Abstract
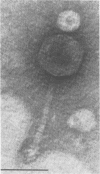
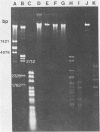
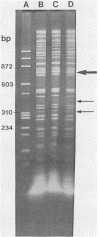
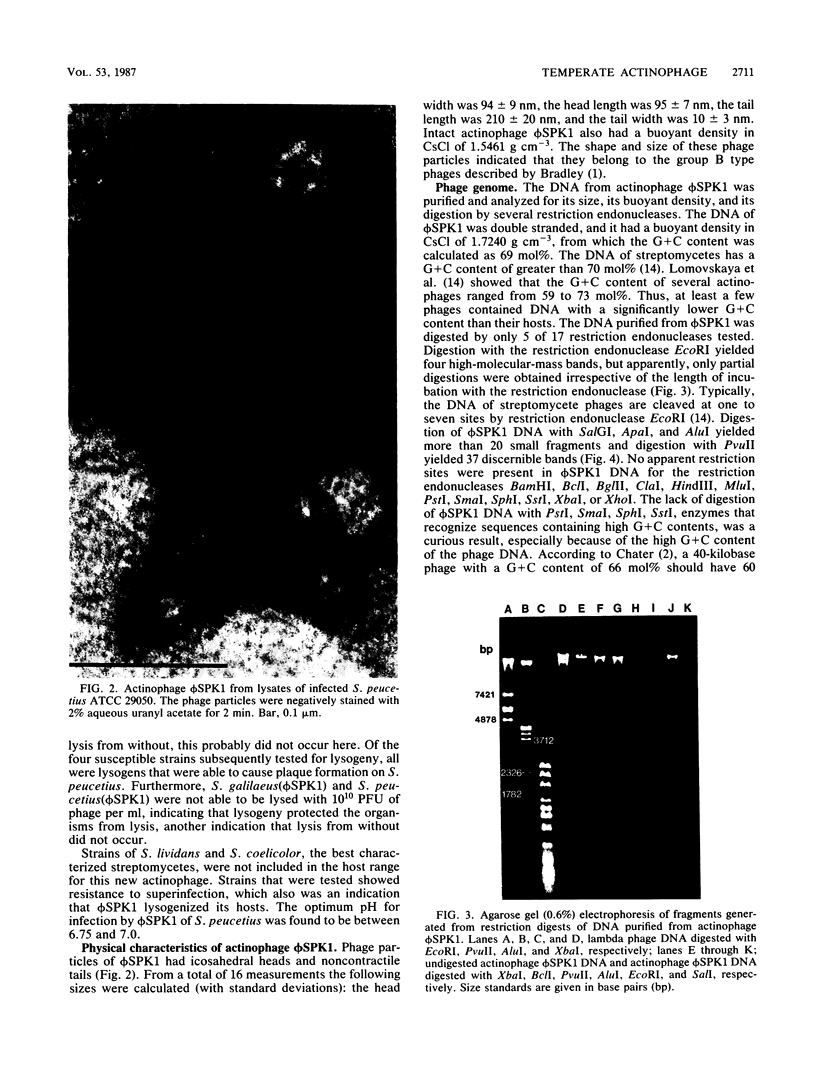
A new temperate actinophage from Streptomyces galilaeus ATCC 31133 was purified after that strain was crossed with S. peucetius ATCC 29050. Sensitive hosts became lysogenized and yielded turbid plaques of 2 to 3 mm in diameter. Host-range analysis indicated that 16 of 27 Streptomyces strains tested were sensitive to infection on solid medium. S. lividans and S. coelicolor A3(2) were among those not infected by this new actinophage. The new actinophage, designated phi SPK1, belongs to the Bradley group B morphological type, the pH optimum for infection is 6.75 to 7.0, it is not efficiently induced by mitomycin C or UV irradiation, it has a circular chromosome of 35.8 +/- 0.5 kilobase pairs in length containing overlapping (cohesive) ends, and the G+C content of its DNA was calculated from the buoyant density of 1.7240 to be 69 mol%. The DNA of phage phi SPK1 was cleaved by the restriction endonucleases ApaI, AluII, EcoRI, PvuII, and SalI, but, in all cases except that with EcoRI, treatment yielded greater than 20 restriction fragments. No sites were detected for BamHI, BclI, BglII, ClaI, HindIII, MluI, PstI, SmaI, SphI, SstI, XbaI, or XhoI.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- Chung S. T. Isolation and characterization of Streptomyces fradiae plasmids which are prophage of the actinophage phiSF1. Gene. 1982 Mar;17(3):239–246. doi: 10.1016/0378-1119(82)90139-1. [DOI] [PubMed] [Google Scholar]
- Dean D. H., Orrego J. C., Hutchison K. W., Halvorson H. O. New temperate bacteriophage for Bacillus subtilis, rho 11. J Virol. 1976 Nov;20(2):509–519. doi: 10.1128/jvi.20.2.509-519.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekleva M. L., Titus J. A., Strohl W. R. Nutrient effects on anthracycline production by Streptomyces peucetius in a defined medium. Can J Microbiol. 1985 Mar;31(3):287–294. doi: 10.1139/m85-053. [DOI] [PubMed] [Google Scholar]
- Donadio S., Paladino R., Costanzi I., Sparapani P., Schreil W., Iaccarino M. Characterization of bacteriophages infecting Streptomyces erythreus and properties of phage-resistant mutants. J Bacteriol. 1986 Jun;166(3):1055–1060. doi: 10.1128/jb.166.3.1055-1060.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tanaka H., Omura S. Isolation and characterization of covalently closed circular DNA associated with chromosomal and membrane fraction from Streptomyces ambofaciens. J Antibiot (Tokyo) 1982 Apr;35(4):497–506. doi: 10.7164/antibiotics.35.497. [DOI] [PubMed] [Google Scholar]
- Lampel J. S., Strohl W. R. Transformation and transfection of anthracycline-producing streptomycetes. Appl Environ Microbiol. 1986 Jan;51(1):126–131. doi: 10.1128/aem.51.1.126-131.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomovskaya N. D., Chater K. F., Mkrtumian N. M. Genetics and molecular biology of Streptomyces bacteriophages. Microbiol Rev. 1980 Jun;44(2):206–229. doi: 10.1128/mr.44.2.206-229.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B. Further studies on the dimensions of viral and protein structures using the catalase crystal internal marker technique. J Ultrastruct Res. 1968 Apr;23(1):178–181. doi: 10.1016/s0022-5320(68)80041-3. [DOI] [PubMed] [Google Scholar]
- Ogata S., Suenaga H., Hayashida S. A temperate phage of Streptomyces azureus. Appl Environ Microbiol. 1985 Jan;49(1):201–204. doi: 10.1128/aem.49.1.201-204.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanishi M., Hamana K., Umezawa H. Factors affecting infection of protoplasts with deoxyribonucleic acid of actinophage PK-66. J Virol. 1968 Jul;2(7):686–691. doi: 10.1128/jvi.2.7.686-691.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanishi M., Manome T., Umezawa H. Isolation and characterization of plasmid DNAs in Actinomycetes. J Antibiot (Tokyo) 1980 Jan;33(1):88–91. doi: 10.7164/antibiotics.33.88. [DOI] [PubMed] [Google Scholar]
- Xue Y., Zhuang Z., Zhu Y., Xu Y., Dong K. Plasmid deoxyribonucleic acid replication in Streptomyces griseus. J Bacteriol. 1981 Apr;146(1):412–414. doi: 10.1128/jb.146.1.412-414.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagisawa M., Huang T. S., Davies J. E. Possible involvement of plasmids in biosynthesis of neomycin. J Antibiot (Tokyo) 1978 Aug;31(8):809–813. doi: 10.7164/antibiotics.31.809. [DOI] [PubMed] [Google Scholar]