Abstract
The goal of this study was to develop a modified fluorescent microsphere-based approach for measuring resting and hyperemic blood flows in individual mouse skeletal muscles. Absolute resting blood flow in the left gracilis posterior was 1.04±0.12 ml·min−1·g−1, while functional hyperemia following muscle activity was 5.94±1.33 ml·min−1·g−1. Measuring absolute blood flow requires sampling arterial blood that serves as a flow-rate and concentration reference to the fluorescent microsphere (FMS) content in the tissue-of-interest for calculating the flow value. Because sampling arterial blood can impair cardiovascular function in the mouse, we also modified our FMS approach to determine relative blood flows in the left gracilis posterior by using the contralateral muscle as our reference in blood flow calculations. Absolute and relative hyperemia measurements detect similar increases in blood flow — 521.93±216.76% and 555.24±213.82%, respectively. However, sampling arterial blood during absolute blood flow measurements significantly decreased mean arterial pressure from the beginning to the end of our experiments, from 102.7±2.18 to 75.5±9.71 mm Hg. This decrease was not seen when measuring relative blood flows. This approach provides critical advantages over contemporary blood flow measurement approaches by allowing blood flow measurements in small and non-superficial tissues.
Keywords: Blood flow, Mouse, Fluorescent microspheres, Skeletal muscle, Hyperemia
1. Introduction
The capacity to match blood flow to tissue need is essential for normal functioning in any tissue. For skeletal muscle, increasing its blood supply through functional hyperemia is essential during locomotion or physical activity. During ischemic revascularization, functional hyperemia in skeletal muscle vasculature is impaired (Hudlicka et al., 1994). Similarly, patients with peripheral artery disease experience intermittent claudication, or ischemic pain, with locomotion (Lumsden and Rice, 2006) that is indicative of an impaired functional hyperemia (Arosio et al., 2002). Additionally, peripheral artery disease is associated with increased mortality due to coronary artery disease (Aronow, 2005). Thus, a deficit in the capacity for functional hyperemia will impair normal tissue function and is suggestive of underlying cardiovascular complications.
Our laboratory utilizes a murine model of chronic hindlimb ischemia (Sullivan et al., 2002) to examine the mechanisms controlling ischemic revascularization. Specifically, we utilize the model to investigate the impairment of functional capacity in newly formed or repaired vasculature following chronic ischemia (Hudlicka et al., 1994).
An important aspect of the mouse model of hindlimb ischemia is that revascularization is spatially segregated; microvascular growth (angiogenesis and arteriolarization) occurs predominately in the lower leg and large vessel remodeling (collateralization) occurs predominately in the upper leg (Sullivan et al., 2002). Because feed arteries and arterioles may utilize different molecular pathways to restore proper vascular reactivity following ischemia, it would be beneficial to assay blood flow in these different regions.
Unfortunately, due to the limited size of the mouse, contemporary methods for determining blood flow lack the resolution to determine blood flow to individual skeletal muscles and/or are limited to the most superficial tissues. For example, MRI is limited to global measures of hindlimb blood flow (Helisch et al., 2006), while ultrasound-based blood flow measurements are limited to large caliber vessels such as the carotid (Sullivan and Hoying, 2002) or femoral (Williams et al., 2006) arteries. At the other end of the spectrum, live tissue imaging with intravital microscopy, which provides the highest level of tissue resolution (Bearden et al., 2004; Duza and Sarelius, 2004), is generally limited to measures of arteriolar reactivity rather than blood flow, due to the requirement that vessel segments must reside in a single plane to obtain sound velocity measurements. Additionally, intra-vital approaches are usually limited to sheet-like tissue, such as the cremaster muscle, due to the challenge of resolving arterioles in optically dense skeletal muscle. The most commonly utilized blood flow measurement technology, laser Doppler perfusion imaging (LDPI), which determines perfusion based on red cell velocity and hematocrit, can be resolved to the single tissue level by post hoc determinations of regions-of-interest from LDPI scans (Chalothorn et al., 2005; Sullivan et al., 2002). However, even when utilizing deep-penetrating probes (Chalothorn et al., 2005; Yu et al., 2005), LDPI is still limited to assessing perfusion in the most superficial tissues.
Another approach for measuring blood flow is with fluorescent microspheres (FMS). For this approach, blood flow is calculated by comparing the tissue deposition of intra-arterially injected FMS and the FMS content of arterial blood that was sampled at a fixed withdrawal rate. Thus, FMS-based approaches can be used to measure blood flow in any tissue receiving arterial blood if the FMS are uniformly distributed in that blood. However, traditional FMS-based approaches have limited tissue resolution in mice. Statistical models indicate that 200–400 FMS must lodge in a tissue to accurately measure blood flow (Prinzen and Bassingthwaighte, 2000) when using fluorimetry, the most commonly used tool for determining tissue fluorescence. This usually requires harvesting most or all hindlimb skeletal muscle to obtain enough FMS (Kubis et al., 2002). Additionally, FMS have not been used to measure resting blood flow and hyperemia in a single mouse, except when alternative referencing approaches are used (Maxwell et al., 1998). This is probably due to the fact that multiple arterial withdrawals have not been successfully taken from mice (Richer et al., 2000), likely due to their low blood volume.
In summary, none of the currently available methods for blood flow measurement allow us to resolve resting blood flow and functional hyperemia in individual skeletal muscles throughout the murine hindlimb. However, FMS present the best alternative in that they can be used to measure blood flow in all skeletal muscles, superficial or deep. Therefore, the goal of this project was to develop a modified fluorescent microsphere-based approach that was capable of measuring resting blood flow and functional hyperemia in individual muscles of the same mouse.
2. Methods
2.1. Animals
Male and female FVB/N transgenic mice, between 2 and 6 months of age, that express green fluorescent protein (GFP) in vascular endothelium (Motoike et al., 2000) were used for all experiments according protocols approved by the University of Arizona Institutional Animal Care and Use Committee. Four mice were used to determine absolute blood flow, while five mice were used to determine relative blood flow. Mice were obtained from a breeding colony maintained at the University of Arizona on a 12:12 light:dark cycle and given water and rodent chow ad libitum.
2.2. Surgical Instruments
S&T forceps (angled 45°), Dumont forceps (#5–45), Castroviejo needle holder, Metzenbaum scissors, Iris scissors (curved, 11.5 cm), S&T clamp applying forceps, and S&T vascular clamp (B-1) were obtained from Fine Science Tools (Foster City, CA, USA).
2.3. Anesthesis and animal preparation
All mice were initially anesthetized in an anesthesia box with 5% isoflurane (Abbott, Abbott Park, Illinois, USA) administered through an isoflurane vaporizer and balanced with oxygen flowing at ~3.5 1·min−1 (VT-110 and VT525, JD Medical, Phoenix, AZ, USA). Following induction, anesthesia was maintained throughout animal preparation and experimentation with 1–2% isoflurane balanced with oxygen flowing at 0.5–1.0 1 min−1 through a small animal anesthesia mask (Harvard Apparatus, Holliston, MA, USA). The mask was modified by removing the mask portion and repositioning the rubber diaphragm over the fitting port to improve access to the ventral surface of the neck during carotid artery catheterizations (see below). Prior to experimentation, hair on the ventral neck and hindlimbs was removed with trimming clippers and depilatory cream (Nair, Princeton, NJ, USA). During experiments, body temperature was maintained with a water-circulating thermal pad (Gaymar, Orchard Park, NY, USA). Additionally, we found it important to cover the animal with two to three gauze sponges or a small towel to prevent ventral heat loss which likely causes hypothermic vasoconstriction and other cardiovascular complications.
2.4. Arterial catheterization
Following exposure from its neurovascular bundle, the right common carotid artery (RCCA) was catheterized with a polyethylene 50 (427411, Thomas Scientific, Swedesboro, NJ, USA) catheter that was tapered over heat to the approximate diameter of the vessel, ~0.5mm (Fig. 1). The catheter was placed through a small incision made 1/2-way through the artery at ~45° to the longitudinal axis. Temporary placement of a vascular clamp downstream to the catheterization site and ligation of the RCCA near the internal and external bifurcation with 6–0 silk suture (Harvard Apparatus, Holliston, MA, USA) prevented blood loss during catheter insertion. Once inserted, the catheter was advanced to near the aortic arch, secured with 6–0 silk suture, and flushed with 100 U·ml−1 heparinized saline (Associated Medical, Scottsdale, AZ, USA) to prevent coagulation. This same approach was repeated for the left common carotid artery (LCCA). The right femoral artery (RFA) (Fig. 1) was catheterized in a similar fashion to the carotid arteries and advanced to the iliac artery; the RFA was not catheterized when determining relative blood flows. For catheterization of the RFA, it was especially important to advance the catheter beyond the site of the temporary vascular clamp, which caused vasospasm and impairs blood referencing. Additionally, placement of the catheter further upstream reduces the chance that hypothermic vasoconstriction will impair blood referencing by positioning the catheter closer to the warmer core of the animal. Catheterization sites were covered with plastic wrap to prevent desiccation (Ellsworth et al., 1995).
Fig. 1.
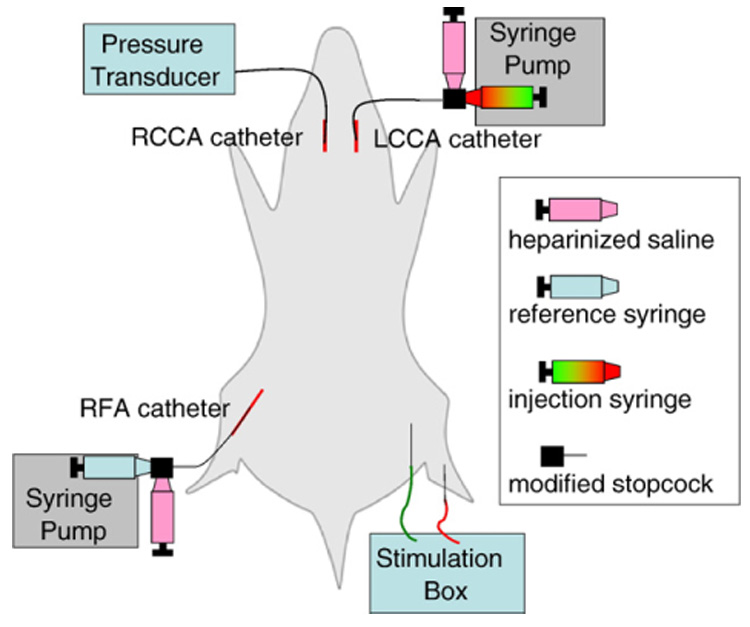
Experimental setup for the determination of absolute blood flow using a reference-catheter approach.
2.5. Modified stopcock
The LCCA and RFA catheters were attached to a modified stopcock, designed to reduce void volume (Fig. 2). The reduced void volume has several advantages. First, a lower void volume allows for fewer FMS to be injected, which reduces the cost of experiments. Second, the reduced void volume creates a space that is easier to flush, which is important for removing blood from the reference stopcock and for removing FMS from the injection stopcock prior to the second FMS injection. Third, and likely most importantly, the reduced void volume greatly reduces the volume of arterial blood that must be sampled and should preserve the cardiovascular integrity of the mouse, which can suffer a significant decrease in MAP following the removal of only 210 µl of arterial blood (Rao and Verkman, 2000).
Fig. 2.
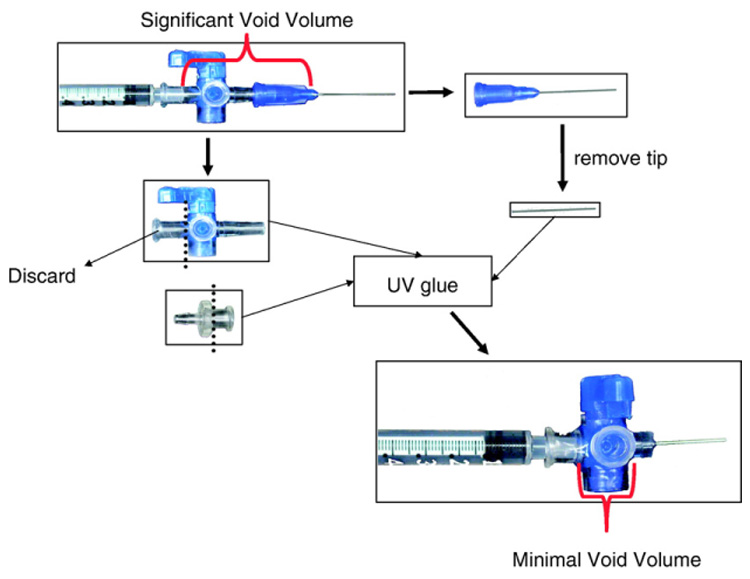
Modified stop-cock used for the delivery of fluorescent microspheres and sampling of arterial blood.
2.6. Blood pressure measurement
The RCCA catheter was attached to a pressure transducer (DTX™ Plus DT-XX, BD, Franklin Lakes, NJ, USA) with a blunt cannula. A computer running Ponemah Physiology Platform (v4.40) collected a conditioned blood pressure signal (Transducer 6600) through an acquisition interface unit (ACQ-16), all of which were purchased from legacy Gould, now DSI (Arden Hills, MN, USA). Mean arterial pressure (MAP) was monitored throughout all experiments.
2.7. Fluorescent microsphere protocol for absolute blood flow
Following catheterizations and electrode placement (see below), the animal was given 30 min to stabilize. At the end of the stabilization period red FMS (15 µm, Molecular Probes/Invitrogen, Carlsbad, CA, USA) were prepared by vortexing for 30 s to resuspend the FMS, sonicating for 5 min to disperse any FMS aggregates, and vortexing for another 30 s to ensure homogenous suspension of FMS. Prior to sonication, the ultrasonic (1510-DHT, Branson, Danbury, CT, USA) was degassed for 5 min. One-hundred µl of FMS was drawn into a glass syringe (1750-LT, Hamilton, Reno, NV, USA), placed in a syringe pump (BS-8000, Braintree Scientific, Braintree, MA, USA), and injected at 200 µl min−1 through the LCCA catheter (Fig. 1). Because the LCCA catheter was not placed in the left ventricle, we used a longer injection time than is common with other murine fluorescent microsphere protocols to increase the likelihood that FMS would be uniformly distributed in the arterial blood. Just prior to injecting FMS, patency of the right femoral artery catheter was checked by drawing a small volume of blood into the catheter and flushing it back into the artery. Withdrawal of arterial blood with a syringe pump at 170 µl min−1 began when FMS had been injected through the 30 µl void volume of the LCCA catheter and continued for 1 min. Following completion of the FMS injection, the LCCA catheter was flushed with 100 µl of heparinized saline (HS). The LCCA catheter port was then closed, the stopcock detached from the glass syringe, and the rear stopcock port flushed with HS to remove any red FMS. After completing the reference collection, blood in the void volume of the RFA catheter (~70 µl) was flushed back into the animal with HS, resulting in a total of only 100 µl of blood permanently withdrawn from the animal. The rear port of the RFA catheter stopcock was then flushed as just described with the LCCA catheter to remove any FMS and arterial blood. The contents of the reference syringe were transferred to a microcentrifuge tube, the syringe flushed with saline, repositioned in the syringe pump, and attached to the RFA catheter via a stopcock. To induce a functional hyperemia, the gracilis posterior muscle, the muscle chosen to be investigated, was electrically stimulated (see below). Immediately following muscle stimulation, green FMS were prepared, injected, and sampled as described for red FMS. Following injection of green FMS, the gracilis posterior was dissected with care to avoid blood loss and potentially FMS loss. Both kidneys were also dissected to confirm uniform distribution of the FMS in the arterial blood; the animals were exsanguinated after removing the kidneys. Following determination of the gracilis posterior and arterial blood FMS content (see below), absolute blood flow was calculated with the following equations:
2.8. Fluorescent microsphere protocol for relative blood flow
The experimental approach for measuring relative blood flow was identical to the absolute blood flow protocol, except that the RFA catheter was not inserted and an arterial reference sample was not taken. Thus, FMS deposition in the right gracilis posterior served as the reference for determining relative blood flow to the left gracilis posterior that was electrically stimulated. Following determination of the gracilis posterior FMS content, relative blood flow was calculated with the following equation:
2.9. Muscle stimulation
Following catheterizations, a small skin incision was made on the medial surface of the left thigh to expose the gracilis posterior muscle (Fig. 1). We chose the gracilis posterior muscle because its central artery is known to form a collateral vessel following chronic ischemia (Sullivan et al., 2002) and the muscle belly is amenable to stimulation. A stimulating electrode (1–5 µm tip diameter and 250 µm shaft diameter from Frederick Haer Co., Bowdoinham, ME, USA) was rested on the muscle, which was then covered with plastic wrap to prevent desiccation and hyperoxic vasoconstriction (Ellsworth et al., 1995). A grounding electrode was placed subcutaneously in the medial lower leg near the Achilles tendon and a brief set of contractions was induced to confirm proper placement of the stimulating electrode. Prior to the second fluorescent microsphere injection, we stimulated the left posterior gracilis with 0.2 ms square waves of 6 V at 4 Hz for 2 min using a Grass SD-9 (Grass-Telefactor, West Warwick, RI, USA). This stimulation frequency produces maximal hyperemia in rat skeletal muscle (Hawker and Egginton, 1999) and is of sufficient time for functional hyperemia to develop (Yu et al., 2005). Maintenance of electrode placement throughout the procedure was determined with a brief set of stimulation contractions following the green FMS injection.
2.10. Determination of FMS concentration
Immediately following completion of the procedure, arterial blood from the reference syringe was vortexed for 30 s and collected by brief centrifugation. The arterial blood was then thoroughly mixed with a pipette, 10 µl transferred to a tared glass slide to obtain the weight of the blood sample, and cover-slipped; 3 × 10 µl samples were prepared in this fashion. FMS in arterial blood were counted by epifluorescent microscopy (Fig. 3D), and averaged between the three slides.
Fig. 3.
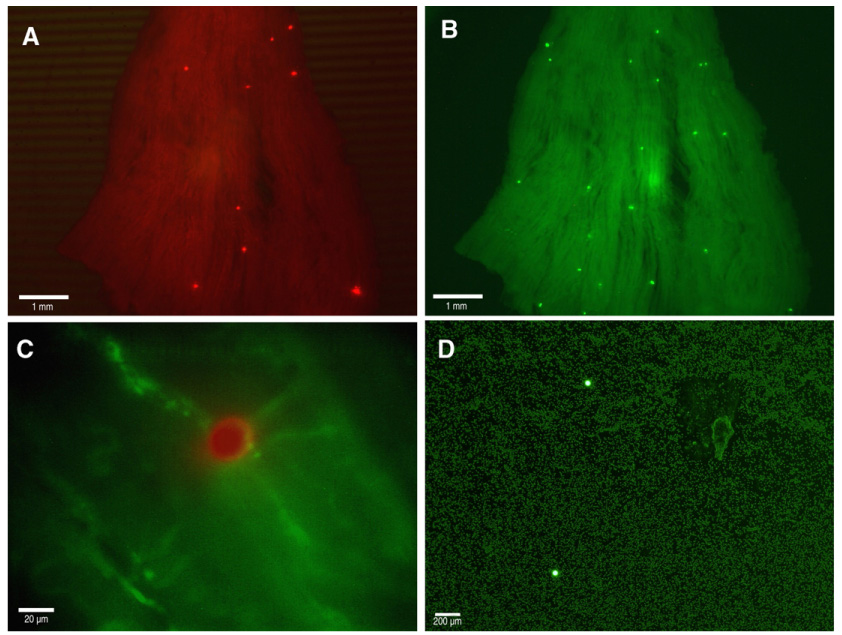
(A) Representative micrograph of red fluorescent microspheres deposited in the gracilis posterior muscle; 1× magnification. (B) Representative micrograph of green fluorescent microspheres in the same gracilis posterior muscle as in (A); 1× magnification. (C) Merged micrograph of a red fluorescent microsphere deposited in a microvessel expressing green fluorescent protein in its endothelium; 40× magnification. (D) Representative micrograph of green fluorescent microspheres in an arterial blood sample; 4× magnification.
Dissected skeletal muscles were blot-dried, weighed, and fixed in 2% paraformaldehyde overnight at 4 °C. Skeletal muscle was then rinsed 3× with cold phosphate-buffered saline and stored in glycerol to clarify. Glycerol displaces the water within the skeletal muscle and serves to “clear” the muscle because its refractive index is more similar to tissue. Clarified muscle was placed on a glass slide and compressed with a cover-slip; the FMS in skeletal muscle were counted by epifluorescent microscopy (Fig. 3A and B). FMS counts were averaged between cover-slip-side up and cover-slip-side down. Because of their resistance to photo-bleaching, no care was made to prevent exposure of intact FMS to light.
Dissected kidneys were stored in saline at 4 °C prior to digestion and fluorescence measurement, as described (Powers et al., 1999). If the right and left total kidney fluorescence values were not within 25% of each other, the animal was eliminated from the study due to lack of homogenous arterial FMS distribution. One animal was removed from each group due to lack to homogenous FMS distribution; one additional animal was removed from the relative blood flow group due to lack of FMS deposition in the right gracilis posterior.
2.11. Statistical analysis
Data are presented as mean±SEM. Statistical significance was set at p≤0.05. Differences between mean blood flow values and mean MAP values were determined by Student’s t-test. Differences in mean MAP between samples from the beginning of experiment to the end were assessed by ANOVA, followed by a Tukey post hoc test.
3. Results
3.1. Absolute blood flow
We calculated absolute blood flow to the left gracilis posterior by intra-arterially injecting fluorescent microspheres (FMS) while sampling arterial blood at a fixed rate from the right femoral artery, as described above. Absolute blood flow in the left gracilis posterior was 1.04±0.12 ml·min−1 g−1 (Fig. 4A). Absolute functional hyperemia in the left gracilis posterior was 5.94±1.33 ml·min−1 g−1 (Fig. 4A).
Fig. 4.
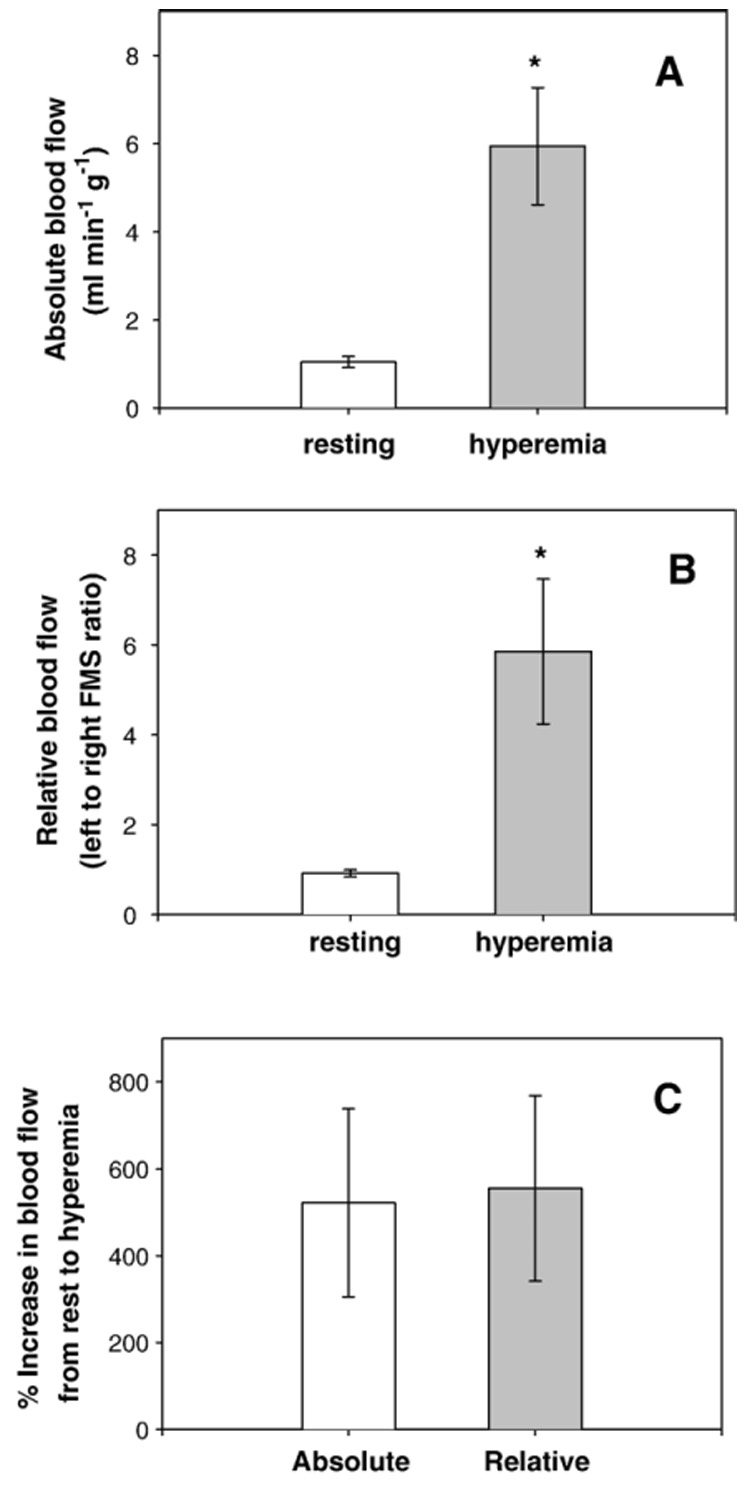
(A) Absolute resting blood flow (left bar) and functional hyperemia (right bar) in the left gracilis posterior,* = p<0.05 for hyperemia versus resting, n=3. (B) Relative resting blood flow (left bar) and functional hyperemia (right bar) in the gracilis posterior muscles, *=p<0.05 for hyperemia versus resting, n=3. (C) Percent increase from resting blood flow to functional hyperemia for absolute blood flow measurements (left bar) and relative blood flow measurements (right bar).
3.2. Relative blood flow
In addition to using sampled arterial blood as a reference for determining absolute gracilis posterior blood flow, we also utilized the contralateral muscle as a reference for determining relative gracilis posterior blood flow. As expected, relative resting blood flow (left:right FMS ratio) for the left gracilis posterior was approximately one, 0.92±0.08 (Fig. 4B). During functional hyperemia, the relative blood flow to the gracilis posterior was 5.85±1.62 (Fig. 4B). To determine if relative blood flows are similar to absolute blood flows, we compared the percent increase with hyperemia determined by the two methods. The percent increase in absolute blood flow from resting to hyperemia of 521.93±216.76% was not significantly different from the increase in relative blood flow from resting to hyperemia of 555.24±213.82% (p=0.64), (Fig. 4C).
3.3. Mean arterial pressure during blood flow measurements
Because the withdrawal of arterial blood to measure absolute blood flows may impact mean arterial pressure (MAP) due to a reduction in blood volume, we monitored MAP throughout all experiments. Interestingly, despite equivalent determinations of hyperemia between measurements of absolute and relative blood flow, mean arterial pressure (MAP) over the course of the experiment was significantly lower when arterial blood was withdrawn to measure absolute blood flow, 90.7±6.08 mm Hg versus 105.3±2.87 mm Hg (Fig. 5A). As shown in Fig. 5B, this difference is due to a significant (p<0.05) decrease in mean arterial pressure from the first 5 min of the experiment (once MAP had stabilized following catheter insertion) to the last 5 min of the experiments measuring absolute blood flow, from 102.7±2.18 to 75.5±9.71 mm Hg.
Fig 5.
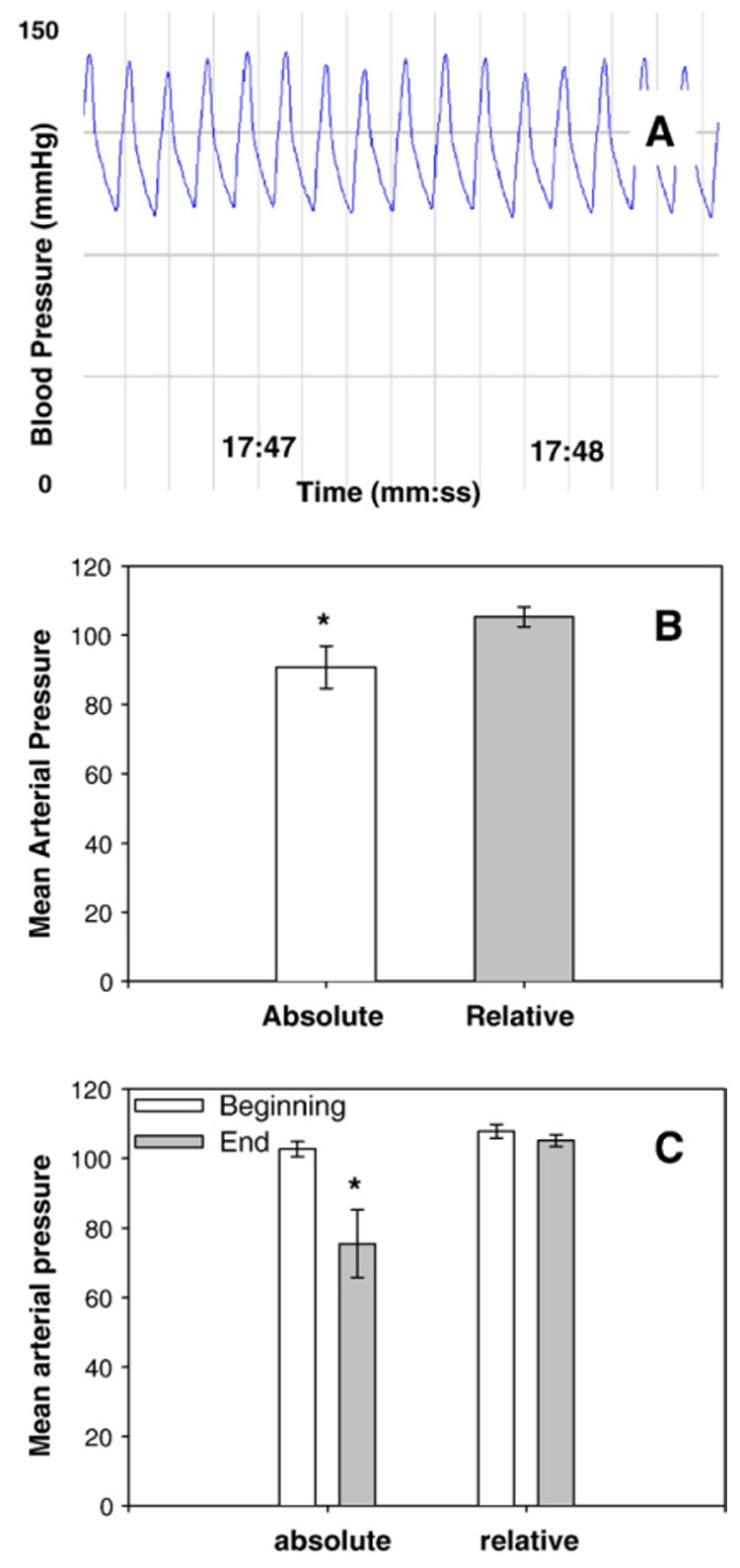
(A) Representative blood pressure trace from right common carotid artery. (B) Mean arterial pressure when measuring absolute blood flow (left bar) and when measuring relative blood flow (right bar) averaged throughout the entire experiment, *=p<0.05 for ratio-based versus reference based. (C) Mean arterial pressure when measuring absolute blood flow for 5 min after stabilization from catheterizations (far left bar) and during last 5 min of the experiment (middle left bar); mean arterial pressure when measuring relative blood flow for 5 min after stabilization from catheterizations (middle right bar) and during last the 5 min of the experiment (right bar), *=p<0.05 for the last 5 min of absolute blood flow experiments versus all other values.
4. Discussion
4.1. Blood flow measurements
We measured absolute and relative blood flows at rest and during functional hyperemia in a single skeletal muscle in the same mouse. Although, both approaches measure roughly equivalent changes in blood flow, the relative measure would be preferred, as it does not result in a decrease in mean arterial pressure (MAP) and might prevent unknown confounding conditions. Extrapolating from data obtained with both radioactive and fluorescent microspheres from other studies that measured blood flow in larger tissue volumes (e.g. all skeletal muscle or entire hindlimb) skeletal muscle blood flows were found to be 0.25 ml·min−1·g−1 (Kubis et al., 2002) or 0.71 ml·min−1·g−1 (Sarin et al., 1990) or 3.2 ml ·min−1 ·g−1 (Maxwell et al., 1998). These are in reasonable agreement with the blood flow value we measured in the gracilis posterior of 1.04±0.40 ml·min−1 ·g−1. The similarity between the blood flow values suggests that our approach produces realistic measurements of skeletal muscle blood flow. Cardiac output values for exercising mice were not available to extrapolate the absolute blood flow in the only study to date to use FMS to measure functional hyperemia in mice (Maxwell et al., 1998), but it is likely that if we are measuring realistic resting blood flow that our functional hyperemia values are also realistic.
4.2. Effect on mean arterial pressure
Although our measurements of absolute blood flow appear to be realistic, the decrease in mean arterial pressure during the experiment raises concern about the cardiovascular integrity of the mouse when measuring absolute blood flows. Even though only ~200 µl of arterial blood, approximately 10% of the mouse blood volume, is permanently removed from the animal, cardiovascular complications are produced. However, because the decrease in mean arterial pressure did not appear to affect gracilis posterior blood flow (Fig. 4), the pressure measured through the right common carotid artery may not be representative of the arterial pressure experienced at the gracilis posterior. It is also possible that a lower resistance in the gracilis posterior during measurements of absolute flow maintained blood flow equivalent to that during relative measurements. Likewise, the observed drop in mean arterial pressure may not be sufficient to significantly change the driving force of blood flow to this muscle.
4.3. Absolute versus relative blood flow
Considering the effect on mean arterial pressure and the additional experimental challenge of catheterizing the right femoral artery, determining absolute blood flow may only be necessary for a handful of applications. For example, absolute flows would be needed to incorporate blood flow data into mathematical models (Pries et al., 2001) or when calculations of resistance/conductance are desired. However, for most applications, measuring relative blood flow provides several advantages over measuring absolute blood flow. First, because a reference blood sample is not required, there is no risk of affecting an animal’s mean arterial pressure through excessive arterial sampling. Second, measuring relative blood flow allows the contralateral hindlimb to serve as an internal control that can reduce animal-to-animal variability. Third, a relative blood flow that is based on left:right ratios allows the investigator to more easily compare results with a primary literature that most commonly utilizes LDPI to determine perfusion ratios in mouse hindlimbs.
4.4. Application of approach
Measuring blood flow in individual skeletal muscle of the mouse increases the utility of this species for assaying blood flow and blood flow control. Specifically, we can now measure blood flow in select, single skeletal muscles throughout the hindlimb, superficial or deep, as well as in small volume tissues. For our interests in blood flow control following ischemic revascularization, we can utilize the features of this method to take advantage of the heterogeneity in vascular repair seen with mouse models of chronic hindlimb ischemia (Sullivan et al., 2002). Because collateralization occurs mainly in the thigh while microvascular growth and repair occurs predominately in the lower leg, we can measure blood flow in muscles with a known vascular response in either region. This allows us to determine any differences in blood flow control due to the type of vascular element that has undergone growth or repair. Additionally, this method could be used to assay differences in blood flow control for skeletal muscles of differing fiber types, innervation patterns, function, or location.
Underlying all of these questions that can now be addressed in mouse models is the ability to utilize genetic manipulation. For example, targeted disruption of genes whose protein products are important in blood flow control may finally provide an answer for the question of what is necessary and sufficient for functional hyperemia.
In addition to examining blood flow control, the capacity for measuring blood flow in individual muscles can be used to assay the effects of cellular, biological, and pharmaceutical therapeutics on the restoration of tissue perfusion or blood flow control following ischemia. Again, the segregation of collateralization and microvascular growth in mouse models of hindlimb ischemia provides an opportunity to determine the effect of therapeutic agents on the restoration of blood flow in muscles that have undergone differing forms of vascular repair. This may lead to more specific therapies for patients with ischemic disease, who may not experience dysfunction in all vascular elements.
4.5. Limitations of the approach
The primary limitation of our approach is its invasiveness, which prevents serial measurements that are possible with noninvasive strategies for measuring blood flow, such as laser Doppler perfusion imaging or MRI. However, it may be possible to apply chronic, in-dwelling catheters that are exteriorized through the dorsal neck for conscious studies (Barbee et al., 1992; Maxwell et al., 1998) or possibly serial experiments, as other species will retain trapped fluospheres within peripheral tissue for many weeks with no obvious cardiovascular complications (Hoffmann et al., 2002).
Additionally, while laser Doppler flow imaging continuously measures perfusion (an index of blood flow) over many minutes, our FMS approach is limited to determining blood flow at discrete times. Thus, if multiple changes in blood flow are expected or if determining the kinetics of an increase in blood flow is desired, our approach is not ideal. It is possible that more than two injections of FMS could be made, but pilot experiments would need to be performed to determine the effects of multiple FMS injections on pressure and resistance in the tissue of interest.
Aside from the invasiveness of the method, our approach shares a limitation with any deposition technique in that an insufficient number of FMS may lodge in the tissue of interest. Although we have improved the floor of detection, from the FMS content of ~50 microspheres that is required for a fluorimeter (Richer et al., 2000) to 1 FMS, some tissues may be sufficiently small as to not receive even 1 FMS during a majority of experiments. Additionally, even if a tissue regularly receives FMS, blood flow variability will increase as fewer FMS are deposited. For example, the difference in blood flow between 15 and 14 FMS is likely negligible, while the difference between 1 and 2 FMS is also likely negligible, or at least much less than the 100% difference in flow that would be calculated. A low number of FMS deposited in the given tissue may be ameliorated by injecting a larger volume or more concentrated volume of FMS. However, pilot experiments should be performed to determine any effects of modifying injection parameters.
5. Conclusions
In conclusion, we have developed a protocol for measuring either absolute or relative blood flows at rest and during hyperemia in individual skeletal muscles of a single mouse. This approach provides critical advantages by allowing blood flow measurements in small skeletal muscles as well as in skeletal muscles that are not superficial. The application of this approach to common models of murine hindlimb ischemia, in combination with genetically modified mice can greatly assist in understanding the molecular mechanisms of blood flow control, as well as how blood flow and its control are modified by ischemia and therapeutic interventions. Although not addressed here, this approach is not limited to blood flow measurements in skeletal muscle, and could be expanded to make blood flow measurements any small volume tissues, such as tumors or specific organ regions.
Acknowledgements
We would like to thank Andrew J Fuglevand for providing the tungsten microelectrodes and for his technical assistance in developing a stimulation protocol. We would also like to thank University of Arizona Animal Care for their care and maintenance of our mouse colony. Funding for this project was supported by HL67067, HL63732, and HL07249.
Abbreviations
- FMS
fluorescent microspheres
- LCCA
left common carotid artery
- RCCA
right common carotid artery
- RFA
right femoral artery
- MAP
mean arterial pressure
References
- Aronow WS. Management of peripheral arterial disease. Cardiol. Rev. 2005;13:61–68. doi: 10.1097/01.crd.0000126082.86717.12. [DOI] [PubMed] [Google Scholar]
- Arosio E, De MS, Zannoni M, Prior M, Lechi A. Effect of glutathione infusion on leg arterial circulation, cutaneous microcirculation, and pain-free walking distance in patients with peripheral obstructive arterial disease: a randomized, double-blind, placebo-controlled trial. Mayo Clin. Proc. 2002;77:754–759. doi: 10.4065/77.8.754. [DOI] [PubMed] [Google Scholar]
- Barbee RW, Perry BD, Re RN, Murgo JP. Microsphere and dilution techniques for the determination of blood flows and volumes in conscious mice. Am. J. Physiol. 1992;263:R728–R733. doi: 10.1152/ajpregu.1992.263.3.R728. [DOI] [PubMed] [Google Scholar]
- Bearden SE, Payne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J. Physiol. 2004;561:535–545. doi: 10.1113/jphysiol.2004.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalothorn D, Zhang H, Clayton JA, Thomas SA, Faber JE. Catecholamines augment collateral vessel growth and angiogenesis in hindlimb ischemia. Am. J. Physiol.: Heart Circ. Physiol. 2005;289:H947–H959. doi: 10.1152/ajpheart.00952.2004. [DOI] [PubMed] [Google Scholar]
- Duza T, Sarelius IH. Increase in endothelial cell Ca(2+) in response to mouse cremaster muscle contraction. J. Physiol. 2004;555:459–469. doi: 10.1113/jphysiol.2003.051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am. J. Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- Hawker MJ, Egginton S. The effect of stimulation frequency on blood flow in rat fast skeletal muscles. Exp. Physiol. 1999;84:941–946. [PubMed] [Google Scholar]
- Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler. Thromb. Vasc. Biol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- Hoffmann JN, Steinhagen S, Kast C, Scheuber HP, Jochum M, Gippner-Steppert C, Inthorn D, Schildberg FW, Nolte D. Chronic left heart catheterization for microvascular blood flow determination in the rabbit: a minimally invasive technique using specially designed port devices. J. Surg. Res. 2002;102:119–125. doi: 10.1006/jsre.2001.6280. [DOI] [PubMed] [Google Scholar]
- Hudlicka O, Brown MD, Egginton S, Dawson JM. Effect of longterm electrical stimulation on vascular supply and fatigue in chronically ischemic muscles. J. Appl. Physiol. 1994;77:1317–1324. doi: 10.1152/jappl.1994.77.3.1317. [DOI] [PubMed] [Google Scholar]
- Kubis N, Richer C, Domergue V, Giudicelli JF, Levy BI. Role of microvascular rarefaction in the increased arterial pressure in mice lacking for the endothelial nitric oxide synthase gene (eNOS3pt-/-) J. Hypertens. 2002;20:1581–1587. doi: 10.1097/00004872-200208000-00021. [DOI] [PubMed] [Google Scholar]
- Lumsden AB, Rice TW. Medical management of peripheral arterial disease: a therapeutic algorithm. J. Endovascular Ther. 2006;13(2):II19–II29. doi: 10.1177/15266028060130S206. [DOI] [PubMed] [Google Scholar]
- Maxwell AJ, Schauble E, Bernstein D, Cooke JP. Limb blood flow during exercise is dependent on nitric oxide. Circulation. 1998;98:369–374. doi: 10.1161/01.cir.98.4.369. [DOI] [PubMed] [Google Scholar]
- Motoike T, Loughna S, Perens E, Roman BL, Liao W, Chau TC, Richardson CD, Kawate T, Kuno J, Weinstein BM, Stainier DY, Sato TN. Universal GFP reporter for the study of vascular development. Genesis. 2000;28:75–81. doi: 10.1002/1526-968x(200010)28:2<75::aid-gene50>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Powers KM, Schimmel C, Glenny RW, Bernards CM. Cerebral blood flow determinations using fluorescent microspheres: variations on the sedimentation method validated. J. Neurosci. Methods. 1999;87:159–165. doi: 10.1016/s0165-0270(98)00180-0. [DOI] [PubMed] [Google Scholar]
- Pries AR, Reglin B, Secomb TW. Structural adaptation of microvascular networks: functional roles of adaptive responses. Am. J. Physiol.: Heart Circ. Physiol. 2001;281:H1015–H1025. doi: 10.1152/ajpheart.2001.281.3.H1015. [DOI] [PubMed] [Google Scholar]
- Prinzen FW, Bassingthwaighte JB. Blood flow distributions by microsphere deposition methods. Cardiovasc. Res. 2000;45:13–21. doi: 10.1016/s0008-6363(99)00252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S, Verkman AS. Analysis of organ physiology in transgenic mice. Am. J. Physiol., Cell Physiol. 2000;279:C1–C18. doi: 10.1152/ajpcell.2000.279.1.C1. [DOI] [PubMed] [Google Scholar]
- Richer C, Domergue V, Gervais M, Bruneval P, Giudicelli JF. Fluospheres for cardiovascular phenotyping genetically modified mice. J. Cardiovasc. Pharmacol. 2000;36:396–404. doi: 10.1097/00005344-200009000-00017. [DOI] [PubMed] [Google Scholar]
- Sarin SK, Sabba C, Groszmann RJ. Splanchnic and systemic hemodynamics in mice using a radioactive microsphere technique. Am. J. Physiol. 1990;258:G365–G369. doi: 10.1152/ajpgi.1990.258.3.G365. [DOI] [PubMed] [Google Scholar]
- Sullivan CJ, Hoying JB. Flow-dependent remodeling in the carotid artery of fibroblast growth factor-2 knockout mice. Arterioscler. Thromb. Vasc. Biol. 2002;22:1100–1105. doi: 10.1161/01.atv.0000023230.17493.e3. [DOI] [PubMed] [Google Scholar]
- Sullivan CJ, Doetschman T, Hoying JB. Targeted disruption of the Fgf2 gene does not affect vascular growth in the mouse ischemic hindlimb. J. Appl. Physiol. 2002;93:2009–2017. doi: 10.1152/japplphysiol.00451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JL, Cartland D, Hussain A, Egginton S. A differential role for nitric oxide in two forms of physiological angiogenesis in mouse. J. Physiol. 2006;570:445–454. doi: 10.1113/jphysiol.2005.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Demuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc. Natl. Acad. Sci. U. S. A. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]


