, Pytel P, Wang JP, Campbell IL, Utset MF, Popko B. Interferon-gamma induced medulloblastoma in the developing cerebellum. J Neurosci. 2004;24:10074–10083. doi: 10.1523/JNEUROSCI.2604-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu L, Zhang LL, Li GJ, Guo W, Liang W, Zheng W. Alteration of serum concentrations of manganese, iron, ferritin, and transferrin receptor following exposure to welding fumes among career welders. Neurotoxicology. 2005;26:257–265. doi: 10.1016/j.neuro.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magisretti PJ, Ransom BR. Astrocytes. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. American College of Neuropsychopharmacology; 2002. (online) [Google Scholar]
- Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood. 2005;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- Mani A, Radhakrishnan J, Farhi A, Carew KS, Warnes CA, Nelson-Williams C, Day RW, Pober B, State MW, Lifton RP. Syndromic patent ductus arteriosus: evidence for haploinsufficient TFAP2B mutations and identification of a linked sleep disorder. Proc Natl Acad Sci U S A. 2005;102:2975–2979. doi: 10.1073/pnas.0409852102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis RL. Bub1, a gatekeeper for Cdc20-dependent mitotic exit. Dev Cell. 2004;7:634–635. doi: 10.1016/j.devcel.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meda L, Baron P, Scarlato G. Glial activation in Alzheimer's disease: the role of Abeta and its associated proteins. Neurobiol Aging. 2001;22:885–893. doi: 10.1016/s0197-4580(01)00307-4. [DOI] [PubMed] [Google Scholar]
- Mense SM, Sengupta A, Zhou M, Lan C, Bentsman G, Volsky DJ, Zhang L. Gene expression profiling reveals the profound upregulation of hypoxia-responsive genes in primary human astrocytes. Physiol Genomics. 2006;25:435–449. doi: 10.1152/physiolgenomics.00315.2005. [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Mimmack ML, Ryan M, Baba H, Navarro-Ruiz J, Iritani S, Faull RL, McKenna PJ, Jones PB, Arai H, Starkey M, et al. Gene expression analysis in schizophrenia: reproducible up-regulation of several members of the apolipoprotein L family located in a high-susceptibility locus for schizophrenia on chromosome 22. Proc Natl Acad Sci U S A. 2002;99:4680–4685. doi: 10.1073/pnas.032069099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Dirnagl U. Role of glial cells in cerebral ischemia. Glia. 2005;50:281–286. doi: 10.1002/glia.20205. [DOI] [PubMed] [Google Scholar]
- Newland MC. Animal models of manganese's neurotoxicity. Neurotoxicology. 1999;20:415–432. [PubMed] [Google Scholar]
- Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Parslow TG, Stites DP, Terr AI, Imboden JB. Medical Immunology. New York: Lange Medical Books; 2001. [Google Scholar]
- Plafker SM, Macara IG. Importin-11, a nuclear import receptor for the ubiquitin-conjugating enzyme, UbcM2. Embo J. 2000;19:5502–5513. doi: 10.1093/emboj/19.20.5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JK. Galanin and cognition. Behav Cogn Neurosci Rev. 2004;3:222–242. doi: 10.1177/1534582305274711. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Common and distinct elements in cellular signaling via EGF and FGF receptors. Science. 2004;306:1506–1507. doi: 10.1126/science.1105396. [DOI] [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Mouri T, Nishiyama K, Fujii N. Study of subacute toxicity of manganese dioxide in monkeys. Tokushima J Exp Med. 1975;22:5–10. [PubMed] [Google Scholar]
- Tait CR, Jones PF. Angiopoietins in tumours: the angiogenic switch. J Pathol. 2004;204:1–10. doi: 10.1002/path.1618. [DOI] [PubMed] [Google Scholar]
- Wang Z, Trillo-Pazos G, Kim SY, Canki M, Morgello S, Sharer LR, Gelbard HA, Su ZZ, Kang DC, Brooks AI, et al. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. J Neurovirol. 2004;10 1:25–32. doi: 10.1080/753312749. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, LoIacono NJ, et al. Environ Health Perspect. 2005. Water Manganese Exposure and Children's Intellectual Function in Araihazar, Bangladesh. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrenn CC, Kinney JW, Marriott LK, Holmes A, Harris AP, Saavedra MC, Starosta G, Innerfield CE, Jacoby AS, Shine J, et al. Learning and memory performance in mice lacking the GAL-R1 subtype of galanin receptor. Eur J Neurosci. 2004;19:1384–1396. doi: 10.1111/j.1460-9568.2004.03214.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.