Abstract
Plasma membrane calcium pumps (PMCAs) are integral membrane proteins that actively expel Ca2+ from the cell. Specific Ca2+-ATPase activity of erythrocyte membranes increased steeply up to 1.5–5 times when the membrane protein concentration decreased from 50 μg/ml to 1 μg/ml. The activation by dilution was also observed for ATP-dependent Ca2+ uptake into vesicles from Sf9 over-expressing the PMCA 4b isoform, confirming that it is a property of the PMCA. Dilution of the protein did not modify the activation by ATP, Ca2+ or Ca2+-calmodulin. Treatment with non-ionic detergents did not abolish the dilution effect, suggesting that it was not due to resealing of the membrane vesicles. Pre-incubation of erythrocyte membranes with Cytochalasin D under conditions that promote actin polymerization abolished the dilution effect. Highly-purified, micellar PMCA showed no dilution effect and was not affected by Cytochalasin D. Taken together, these results suggest that the concentration-dependent behavior of the PMCA activity was due to interactions with cytoskeletal proteins. The dilution effect was also observed with different PMCA isoforms, indicating that this is a general phenomenon for all PMCAs.
Keywords: PMCA, calmodulin, calcium, membrane, cytoskeleton, Cytochalasin D
INTRODUCTION
Plasma membrane Ca2+ pumps (PMCAs) expel Ca2+ from all eukaryotic cells to help them maintain low concentrations of cytosolic Ca2+. PMCAs consist of a single polypeptide chain of 127,000 to 137,000 daltons. PMCAs are calmodulin-regulated P-type ATPases encoded by a multigene family. In humans, four genes encode PMCA isoforms 1–4, and alternative splicing augments the number of variants to over 20 (for a review, see Zacharias and Strehler [1]).
To characterize the mechanism of the PMCAs under different conditions, it is necessary to measure Ca2+-ATPase activity and Ca2+ transport [2]. Although Chaudhary et al [3] have synthesized natural occurring extracellular Ca2+ pump inhibitor peptides named caloxins, they are not as useful as ouabain for Na+/K+-ATPase or thapsigargin for the SERCA pumps, and such measurements are challenging for the PMCA in its natural environment.
We describe here unexpected changes of the specific activity of the PMCA, showing an activation-inhibition behavior due to dilution of membranes containing native embedded PMCAs. It has been shown for other membrane proteins, e.g. for Na+/K+-ATPase, that cytoskeletal proteins such as actin, modulate their activity by a mechanism that apparently involves the direct binding of actin to the enzyme [4]. The focus of this work was to characterize the activation-inhibition phenomenon and to explore a possible interaction of the PMCA with the cytoskeleton.
The most obvious practical consequence of the observed dilution effects is that when making comparisons between PMCA activities in different conditions, the activities should always be measured at similar membrane protein concentrations to be meaningful.
MATERIALS AND METHODS
Reagents
All the chemicals used in this work were of analytical grade and purchased from Sigma Chemical Co. (USA). Recently drawn human blood for the isolation of PMCA was obtained from the Hematology Section of the Hospital de Clínicas General San Martín (Argentina).
Isolation of membranes from human erythrocytes
Red cells were washed three times with 10 volumes of 150 mM NaCl at 10°C. Calmodulin-depleted erythrocyte membranes were prepared according to González Flecha et al [5] using 15 mM 3-(N-morpholino)-propanesulfonic acid (MOPS) and 1 mM ethylene glycol bis-(β-aminoethyl ether)-N, N, N′, N′-tetraacetic acid EGTA (pH 7.4 at 4°C) as hypotonic solution, and finally 15 mM MOPS and 5 μM CaCl2 (pH 7.4 at 4°C). The membranes were stored in liquid nitrogen until use.
Purification of PMCA from human erythrocytes
PMCA was isolated in pure form by calmodulin-affinity chromatography as described elsewhere [6], and stored in liquid nitrogen until use. PMCA was kept in a buffer containing 20% (w/v) glycerol, 0.005% C12E10, 120 mM KCl, 1 mM MgCl2, 10 mM MOPS-K, pH 7.4 at 4°C, 2 mM EDTA, 2 mM CaCl2, 2 mM 1,4-dithiothreitol (DTT).
Cell culture
The Sf9 or Sf21 cells (Spodoptera frugiperda) were grown in suspension at 27 °C in Grace Medium supplemented with 10% fetal bovine serum, 10 μg/ml penicillin and 0.25 μg/ml streptomycin.
Expression of PMCA in Sf9 or Sf21 cells
The expression for protein production was carried out by infecting Sf9 or Sf21 cells in complete Grace Medium with the appropriate recombinant virus at a multiplicity of infection (MOI) of 1 or 2. The preparation of recombinant baculovirus for PMCA4b, PMCA2b and the truncated mutant ct-120 has been described earlier [7]. After 48 hours of incubation at 27°C, the cells were harvested. The cells were washed with phosphate buffered saline (PBS) containing 1 mM EGTA and protease inhibitors, quick frozen, and kept at −80°C until microsomal preparation.
Microsomal preparation
Crude microsomal membranes from Sf9 or Sf21 cells (either untransfected or transfected with PMCA4b, 2b, 4a, PMCA-CT120 or null virus), were prepared as described previously for COS cells with minor modifications [8]. Briefly, the frozen cells were washed in PBS containing 1 mM EGTA and protease inhibitors. After equilibration on ice for 10 min in hypotonic solution of 10 mM Tris-HCl, pH 7.5, containing 1 mM MgCl2, 0.5 mM EGTA, 2 mM DTT and protease inhibitors, the cells were homogenized with a Potter-Elvehjem homogenizer. An equal volume of 10 mM Tris-HCl, pH 7.5, containing 0.3 M KCl, 0.5 M sucrose, 2 mM DTT and protease inhibitors was added to the homogenate and the mixture further homogenized. Per 200 × 106 cells, 6 ml hypotonic and 6 ml homogenization buffer were used. Unbroken cells, tissue debris and nuclei were removed by centrifugation at 4000 g for 20 min at 4 ºC. The supernatant was mixed (at 1:1 ratio) with 2.5 mM EDTA and 0.5 M KCl and centrifuged at 100,000 g for 40 min at 4°C and the pellet suspended in 10 mM Tris-HCl, pH 7.5, 0.25 M sucrose, 0.15 M KCl, 2 mM DTT and protease inhibitors. Aliquots of the microsomes were stored in liquid nitrogen.
Measurement of Ca2+-ATPase activity
ATPase activity was measured at 37°C by following the release of inorganic phosphate from ATP as described previously [9]. The incubation media were: a) erythrocyte membranes: 120 mM KCl, 30 mM MOPS-K (pH 7.4), 3.75 mM MgCl2, 1 mM EGTA, and enough CaCl2 to give the desired final free [Ca2+]; b) Sf9 microsomes: 120 mM KCl, 30 mM MOPS-K (pH 7.4), 3.75 mM MgCl2, 5 mM NaN3, 0.5 mM ouabain, 200 nM thapsigargin, 1 mM EGTA, and enough CaCl2 to give the desired final free [Ca2+]; c) purified PMCA: 120 mM KCl, 30 mM MOPS-K (pH 7.4), 3.75 mM MgCl2, 80 μg/ml C12E10, 60 μg/ml phosphatidylcholine, and enough CaCl2 to give the desired final free [Ca2+]. The reaction was started by the addition of ATP (final concentration 2 mM). Release of Pi was estimated according to the procedure of Fiske and Subbarow [10]. Alternatively (experiments in figures 5 and 6) ATPase activity was measured by continuous monitoring of the phosphorolysis of 2-amino-6-mercapto-7-methylpurine riboside (MESG) in a coupled assay system. In this case, the activity was measured in media containing 120 mM KCl, 30 mM N-[Tris(hydroxymethyl)methyl]-2-aminoethanesulfonic acid-(tetraethylammonium salt) (Tes-TEA) (pH 7.2), 5 mM MgCl2, 2.5 mM ATP, 0.2 mM MESG, 5 u/ml of phosphoenol pyruvate (PEP), 5 mM NaN3, 0.5 mM ouabain, 200 nM thapsigargin, 0.2 mM EGTA, and enough CaCl2 to give the free Ca2+ concentration indicated in the figure [11]. When present, calmodulin was 300 nM.
Figure 5.


The rate of activation of PMCA by CaM is not affected by dilution. Panel A: ATP hydrolysis by PMCA was measured continuously by the procedure of Webb [11]. Upon addition of calmodulin the data were fitted by the following equation:
| (3) |
where Y0 is the amount of product present at time = 0, v0 is the ATPase activity in the absence of calmodulin, vc is the increase in activity upon calmodulin addition, and k (app k in the figure) is the rate constant for the change in activity. A detailed justification for this equation is provided in ref. [19]. Panel B: k for the different conditions presented as bar graphs.
Figure 6.

The dilution effect is present in different isoforms of PMCA. ATPase activity was measured as a function of membrane protein concentration at 10 μM Ca2+ and in the absence of calmodulin by following the phosphorolysis of MESG, as described in Materials and Methods. Panel A: PMCA4a and 4b; panel B: PMCA4-CT120; panel C: PMCA2b.
Measurement of free Ca2+ concentrations
The Ca2+ concentration in the incubation medium was measured using a selective Ca2+ electrode, as described by Kratje et al. [12].
Measurement of Ca2+ uptake
Ca2+ transport was measured by the incorporation of 45Ca2+ into microsomes prepared from Sf9 or Sf21 cells over-expressing PMCA4b. Microsomes were incubated at 15 °C in a medium containing 120 mM KCl, 30 mM MOPS-K (pH 7.4), 3.75 mM MgCl2, 5 mM NaN3, 0.5 mM ouabain, 200 nM thapsigargin and 45Ca2+ (at 100 μM free Ca2+ concentration) and 5 minutes later the reaction was started by addition of ATP (final concentration 2 mM). Samples were taken at different times and filtered immediately through Millipore filters with a pore diameter of 0.45 μm, which retained all protein [13]. The experimental set up was adjusted to ensure initial velocity conditions all along the experiment. The filters were washed twice with 10 ml of a cold solution of 150 mM KCl and 10 mM Tris-HCl, pH 7.4, and then transferred to counting vials, containing PPO/POPOP (0.4% and 0.015 % respectively) in toluene. Radioactivity retained in the filters was measured in a liquid scintillation counter. The 45Ca2+ pumped into the microsomes as a consequence of PMCA activity was calculated as the difference in radioactivity measured with or without ATP.
Gel Electrophoresis
SDS gel electrophoresis was carried out according to the Tris/tricine method [14]. After electrophoresis, the gels were stained with Coomasie Brilliant Blue G.
Determination of protein concentrations
The protein concentration was estimated by the method described by Lündahl [15] and by the method of Peterson [16].
Phospholipid quantification
Phospholipid concentration was measured according to Chen et al. [17] with some modifications. Samples and standards containing 10–100 nmol phosphate were dried by heating at 100° C. Mineralization was carried out by adding 0.1 ml HNO3, 0.9 ml HClO4 and incubating at 190 °C for 30 min. Inorganic phosphate was determined by the method of Fiske and Subbarow [10].
Incubation of erythrocyte membranes with Cytochalasin D
Erythrocyte membranes were incubated with 20 μM Cytochalasin D or the same amount of DMSO as a control (vehicle for Cytochalasin D solutions) for 10 minutes at 37 °C in a medium containing 120 mM KCl (which promotes polymerization), Tris-HCl 30 mM, pH 7.4 at 25 °C, and 2 mM MgCl2. After incubation, membranes were diluted to reach the final protein concentration indicated in the figure, and Ca2+-ATPase activity was measured as described above by the procedure of Fiske and Subbarow [10].
RESULTS
Effect of total membrane protein concentration on Ca2+-ATPase activity
Figure 1 shows the effect of decreasing the concentration of membrane protein on the Ca2+-dependent ATPase activity of human red cell membranes. The activity increased from about 0.8 μmol Pi (mg of protein h)−1 at a concentration above 50 μg/ml, to about 2.0 μmol Pi (mg of protein h)−1 at 5 μg/ml of membrane protein. The half-maximal protein concentration for this effect was 8.5 μg/ml. Thus, diluting the membrane preparation increases the activity at least 2-fold. The dilution effect was observed at all ionic strengths from 0.06 to 0.6 M (modified by the addition of KCl to the media). The activity at 0.06 M KCl was 1.3 ± 0.1 versus 0.8 ± 0.1 μmol Pi. (mg protein. h)−1 at 10 and 50 μg/ml total protein concentration, respectively, while at 0.6 M it was 1.3 ± 0.3 versus 0.9 ± 0.09 μmol Pi. (mg protein. h)−1 at 10 and 50 μg/ml total protein concentration, respectively. This suggests that the phenomenon is not dependent on the ionic strength. Additionally, the effect was also observed when the activity was measured at 20 μM ATP (instead of 2000 μM as in the rest of the experiments), using the method of Richards et al, which involves the use of 32γ-P-ATP [18]. In this case, the activity was 0.81 ± 0.3 versus 0.43 ± 0.1 μmol Pi. (mg protein. h)−1 at 0.8 and 3.3 μg/ml total protein concentration, respectively, an effect that is similar to that observed in Figure 1.
Figure 1.
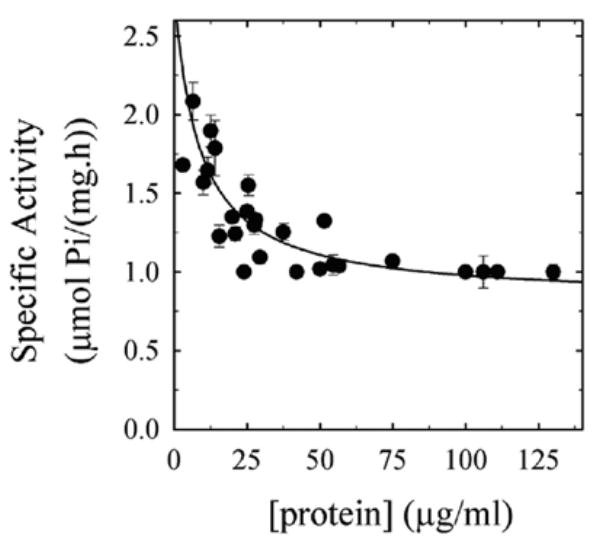
Ca2+-dependent ATPase activity decreases as membrane protein concentration increases. The membranes were obtained from human erythrocytes as described in Methods. The experimental points were fitted by the following equation:
| (1) |
where Vres is the specific activity when protein concentration is maximal, Vm is the maximal change in specific activity when protein concentration goes from zero to infinity and K0.5 is the protein concentration that gives half maximal inhibition. Parameter values are: Vres: 0.8 ± 0.2 μmol Pi (mg of protein h)−1; Vm: 2.0 ± 0.2 μmol Pi (mg of protein h)−1; and K0.5: 8.5 μg/ml. Results are shown as mean (± 1 SD) of seven independent experiments performed in triplicate. Media composition was as indicated in the Materials and Methods section. Ca2+ concentration was 100 μM.
To test if the above effect was apparent only in red cells or whether it was present in PMCAs in different cells/membrane systems, we expressed human PMCA from different isoforms in insect cells using the baculovirus over-expression system. In this system, we were able to express different amounts of PMCA protein by varying the multiplicity of infection (MOI), and then testing the specific Ca2+-dependent ATPase activity as a function of the membrane protein concentration. As shown in Figure 2A, when Sf21 cells are transfected with a null virus (negative control), the amount of PMCA in the membranes is too low to be detected by Coomasie staining, preventing calculation of the amount of PMCA protein by densitometry. By using recombinant PMCA4b-expressing virus at MOIs of 1 and 2, the amount of PMCA increases to 8.6% and 13.1% respectively, of the total membrane protein. For comparison, PMCA comprises approximately 0.1% of the protein in native membranes. Panel B shows the effect of increasing the amount of microsomal protein on the specific ATPase activity in the three conditions described in Panel A (null, MOI = 1, and MOI =2). In all three cases, the specific activity first increases with the protein concentration, reaches a maximum, and then decreases to a finite value. The data also show that the higher the amount of PMCA in the membranes, the higher the concentration of membranes at which the maximum is reached and the setting of inhibition is observed.
Figure 2.


Membrane protein concentration has a biphasic effect on activity when PMCA is over-expressed. Panel A. SDS-PAGE. Lane 1: microsomes without PMCA over-expression (cells infected with null virus) as a negative control. Lane 2: microsomes with PMCA4b over-expression (cells infected with MOI=1). Lane 3: microsomes with PMCA4b over-expression (cells infected with MOI=2). Lane 4: purified PMCA from human red cells (positive control), and a BSA aliquot. The gel was stained with Coomassie Brilliant Blue. The amount of PMCA in the membranes was calculated densitometrically by comparison to known amounts of BSA. The arrow on the right shows the 135 kD band characteristic of PMCA4b, which can be seen in lanes 2 to 4. This band cannot be seen when the enzyme is not over-expressed, since the endogenous PMCA does not exceed 0.1% of total membrane protein (lane 1). According to the densitometry, in cells infected at MOI = 1 (lane 2), PMCA represented 8.6% and at MOI = 2 (lane 2), 13.1% of the total membrane protein.
Panel B. Ca2+-ATPase activity as a function of total protein concentration of microsomes prepared from cells infected with null virus (solid squares), PMCA4b at MOI=1 (open circles), and PMCA4b at MOI=2 (solid circles). The activity was measured in the absence of calmodulin and in the presence of 100 μM Ca2+.
Effect of membrane dilution on Ca2+ uptake
To further confirm that the dilution effect was a functional property of PMCA, and was not due to the presence of other (unknown) ATPase activity in the membrane, we measured active Ca2+ uptake into microsomes prepared from Sf9 insect cells over-expressing PMCA 4b, at 4 and 12 μg/ml final membrane protein concentrations. Results are shown in Figure 3. In both cases, Ca2+ uptake was linear with time, indicating that measurements were performed under initial velocity conditions. The slope, however, was three times higher at the lower protein concentration, showing that the dilution effect is also observed for Ca2+ transport, thus confirming that the effect is attributable to PMCA.
Figure 3.

The effect of dilution is observed on active Ca2+ transport into Sf9 vesicles. The activity was measured as described in Materials and Methods, using 12 μg/ml (solid symbols) or 4 μg/ml (open symbols) final protein concentrations. The slope of the linear regression with time is the Ca2+ transport activity: 0.0287 and 0.116 nmol Ca2+ (mg of protein h)−1 for high and low protein concentration, respectively.
Effect of detergent on the dilution effect
To study the relationship between the dilution effect and the accessibility of the membranes to the reactants, we carried out experiments on the effect of the detergent C12E10 on the Ca2+-ATPase activity of red cell membranes as a function of the concentration of this detergent. Media contained 10, 50 and 200 μg/ml of membrane protein, so as to compare meaningfully the results obtained at three different concentrations of membrane protein. Figure 4 shows the results of 3–5 independent experiments, expressing the activity as a function of the concentration of the detergent (panel A) and of the molar fraction of the detergent in the membrane (panel B). In all three cases, the detergent activated the Ca2+-ATPase following a biphasic curve (activation-inactivation behavior). The peak of specific activity was found at lower detergent concentrations when the protein concentration was the lowest (Fig. 4A). However, comparing the effect of detergent as a function of its molar fraction relative to the total phospholipid content (Panel B), a similar pattern was found regardless of the concentration of protein: the specific activity rises above a molar fraction of 0.3–0.5 and is maximal around 0.7–0.8. This implies that the increase in specific activity due to membrane protein dilution was not abolished by the detergent, suggesting that the dilution effect is not due to impairment of accessibility of reactants to the membranes at higher protein concentration.
Figure 4.

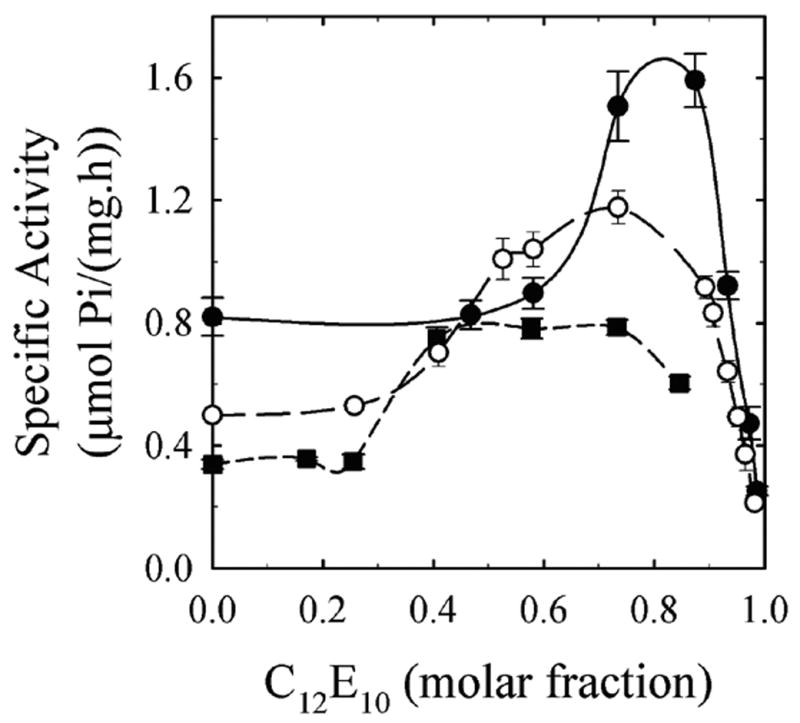
Addition of C12E10 to erythrocyte membranes does not abolish the dilution effect. Panel A: Specific activity as a function of detergent concentration (% w/v). Panel B: Specific activity as a function of molar fraction of detergent (Xdet) at 10 μg/ml (solid circles), 50 μg/ml (open circles), 200 μg/ml (solid squares) protein concentration.
| (2) |
Characterization of the phenomenon
To further characterize the above effect on PMCA specific activity, we analyzed the influence of membrane protein concentration on the Ca2+ and calmodulin dependence of the ATPase activity in red cell membranes. We observed that membrane concentration had no significant effect on the apparent affinity for either Ca2+ or calmodulin. The apparent affinity for Ca2+ was 39.5 ± 9.2 and 22.3 ± 5.1 μM for 20 and 100 μg/ml protein concentration, respectively, in the absence of CaM, while it was 1.03 ± 0.42 and 0.59 ± 0.29 μM for 20 and 100 μg/ml protein concentration, respectively, in the presence of CaM. The apparent affinity for CaM was 7.04 ± 2.72 and 7.84 ± 4.1 μM for 20 and 40 μg/ml protein concentration, respectively. The result is in keeping with the idea that the dilution effect is not due to a change in accessibility for these ligands at higher membrane concentrations.
The apparent affinity of PMCA4b for calmodulin is determined by a slow activation step, and an even slower dissociation of calmodulin from PMCA [19]. To check whether these steps were modified by dilution of the membranes (without changing the overall apparent affinity for calmodulin), we performed experiments in which the activity of PMCA4b, expressed in insect cells, was measured by continuously monitoring the hydrolysis of ATP at a low and high microsomal concentration (Figure 5). At the time shown by the vertical arrows in Fig. 5A, calmodulin was added and the rate at which calmodulin activated PMCA was obtained from the rate of change of slope by fitting the data according to the equation in the legend to Figure 5. The bar graph in Fig. 5B is a summary of three independent experiments like the one shown in Fig. 5A. From the results it is evident that: (a) the hydrolysis of ATP is linear with time both before addition of calmodulin and after calmodulin activation of the pump is completed, and (b) the rate at which calmodulin activates the pump is approximately the same at low and high microsomal protein concentration.
Effect of membrane dilution on the activity of other isoforms of the PMCA
We compared the dilution effect for several isoforms and constructs of PMCA expressed in the baculovirus/Sf9 cell system. Figure 6A shows that both alternative splicing products of PMCA4, i.e. PMCA4a and PMCA4b, display the dilution effect. To test whether the C-terminal tail of PMCA4 is necessary for this effect we measured the activity of the construct PMCA4-CT120 as a function of membrane protein concentration. PMCA4-CT120 is a deletion mutant of PMCA4 that lacks the entire regulatory C-terminal end [20]. The results shown in Fig. 6B suggest that the dilution effect is not dependent on the C-terminal end of PMCA. We also tested a different isoform, PMCA2b, and obtained a similar behavior (Fig. 6C). These results support the idea that the dilution effect is a general phenomenon for all PMCAs.
Possible interaction of PMCA with the cytoskeleton
Figures 3, 4 and 5 demonstrate that different accessibility to ligands is not responsible for the dilution effect. Figure 7A shows the results of an experiment designed to test the idea that interaction with the cytoskeleton might be at least in part responsible for the dilution effect. Ca2+-ATPase activity from red cell membranes was measured at 37 °C after incubation with Cytochalasin D. This toxin is known to promote or inhibit the polymerization of actin depending on the concentration of Mg2+ and K+ ions present in the media [21]. To promote actin polymerization, 120 mM KCl was added to the medium. Control membranes were incubated with DMSO (vehicle for Cytochalasin D solutions) alone. It can be observed in Fig 7A that when Cytochalasin D was present, no activation (but rather inhibition) by dilution of the membranes was obtained. This suggests that the cytoskeleton, or at least actin, depending on its state of polymerization, is involved in the membrane concentration-dependent activation/inhibition of PMCA.
Figure 7.


Actin cytoskeleton is involved in the dilution effect. Panel A. Effect of Cytochalasin D on specific Ca2+-ATPase activity in membranes prepared from human erythrocytes. Membranes from human erythrocytes were pre-incubated with 20 μM Cytochalasin D (open circles) or the same amount of DMSO as a control (solid circles) at 37 °C, in medium with 120 mM KCl (a polymerizing condition). After 10 min of incubation, membranes were diluted to reach the final protein concentration indicated in the figures and Ca2+-ATPase specific activity was measured as described before. Panel B. Effect of Cytochalasin D on purified PMCA from human erythrocytes. PMCA was incubated for 1.5 hours in the medium used to measure Ca2+-ATPase activity (see Materials and Methods), containing 20 μM Cytochalasin D (open circles) or the same amount of DMSO (solid circles). PMCA was supplemented with phosphatidylcholine and C12E10 which were kept constant at 60 and 80 μg/ml respectively for all concentrations of PMCA assayed.
Figure 7B shows that, compared to the native embedded enzyme, the PMCA activity of the purified enzyme from human erythrocytes was not influenced by protein concentration, provided that enough phosphatidylcholine was added. This is consistent with the experiments shown by Bredeston and Rega [22] and indicates that other proteins associated with PMCA in the native membranes are needed to activate the enzyme at low protein concentrations. Figure 7B also shows that Cytochalasin D does not contribute to PMCA activation or inactivation by itself.
DISCUSSION
Results described in this work reveal an unexpected phenomenon: the specific catalytic activity of PMCA is not independent of the membrane concentration in the media, but rather increases as the total membrane protein concentration decreases. The phenomenon was confirmed with PMCA from different sources, including PMCA in red cell membranes as well as different isoforms of PMCA over-expressed in insect cells: the ubiquitous PMCA4b, PMCA4a (which has a different C-terminal tail than PMCA4b), PMCA2b, which is of interest because of its location in neuronal tissue and the inner ear, and the mutant PMCA4-CT120 which lacks the C-terminal end including the calmodulin-binding region. Therefore, the observed “dilution effect” of PMCA activity seems to be a general property rather than a phenomenon specific to a particular PMCA preparation. Besides, similar changes of ATPase activity with membrane concentration were also observed in Na+/K+-ATPase [4].
The most obvious explanation for such a phenomenon would be that, upon concentration, the membrane fragments either form sealed vesicles or present accessibility problems for the different ligands (namely Ca2+, ATP, Mg2+ and calmodulin), therefore decreasing the apparent specific activity of the enzyme. However, several arguments allow ruling out this explanation: a) the “dilution effect” was observed by measuring both the ATPase activity of membrane fragments and Ca2+ uptake into vesicles; b) the apparent affinities for Ca2+ and calmodulin, as well as the rate of activation by calmodulin, were independent of the concentration of membranes. Moreover, they were comparable to the values found in the literature [7, 19, 23, 24], suggesting that the effective concentration of these ligands in the vicinity of the pump molecules was unaltered, and that there were no accessibility problems; c) the “dilution effect” was observed even in the presence of non-ionic detergents.
A different possible explanation is related to “molecular crowding” of PMCA molecules. However, activation by dilution has never been observed in preparations of purified pump, a result that we confirmed here in the experiment shown in Figure 7. If molecular crowding was the reason for our results, we would expect to see activation by dilution in preparations of purified PMCA. On the contrary, it has been documented [22] that the purified enzyme shows concentration-dependent activation, as we observed here for extremely low membrane concentrations. In this case, the effect was attributed to the concentration of phosphatidylcholine.
When we tested the dilution effect after incubating the erythrocyte membranes with Cytochalasin D, under conditions that promote actin polymerization, there was no activation by dilution. No dilution effect was observed in the purified enzyme, which lacks cytoskeleton proteins. Indeed, Cytochalasin D had no effect on such preparations. Taken together, these results strongly suggest that the activation-inhibition behavior is a consequence of interaction of cytoskeleton proteins with PMCA. Therefore, one of the possible causes of the variability observed between preparations in the fold-increase of activity due to dilution could be the differences in concentration of protein related to the cytoskeleton, e.g. in inside-out vesicles (not shown) the activity increased at least 10 fold, and this can be also related to how thorough is the washing of the membranes.
We can estimate the concentration of actin in our preparations by SDS-PAGE using BSA as a control (data not shown). For the concentration levels we tested, actin was estimated to be around 0.5 μM in the concentrated membranes, dropping to less than 0.05 μM in diluted membranes. According to the literature, the critical concentration of actin where the growth rate of actin filaments falls below 0 is 0.05 μM [25], which is similar to the estimated concentration of actin in the diluted samples in our experiments. Based on this, we hypothesize that at the low actin concentration in our diluted membrane samples, actin exists in short filaments or as monomers. Monomeric actin would thus be responsible for the activation observed at low concentrations of membranes or, alternatively, oligomeric actin could act as an inhibitor. This hypothesis is consistent with the results obtained using Cytochalasin D: when incubating erythrocyte membranes with Cytochalasin D under conditions that promote actin polymerization (120 mM KCl, 3.75 mM MgCl2) the dilution effect is not observed because actin would remain in its filamentous form, despite the dilution of the membranes. This hypothesis is currently being tested in experiments using monomeric and filamentous actin on purified PMCA. Interestingly, evidence in the literature has suggested that short filaments of actin activate the Na+/K+-ATPase in a mechanism that apparently involves the direct binding of actin to the enzyme [4].
PMCA in activated platelets has been postulated to be recruited to the cytoskeleton by interaction with PDZ domain proteins via the pump’s C-terminal PDZ-binding tail [26] (the PDZ domain is a common structural domain of 80–90 amino-acids that constitute a ubiquitous protein-protein interaction motif [27]). The fact that the CT-120 truncated mutant of PMCA4 also shows the dilution effect indicates the involvement of a region other than the C-terminal end. Although the C-terminal end of PMCA4b and 2b contains a PDZ-binding motif -which is involved in recruitment of scaffolding proteins [28] this is not the only way of interaction with the cytoskeleton complex. Recent work has shown that the PMCA can interact via its N-terminal tail as well as through its large catalytic loop with signaling and anchoring proteins [29–31]. From the results presented here it is not possible to determine the precise region involved in the dilution effect.
When measuring activity in Sf9 over-expressing PMCA, the dilution is not as evident as in the same native cells. It could be possible that over-expression of PMCA overwhelms the capacity for cytoskeletal interactions, i.e. the PMCA/actin ratio is greater in the over-expressed cells, and this could alter the dilution effect.
A different explanation for the observed effect is the presence of a hydrophobic inhibitor in the native membranes. Regarding this possibility, it was recently reported that certain sphingolipids are inhibitors of PMCA [32–34]. Besides, lipid composition of the membranes was shown to be important for PMCA activity in human ocular lens [35]. Other hypothetical inhibitors are small membrane proteins, analogous to phospholamban in the case of SERCA. Relevant to this, a family of hydrophobic peptides has been reported to regulate the Na+/K+ pump (the FXYD proteins [36, 37]). To date, no such regulatory peptides have been proposed for the PMCA, although Chaudhary et al [3] have synthesized natural occurring extracellular Ca2+ pump inhibitor peptides named caloxins. The fact that the dilution effect remained even after the membranes were treated with the detergent C12E10, which extracts phospholipids and other hydrophobic components, makes this interpretation less likely.
The dilution effect has experimental implications when measuring functional properties of the pump. Most importantly, when making comparisons between PMCA activities in different conditions, the activities should be measured at equal membrane concentrations, a precaution that is often overlooked. Besides adding a novel twist to the study of membrane-embedded enzymes, the findings described here are important for the study of PMCA in particular, and of cellular Ca2+ homeostasis in general. Future studies will attempt to elucidate the mechanism underlying the observed membrane concentration-dependence of PMCA activity.
Acknowledgments
The present work was supported by the NIH, Fogarty International Center Grant R03TW006837 to EES and JPFCR, NIH grant GM28835 to EES, American Heart Association grant 0130531Z to AJC, and by ANPCYT, CONICET and UBACYT from Argentina. LV is a doctoral fellow of CONICET. RCR and JPFCR are established investigators of CONICET, Argentina.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among Plasma Membrane Calcium Pumps. Physiol Rev. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Rega AF, Garrahan PJ. The Ca2+ Pump of Plasma Membrane. CRC Press; Boca Raton, F.L.: 1986. [Google Scholar]
- 3.Chaudhary J, Walia M, Mantharu JM, Escher E, Grover AK. Caloxin: a novel plasma membrane Ca2+ pump inhibitor. Am J Physiol Cell Physiol. 2001;280:C1027–C1030. doi: 10.1152/ajpcell.2001.280.4.C1027. [DOI] [PubMed] [Google Scholar]
- 4.Cantiello HF. Actin filaments stimulate the Na+-K+-ATPase. Am J Physiol. 1995;269:F637–F643. doi: 10.1152/ajprenal.1995.269.5.F637. [DOI] [PubMed] [Google Scholar]
- 5.González Flecha FL, Castello PR, Caride AJ, Gagliardino JJ, Rossi JPFC. The erythrocyte calcium pump is inhibited by non-enzymic glycation: studies in situ and with the purified enzyme. Biochem J. 1993;293:369–375. doi: 10.1042/bj2930369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filomatori CV, Rega AF. On the mechanism of activation of the Plasma Membrane Ca2+-ATPase by ATP and acidic phospholipids. J Biol Chem. 2003;278:22265–22271. doi: 10.1074/jbc.M302657200. [DOI] [PubMed] [Google Scholar]
- 7.Caride AJ, Penheiter AR, Filoteo AG, Bajzer Z, Enyedi A, Penniston JT. The plasma membrane calcium pump displays memory of past calcium spikes. J Biol Chem. 2001;276:39797–39804. doi: 10.1074/jbc.M104380200. [DOI] [PubMed] [Google Scholar]
- 8.Verma AK, Enyedi A, Filoteo AG, Strehler EE, Penniston JT. Plasma Membrane Calcium Pump Isoform 4a has a longer Calmodulin-binding Domain than 4b. J Biol Chem. 1996;271:3714–3718. doi: 10.1074/jbc.271.7.3714. [DOI] [PubMed] [Google Scholar]
- 9.González Flecha FL, Castello PR, Gagliardino JJ, Rossi JPFC. Molecular characterization of the glycated Plasma Membrane Calcium Pump. J Membr Biol. 1999;171:25–34. doi: 10.1007/s002329900555. [DOI] [PubMed] [Google Scholar]
- 10.Fiske CH, Subbarrow Y. The colorimetric determination of phosphorus. J Biol Chem. 1925;66:375–400. [Google Scholar]
- 11.Webb MR. A continuous spectrophotometric Assay for inorganic phosphate and for measuring phosphate release kinetics in biological systems. Proc Natl Acad Sci. 1992;89:4884–4887. doi: 10.1073/pnas.89.11.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kratje RB, Garrahan PJ, Rega AF. The effects of alkali metal ions on active Ca2+ transport in reconstituted ghosts from human red cells. Biochim Biophys Acta. 1983;731:40–46. doi: 10.1016/0005-2736(83)90395-4. [DOI] [PubMed] [Google Scholar]
- 13.Rossi JPFC, Schatzmann HJ. Is the red cell calcium pump electrogenic? J Physiol. 1982;327:1–15. doi: 10.1113/jphysiol.1982.sp014215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaegger H, Jagow Von. Tricine-sodium dodecyl sulfate polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 15.Lündahl P. Proteins selectively released from water-extracted human erythrocyte membranes upon citranylation or maleylation. Biochim Biophys Acta. 1975;379:304–316. doi: 10.1016/0005-2795(75)90033-1. [DOI] [PubMed] [Google Scholar]
- 16.Peterson GL. Determination of total protein. Methods Enzymol. 1983;91:95–121. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- 17.Chen PS, Jr, Toribara TY, Warner H. Microdetermination of phosphorus. Anal Chem. 1956;28:1756–1758. [Google Scholar]
- 18.Richards DE, Rega AF, Garrahan PJ. Two classes of site for ATP in the Ca2+-ATPase from human red cell membranes. Biochim Biophys Acta. 1978;511:194–201. doi: 10.1016/0005-2736(78)90313-9. [DOI] [PubMed] [Google Scholar]
- 19.Caride AJ, Elwess NL, Verma AK, Filoteo AG, Enyedi A, Bajzer Z, Penniston JT. The rate of activation by calmodulin of isoform 4 of the plasma membrane Ca2+ pump is slow and is changed by alternative splicing. J Biol Chem. 1999;274:35227–35232. doi: 10.1074/jbc.274.49.35227. [DOI] [PubMed] [Google Scholar]
- 20.Enyedi A, Verma AK, Filoteo AG, Penniston JT. A highly active 120-kDa truncated mutant of the plasma membrane Ca2+ pump. J Biol Chem. 1993;268:10621–10626. [PubMed] [Google Scholar]
- 21.Brenner SL, Korn ED. The effects of cytochalasins on actin polymerization and actin ATPase provide insights into the mechanisms of polymerization. J Biol Chem. 1980;255:841–844. [PubMed] [Google Scholar]
- 22.Bredeston LM, Rega AF. Phosphatidylcholine makes specific activity of the purified Ca2+-ATPase from plasma membranes independent of enzyme concentration. Biochim Biophys Acta. 1999;1420:57–62. doi: 10.1016/s0005-2736(99)00084-x. [DOI] [PubMed] [Google Scholar]
- 23.Niggli V, Adunyah ES, Penniston JT, Carafoli E. Purified (Ca2+-Mg2+)-ATPase of the erythrocyte membrane. Reconstitution and effect of calmodulin and phospholipids. J Biol Chem. 1981;256:395–401. [PubMed] [Google Scholar]
- 24.Rossi JPFC, Rega AF. A study to see whether phosphatidylserine, partial proteolysis and EGTA substitute for calmodulin during activation of the Ca2+-ATPase from red cells membranes by ATP. Biochim Biophys Acta. 1989;996:153–159. doi: 10.1016/0167-4838(89)90241-0. [DOI] [PubMed] [Google Scholar]
- 25.Vavylonis D, Yang Q, O’Shaughnessy B. Actin polymerization kinetics, cap structure, and fluctuations. PNAS. 2005;102:8543–8548. doi: 10.1073/pnas.0501435102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zabe M, Dean WL. Plasma Membrane Ca2+-ATPase associates with the cytoskeleton in activated platelets through a PDZ-binding domain. J Biol Chem. 2001;276:14704–14709. doi: 10.1074/jbc.M009850200. [DOI] [PubMed] [Google Scholar]
- 27.Ponting CP. Evidence for PDZ domains in bacteria, yeast and plants. Prot Sci. 1997;6:464–468. doi: 10.1002/pro.5560060225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Marco SJ, Strehler EE. Plasma membrane Ca2+-ATPase isoforms 2b and 4b interact promiscuously and selectively with members of the membrane-associated guanylate kinase family of PDZ (PSD95/Dlg/ZO-1) domain-containing proteins. J Biol Chem. 2001;276:21594–21600. doi: 10.1074/jbc.M101448200. [DOI] [PubMed] [Google Scholar]
- 29.Rimessi A, Coletto L, Pinton P, Rizzuto R, Brini M, Carafoli E. Inhibitory interaction of the 14-3-3{epsilon} protein with isoform 4 of the plasma membrane Ca2+-ATPase pump. J Biol Chem. 2005;280:37195–37203. doi: 10.1074/jbc.M504921200. [DOI] [PubMed] [Google Scholar]
- 30.Buch MH, Pickard A, Rodriguez A, Gillies S, Maass AH, Emerson M, Cartwright EJ, Williams JC, Oceandy D, Redondo JM, Neyses L, Armesilla AL. The sarcolemmal calcium pump inhibits the calcineurin/nuclear factor of activated T-cell pathway via interaction with the calcineurin A catalytic subunit. J Biol Chem. 2005;280:29479–29487. doi: 10.1074/jbc.M501326200. [DOI] [PubMed] [Google Scholar]
- 31.Williams JC, Armesilla AL, Mohamed TM, Hagarty CL, McIntyre FH, Schomburg S, Zaki AO, Oceandry D, Cartwright EJ, Buch MH, Emerson M, Neyses L. The sarcolemmal calcium pump, alpha-1 syntrophin, and neuronal nitric-oxide synthase are parts of a macromolecular protein complex. J Biol Chem. 2006;281:23341–23348. doi: 10.1074/jbc.M513341200. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y, Fan X, Yang F, Zhang X. Gangliosides modulate the activity of the plasma membrane Ca2+-ATPase from porcine brain synaptosomes. Arch Biochem Biophys. 2004;427:204–212. doi: 10.1016/j.abb.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Pang Y, Zhu H, Wu P, Chen J. The characterization of plasma membrane Ca2+-ATPase in rich sphingomyelin-cholesterol domains. FEBS Lett. 2005;579:2397–2403. doi: 10.1016/j.febslet.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 34.Colina C, Cervino V, Benaim G. Ceramide and sphingosine have an antagonistic effect on the plasma-membrane Ca2+-ATPase from human erythrocytes. Biochem J. 2002;362:247–251. doi: 10.1042/0264-6021:3620247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang D, Dean WL, Borchman D, Paterson CA. The influence of membrane lipid structure on plasma membrane Ca2+-ATPase activity. Cell Calcium. 2006;39(3):209–216. doi: 10.1016/j.ceca.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Zouzoulas A, Therien AG, Scanzano R, Deber CM, Blostein R. Modulation of Na, K-ATPase by the γ-subunit. J Biol Chem. 2003;278:40437–40441. doi: 10.1074/jbc.M308610200. [DOI] [PubMed] [Google Scholar]
- 37.Feschenko MS, Donnet C, Wetzel RK, Asinovski NK, Jones LR, Sweadner KJ. Phospholemman, a single-span membrane protein, is an accessory protein of Na, K-ATPase in cerebellum and choroids plexus. J Neurosci. 2003;23:2161–2169. doi: 10.1523/JNEUROSCI.23-06-02161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


