Abstract
Objective
We tested a hypothesis on two patterns of anticipatory postural adjustments (APAs) in neck muscles, reciprocal and co-activation, that may be used in a task-specific way. We also explored possible relation of APAs in leg and trunk muscles to head stabilization.
Methods
Load perturbations (loading and unloading) were applied to the head, trunk, and head and trunk simultaneously using similar hand actions by standing persons. Electromyographic signals (EMGs) from ten muscles were recorded. Shifts of the center of pressure and EMG indices were computed over typical for APA time intervals.
Results
Time-shifted (reciprocal) activation of neck flexor and extensor muscles during APAs was seen when perturbations were applied directly to the head. Simultaneous activation dominated when the perturbations were applied to the trunk. Minimal APAs were seen in the leg/trunk muscles during head perturbation tests. APAs during trunk perturbation were not different from those during trunk and head perturbation.
Conclusions
The results confirm the existence of two different patterns of APAs in neck muscles. A time-shifted (reciprocal) pattern is more likely to be used in anticipation of a perturbation acting directly on the head. A simultaneous activation (co-activation) pattern is used when direction of head perturbation cannot be predicted with certainty. Leg/trunk APAs are unlikely to help stabilize head posture.
Significance
These results are important for better understanding of feed-forward mechanisms of the control of head posture with possible implications for neurological patients who suffer from impaired feed-forward postural control.
Keywords: Posture, anticipatory postural adjustments, head posture, electromyography, human
1. Introduction
When a standing person performs a fast arm movement, the vertical postural is perturbed. A major feed-forward mechanism of postural stabilization in such conditions is the anticipatory postural adjustments (APAs) that represent changes in the activity of postural muscles prior to the initiation of voluntary motor actions (Belen’kii et al. 1967; Marsden et al. 1978; Cordo and Nashner 1982; Bouisset and Zattara 1987). In particular, APAs have been described in the leg and trunk muscles prior to a fast arm action or load manipulation (Lee 1980; Horak et al 1984; Bouisset and Zattara 1987; Aruin and Latash 1996; Shiratori and Latash 2000; Slijper et al 2002; Shiratori and Aruin 2004). Many of the mentioned studies quantified APAs as changes in the muscle activation levels within a time window selected to avoid action of stretch reflexes and other feedback mechanisms.
The purpose of APAs has been commonly considered as producing appropriate shifts of the point of application of the resultant force acting on the body from the supporting surface (center of pressure, COP, Bouisset and Zattara 1987; Massion 1992). These shifts are produced by coordinated changes in the muscle activity (Krishnamoorthy et al. 2003). COP shifts are viewed as a major mechanism to produce shifts of the center of mass (COM) that is commonly viewed as a major controlled variable in postural tasks (Winter et al. 1996). During APAs, however, the purpose of COP shifts is to avoid COM motion that otherwise could be induced by the perturbation.
Most studies of APAs in standing persons have naturally focused on trunk stabilization (reviewed in Massion 1992). It has been suggested, however, that during daily activities such as walking and running, and also during acrobatic movement such as salto, the head posture with respect to the trunk is well stabilized to ensure a reliable reference frame (Berthoz and Pozzo 1994; Pozzo et al. 2001). The importance of head stability during whole-body actions performed by standing persons has received support in another recent study (Freitas et al. 2006). However, only a couple of studies addressed the role of APAs in head stabilization, and their results are controversial.
Gurfinkel et al. (1988) described increased activation of neck extensors and a drop in the activity of neck flexors about 60 ms prior to the activation of the prime mover (deltoid muscles). This pattern was time locked with APAs in leg muscles, and the authors suggested that the combined APAs in the neck, trunk, and leg muscles formed a complex posture-stabilizing pattern. Van der Fits et al. (1998) used a similar task in a variety of subject postures, standing, sitting, and supine. These authors described a co-contraction pattern of neck flexors and extensors across all conditions.
The discrepancy in the results of the two mentioned studies led us to the following hypothesis. We suggest that APA patterns in neck muscles may be defined by two factors, local (a predictable perturbation acting on the head) and global (a perturbation acting on the center of mass of the body and affecting the head indirectly, due to the mechanical coupling between the trunk and the head). If the direction of a perturbation acting on the head is well predictable, a reciprocal APA pattern in neck muscles can be used to counteract the mechanical effects of the perturbation on the head posture. In contrast, if a self-triggered perturbation acts on the trunk, APAs in leg and trunk muscles stabilize the trunk posture, but the combined action of those APAs and the original perturbation may perturb the head posture due to the mechanical coupling. Since the direction and magnitude of such a perturbation acting on the head may not be well predictable, an increase in the apparent neck stiffness (co-contraction) may be used as an APA to increase resistance to perturbation in any direction.
To test the main hypothesis on two APA patterns, we performed experiments where similar initial positions of the body and similar actions by the subject were associated with perturbations acting predominantly on the head, predominantly on the trunk, and on the trunk and the head together. Our first specific hypothesis (Hypothesis #1) was that APA patterns in the neck flexor-extensor muscles would change from a time-shifted (reciprocal) pattern to a synchronized (co-activation) pattern when the source of the perturbation changes from that applied directly to the head to resulting from joint coupling. We also explored a possibility that APAs in trunk and leg muscles contributed to head stabilization. If so, a combined perturbation to the trunk and to the head could be expected to lead to significantly larger APAs in those muscles than a perturbation applied to the trunk alone (Hypothesis #2). Large APAs in the leg/trunk muscles could also be expected when a perturbation is applied directly to the head (Hypothesis #3).
2. Methods
2.1. Subjects
Seven subjects (four males and three females) with the mean age 30.1 years (± 2.9, SD), mean mass 77.7 kg (± 20.3, SD), and mean height 170.4 cm (± 7.9, SD) participated in the study. All the subjects were healthy, without any known neurological or muscular disorders. All subjects were right-handed based on their preferential hand usage during writing and eating. The subjects gave informed consent based on the procedures approved by the Office for Research Protection of The Pennsylvania State University.
2.2. Apparatus
A force platform (AMTI, OR-6) was used to record the moment of force around the frontal and sagital axes (MY and MX, respectively) and the vertical component of the ground reaction force (FZ). These signals were used to compute the displacement of the body center of pressure (COP).
Disposable self-adhesive electrodes (3M Corporation) were used to record the surface electromyogram (EMG) of the following muscles: gastrocnemius lateralis (GL), tibialis anterior (TA), biceps femoris (BF), rectus femoris (RF), lumbar erector spinae (ES), rectus abdominis (RA), and sternocleidomastoid as the neck flexor (NF). We also recorded the surface EMG activity at the dorsal part of the neck. Since the dorsal part of the neck has multiple layers of muscles, the recorded signals reflected the activity not only of the most superficial muscle (superior fibers of trapezius) but also of deeper muscles (semispinalis, splenius cervicis, and splenius capitis). We will refer to this signal as reflecting neck extensor (NE) activity.
All muscles recorded were chosen based on their potential role in postural stabilization of the head and the trunk and their accessibility to surface EMG recording. The electrodes pairs were placed over the muscle bellies; the distance between the two electrodes of each pair was 3 cm. Lower limb and trunk electrodes were placed only unilaterally on the right side of the subject’s body due to the limitation in the number of channels. Neck electrodes were placed bilaterally over the right (R) and the left (L) muscles (NFL, NFR, NEL, and NER). The electrode pairs recording the NE activity were placed parallel to the spine with the upper electrodes positioned about 2 cm below the occipital protuberance. Electrodes recording NF activity were placed along the sternocleidomastoid midway between its sternum and mastoid insertions.
The EMG signals were amplified (×3000) and band-pass filtered (60–500 Hz). All the EMG signals were sampled at 1000 Hz with a 12-bit resolution. A personal computer (Gateway 450Mhz) was used to control the experiment and to collect the data with a customized Labview-based software (Labview-5 – National Instruments, Austin TX, USA).
A ProReflex motion analysis system (Qualysis Track Manager vs 1.7.187 – Qualysis Medical) was used to capture the coordinates of a passive marker placed on the lateral aspect of the index finger metacarpophalangeal joint on the right hand. Three more markers were placed laterally on the customized helmet (described later) and their coordinates were used to detect angular displacement of the head in a sagittal plane (these data were collected only in four subjects). This motion analysis system was synchronized with the computer collecting the force plate and EMG data; it recorded 3D coordinates of the marker at 200 Hz. An accelerometer was placed on the dorsal side of the right hand; its signal was used to detect the moment of hand movement initiation during off-line data processing.
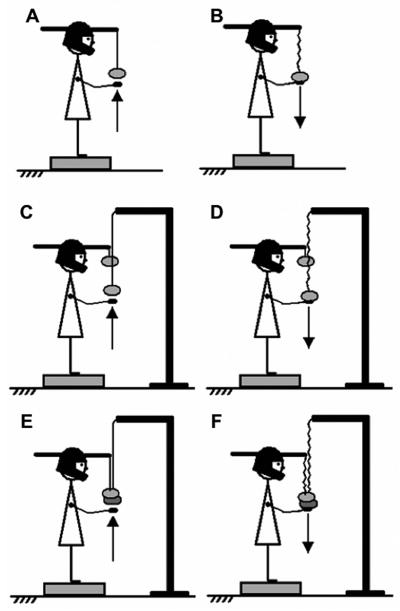
A customized hockey helmet was used to apply perturbations to the subject’s head. This helmet was firmly fitted onto the subjects’ head; the chin support piece was used to improve helmet stability. Two levers (50 cm long) were attached to the front and back portions of the helmet about 2 cm above the eye level line; they were used to attach loads during the experiment (see the next section). The distance between the center of the helmet’s top and the point where the load was attached to the lever was 0.6 m. A pole (1.77 m height) was positioned 0.7 m in front of the subject for series of trials involving trunk perturbation (Figure 1). A horizontal lever was attached to the pole, and a load (0.5 kg) suspended on a flexible fishing line was aligned with the inferior portion of the sternum (see the next section).
Figure 1.
An illustration of the six main experimental series: Head perturbations (HP, panels A and B), trunk perturbation (TP, panels C and D), and head and trunk perturbation together (HTP, panels E and F). Arm upward and downward movements were used to produce perturbations in different directions.
2.3. Procedure
2.3.1 Control tests
The experiment started with three control tests used for normalization of the postural sway data (COP displacement) and the EMG signals as described in the following section (Data processing). The first control test involved a single trial with quiet standing. This test was used to obtain measures of natural postural sway that were later used to normalize COP migration during APAs for across-subjects comparisons. The subjects were instructed to stand quietly on the force plate for 10 s while looking at the target placed 2.3 m in front of them at the eye level.
The other two tests were used to get quantitative indices of activity of postural muscles associated with a standard task of quiet standing with an additional load that generated torque in a sagittal plane acting to rotate the head and the trunk either forward or backwards. These data were used to normalize integral EMG indices during APAs for across-subjects comparisons. In these tests the subjects were instructed to stand quietly and to hold a 5 kg load for 10 s with the arms fully extended while another load (1 kg) was attached to one of the helmet’s levers. The subjects were asked to keep both the head and the trunk vertical; this was controlled by the experimenter. In one of the tests both loads created moments of force that tended to rotate the head and the trunk forward. In the other test the same loads held through a pulley system created moments of force that tended to rotate the head and the trunk backwards.
2.3.2 Experimental conditions
The main portion of the experiment involved three tasks (Figure 1). The tasks required the subjects to produce hand actions that triggered three types of perturbations in the sagittal plane. The three perturbations were; a) head perturbation (HP) created by a fast, low-amplitude arm movement with the arm moving the load acting on the head; b) trunk perturbation (TP) created by a fast, relatively large-amplitude movement of the arms that moved the load acting on the trunk; and c) both head and trunk perturbation (HTP) by a fast, relatively large-amplitude movement of the arms that moved both loads acting on the head and on the trunk. For each task, two different conditions were used involving arm movement in opposite directions (upward and downward) and resulting in different perturbations to the posture. As a result, there were six different series of trials: three tasks (type of perturbation) and two actions (direction of arm movement).
In all series, the subjects were instructed to stand on the force plate with their feet parallel to each other, spaced by 18 cm, with the body weight evenly distributed between the feet and look straight at the same target that was used in the control tests. The foot position was marked on the top of the platform and reproduced across all trials. The arms were extended forward but not outstretched completely, with the elbows flexed and wrists extended, such that the hands were at the level of the load(s) (see Figure 1). Before the upward arm movement series, the two hands formed a “cup” placed just under the load without touching it (Figure 1A, C, E). Prior to the series with downward arm movements, the load rested on the hands such that the line connecting the load to the helmet and/or the pole was slightly loose (Figure 1B, D, F). In all series, the instructed arm movement was a very fast bilateral shoulder flexion (upwards) or extension (downward).
In order to generate perturbation acting mainly on the head (HP), a standard load (0.5 Kg) was attached to the helmet’s front lever and suspended at about the level of the inferior sternum extremity with a flexible fishing line. In one series, the subjects were instructed to move the load quickly upwards over the nominal distance of 10 cm in a self-paced manner 1–2 s after an auditory signal, a “beep” (Figure 1A). This action unloaded the helmet leading to a head extension perturbation. In another series, the subjects were instructed to hold the load in the hands such that the fishing line was slightly lose and then perform a quick hand motion downwards over the nominal distance of 10 cm in a self-paced manner 1–2 s after a “beep” (Figure 1B). This action resulted in loading the helmet and generating a head flexion perturbation.
To generate perturbation acting mainly on the trunk (TP), the same 0.5 kg load was attached to the helmet’s front lever. Another 0.5 kg load was attached to the pole standing on the floor in front of the subject. The subjects were instructed to perform similar series of trials as in the HP series: to move the load attached to the pole quickly upward (Figure 1C) and downward (Figure 1D) in different series. In order to increase the magnitude of the perturbation acting on the trunk, the instruction for these two series was to perform a very fast hand movement over the nominal distance of 25 cm. We used this relatively small load (leading to relatively small trunk perturbations) to avoid fatigue and to have comparable magnitudes of the perturbation in cases when it was applied to the head and when it was applied to the trunk. Therefore, we were limited by perturbations that were safe and not uncomfortable when applied to the head.
The last task (HTP) was a combination of the two already described in order to generate a perturbation acting on both the head and trunk at the same time. Both 0.5 kg loads were used. The first was attached to the helmet’s front lever and the second load was attached to the pole. Both loads were kept close to each other and suspended at the level of the sternum. The instructions were to perform a very fast movement over the nominal distance of 25 cm (same as in the TP task) upward (Figure 1E) and downward (Figure 1F) lifting and releasing the two loads together in two different series.
A familiarization period was given to each subject prior to data collection. During the familiarization period, subjects were asked to perform at least five trials for each of the tasks and actions. The experimenter paid particular attention to the initial vertical posture of both head and trunk and to reproducible arm movement over trials within each series. Seven trials were performed for each series, and the order of the series was balanced across subjects. Resting periods of 30 s were given between trials, and at least 1 min between series. The average duration of the experiment was 45 min, and after the procedures all the subjects were asked about feeling fatigue. None of the subjects complained about any type of discomfort or fatigue.
2.4. Data processing
All signals were processed off-line using LabView-5 and MatLab 6.5 software packages. Signals from the accelerometer were not filtered to allow better detection of movement initiation. For all trials and conditions, all data from force plate, EMG, and 3D motion capture system were aligned according to the first visible change in the signal from the accelerometer attached to the right hand. This moment of the hand movement initiation will be referred as ‘time zero’ (t0=0).
Signals from the force plate were filtered with a 20 Hz low-pass, second-order, zero-lag Butterworth filter. Center of pressure coordinates in the anterior-posterior (AP) and medial-lateral (ML) directions (COPAP and COPML, respectively) were computed using an approximation:
| (1A) |
| (1B) |
As commonly accepted in studies of COP shifts, the effects of the shear forces on the moments of force measured by the platform were ignored because of the small lever arm of those forces (the AMTI platforms record the moments with respect to the platform center located 36 mm beneath the surface). The assessments suggest that the contribution of shear forces to the estimated COP shifts was always well under 10%.
Changes in the COP displacement happen in two directions, anterior-posterior and medio-lateral, even when the perturbation is mostly limited to a sagittal plane. The COP shifts are typically rather small during the APAs (e.g., Aruin and Latash 1995). Therefore, to quantify them we have used a general measure of COP migration during the time window typical of APAs – the area of the ellipse that included most of the COP migration data. To quantify COP migration prior to the perturbation, the COP time series were processed as follows. Ellipses were fitted to the COP data collected over two time intervals: from −500 ms to −350 ms (baseline COP migration) and from −100 ms to +50 ms with respect to t0 (COP migration during APAs). Each ellipse contained 85.3 % of the COP data points (cf. Oliveira et al. 1996). The area of each ellipse was computed. The first time interval captured the COP baseline migration (COPBL) and the second interval captured possible anticipatory changes in the COP trajectory associated with the action (COPAPA). COPAPA was then corrected by COPBL. For across-subjects comparison, the difference (COPAPA-COPBL) was normalized by the area of a third ellipse computed for the data collected over the same period of 150 ms in the middle of the control trial when the subject stood quietly without any load, COPCONTROL.
An index, ICOP, was computed as follows:
| (2) |
All EMG signals were rectified and filtered with a 50 Hz low-pass, second-order, zero-lag Butterworth filter. The accelerometer signal was used to align the rectified EMG by t0. After the alignment, all seven trials within each series were averaged and APAs were quantified as follows. EMG signals for each muscle were integrated over two time intervals: from −500 ms to −350 ms and from −100 ms to +50 ms with respect to t0. The first time interval captured the muscle background activity (∫EMGBG) and the second interval captured possible anticipatory changes in the activity (∫EMGAPA) associated with the action. The interval of EMG integration associated with APAs was selected to include most of the changes in muscle activity that occur in a feed-forward manner while avoiding possible effects of stretch reflexes. ∫EMGAPA was then corrected by ∫EMGBG.
In order to compare the EMG indices (∫EMG) across muscles and subjects, we normalized them by the EMG signals integrated over the same period of 150 ms in the middle of the control trial (∫EMGCONTROL) when the subjects held the two loads while standing quietly. ∫EMG indices for the dorsal muscles (GL, BF, ES, and NE) were divided by EMG integrals obtained when the loads were held in front of the body. ∫EMG indices for the ventral muscles (TA, RF, RA, and NF) were divided by the EMG integrals obtained when the loads were suspended behind the body:
| (3A) |
| (3B) |
2.5. Statistics
Standard methods of descriptive statistics and parametric statistical methods were used (SPSS-13). Four sets of analysis were performed:
The COP index (ICOP) was analyzed with a two-way mixed design ANOVA with factors Perturbation (HP, TP, and HTP) and Direction (Up and Down).
EMG indices (IEMG) of the leg and trunk muscles (GL, TA, BF, RF, ES, and RA) were analyzed using a two-way MANOVA with factors Perturbation and Direction.
EMG indices (IEMG) of the neck muscles (NFL, NFR, NEL, and NER), were analyzed using a three-way MANOVA with factors Perturbation, Direction, and Side (Right and Left).
To investigate the relative timing of changes in the activity of neck muscles, a cross-correlation function between the neck flexor and extensor muscles over a time period from −200 ms to +200 ms with respect to t0 was computed. This analysis was done separately for the right and left muscle pairs. Prior to this analysis, the EMG signals were filtered at 20 Hz with a low-pass, second-order, zero-lag Butterworth filter to obtain EMG envelopes. For each trial, the peak magnitude of the correlation coefficient (R-peak) and the time lag (Δt) of R-peak were computed. In order to normalize the R data these data were log-transformed into z-scores by Fisher’s transformation:
| (4) |
Further, the average z-score and Δt data for each condition were calculated across the trials. Two one-way ANOVAs (Perturbation as the factor) were performed on the z-score and Δt variables.
In all sets of analysis, Tukey’s honestly significant difference (HSD) tests and pairwise contrasts were used as post-hocs for significant effects. The significance level for all analyses was kept at 0.05.
3. Results
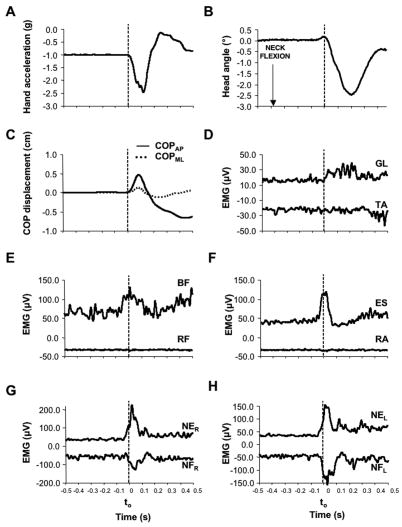
All subjects were able to accomplish successfully all tasks. Figure 2 shows a data set from a TP trial performed by a typical subject (subject #1). Time zero was defined as the earliest signal from the accelerometer (panel A). For better interpretation of this figure, EMGs of pairs of muscles are displayed together (panels D through H). In the Figure, the rectified EMG signals of TA, RF, RA, NFr, and NFl muscles are inverted (turned into negative values) to avoid superimposed lines. Note that anticipatory changes in muscle activity (APAs) started before time zero in BF, ES, NE, and NF. Note the simultaneous changes in the activity of NE and NF. Note also a relatively small head displacement associated with the arm movement (panel B).
Figure 2.
A typical data set from a representative subject (subject #1) during downward arm movement in a TP trial. Hand acceleration (Panel A), Head angular displacement (Panel B), displacement of COP in both directions (Panel C), and EMGs of all ten muscles recorded (Panels D through H) are displayed. Time zero (t0) corresponds to the initiation of hand action. Note: TA, RF, RA, NFR, and NFL EMG time profiles were inverted (turned into negative values) to avoid superposition of lines; for these muscles negative values of larger magnitude indicate increased muscle activation.
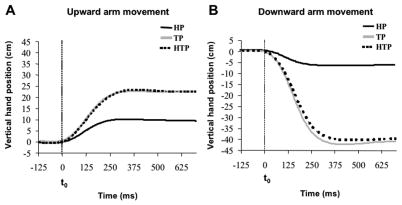
3.1. Upper limb movements and COP displacements
Off-line analysis of the vertical displacement of the passive marker placed on the subject’s hand showed a sigmoid trajectory (Figure 3). Figure 3 shows the vertical hand displacement during upward (panel A) and downward (panel B) arm movements averaged across subjects for each task separately. The amplitude of the movement was much smaller in the head perturbation series (HP) as compared to the trunk perturbation (TP) and head and trunk perturbation (HTP) tasks. The amplitude of the vertical hand displacement was 10.0 cm (± 0.8 SE), 23.0 cm (± 1.8 SE), and 22.5 cm (± 1.5 SE) during upward arm movements under the HP, TP and HTP tasks, respectively. During downward movements, the average displacements were 7.0 cm (± 0.9 SE), 41.0 cm (± 2.0 SE), and 39.8 cm (± 1.9 SE) for the HP, TP and HTP tasks, respectively. Movement time was close to 250–300 ms across all series.
Figure 3.
Vertical hand displacement during upward and downward arm movements (panels A and B, respectively). Each panel shows the average time profiles across subjects for head perturbation (HP), trunk perturbation (TP), and head and trunk perturbation (HTP) tasks. The vertical dashed lines indicate the moment of movement initiation (t0). Standard error bars are not presented to make the Figure readable.
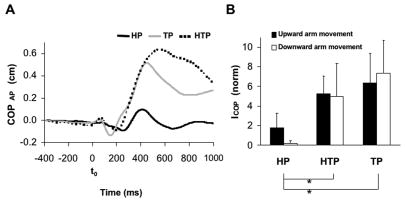
The hand motion was accompanied by relatively small COP displacements. Peak-to-peak COP displacements in both anterior-posterior (AP) and medio-lateral (ML) directions were typically under 1 cm. Figure 4A illustrates averaged across subjects COPAP time profiles during downward arm movements for the three tasks, HP, TP, and HTP. In TP and HTP tasks, COPAP showed larger displacement for the period starting about 100 ms before t0 and ending about 300 ms after t0, as compared to the HP task. Figure 4A also illustrates larger COP displacements forward in the TP and HTP tasks at about 400 ms after the movement initiation. COP migration was quantified with an index ICOP computed for the time window from − 100 ms to + 50 ms with respect to t0 (see Methods). Figure 4B shows larger ICOP in the TP and HTP tasks as compared to HP. This finding was confirmed by a two-way ANOVA (Perturbation × Direction) that showed a significant effect of Perturbation (F[2,36] = 3.35, p<0.05). Tukey’s HSD tests confirmed significant differences between HP and TP and also between HP and HTP (p<0.05). There were no significant effects of Direction and no significant interaction.
Figure 4.
Panel A shows the anterior-posterior center of pressure displacement (COPAP) for downward arm movement condition under head, trunk, and head and trunk perturbations (HP, HTP, and TP, respectively). The vertical dashed line indicates the moment of movement initiation (t0). Average time profiles across subjects are shown and standard error bars are not presented to make the Figure readable. Positive values indicate anterior COP displacement. Panel B shows the index of COP shift (ICOP) over the period from − 100 ms to + 50 ms with respect to t0 during upward and downward arm movements and the HP, HTP, and TP tasks. Averages across subjects with standard error bars are shown; * means p<0.05. Lines connecting the mean bars indicate significant differences.
3.2. Muscle activation during APAs: Neck muscles
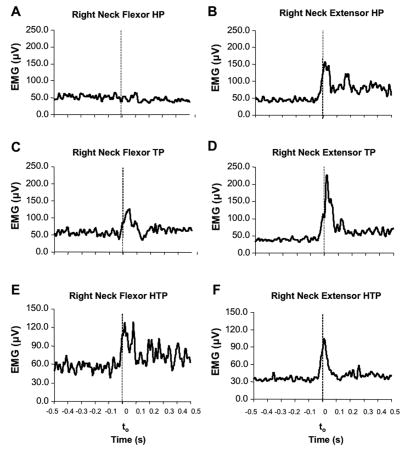
The patterns of changes in the neck muscle activity differed across the HP, TP, and HTP tasks. Figure 5 illustrates typical EMG time profiles for the right neck flexor and extensor muscles in a representative subject (subject #1) performing downward arm movements in the HP, TP, and HTP tasks. The subject showed APAs only in the extensor muscle for the HP task and in both flexor and extensor muscles in the TP and HTP tasks. Note that the flexor and extensor both showed an increase in the activity during APAs in the TP and HTP tasks. The left neck muscles presented similar pattern to those seen in the right neck muscles.
Figure 5.
EMG of the right neck muscles (averages across trials by a representative subject, subject #1) during downward upper limb movements. The neck flexor (panels A, C, and E) and neck extensor (panels B, D, and F) activity under the head perturbation (HP), trunk perturbation (TP), and head and trunk perturbation (HTP) tasks, respectively. Note the APAs only in the extensor muscle for the HP task and in both flexor and extensor muscles in the TP and HTP tasks. The vertical dashed lines indicate the moment of the movement initiation (t0).
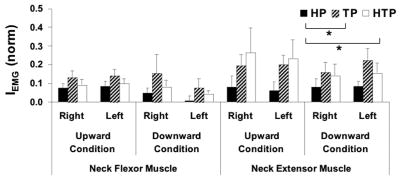
Figure 6 shows the index of integrated muscle activity, IEMG (see Methods) for the neck muscles across all tasks and conditions (averages across subjects with standard error bars). IEMG for both flexors and extensors was higher in the TP and HTP tasks as compared to the HP task. In general, IEMG for the neck extensor muscles were, on average, 2.5 times larger for the TP tasks and 2.6 times larger for the HTP tasks as compared to HP task. A similar trend was seen for the flexor muscles, where IEMG in TP and HTP tasks was 2.9 and 1.9 times larger than in HP task, respectively.
Figure 6.
Integrated EMG index (IEMG) of the left and right neck flexor and extensor muscles (NFL, NFR, NEL, and NER, respectively) during upward and downward arm movements, and under head, trunk, and head and trunk perturbations (HP, TP, and HTP, respectively). Average time profiles across subjects and standard error bars are shown; * means p<0.05. Lines connecting the mean bars indicate significant differences across conditions.
Three-way MANOVA (Perturbation, Direction, and Side as factors) on IEMG showed no effect of Direction and Side and no significant interactions. However, a significant effect of Perturbation was confirmed (F[4,142]=3.06, Wilks’ Lambda p<0.05). Two three-way mixed designs ANOVAs were conducted on IEMG of the neck flexor and extensor muscles separately. The results confirmed a significant main effect of Perturbation for both muscles (F[2,72]>2.98, p<0.05) without significant effects of Direction or Side, and no significant interactions. Tukey’s HSD tests confirmed larger IEMG for both neck extensor and flexor muscles under the TP and HTP tasks as compared to the HP task (p<0.05).
3.3. Relative timing of neck muscle activation during APAs
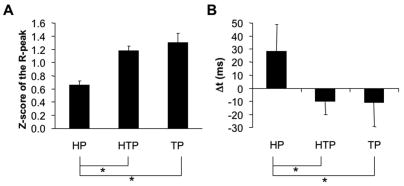
To explore the relative timing of the changes in the neck flexor and extensor muscle activity, cross-correlation analyses were run separately for the EMGs in the left and right side muscle pairs. Figure 7A shows z-scores of the average peak correlation coefficients (R-peak) computed from the cross-correlation between neck flexor and extensor muscles. This figure shows combined data from the right and left side muscles because there were no significant differences between the two sides (described below). TP and HTP tasks showed higher R-peak values between the neck flexor and extensor muscle activation patterns for both right and left sides and for both upward and downward arm movements, as compared to the HP task.
Figure 7.
Results of the cross-correlation analysis between neck flexor and extensor EMGs. Panel A shows averaged across subjects z-scores with standard error bars of the peak correlation coefficient (R-peak). Panel B shows the average time lag (Δt) at R-peak under the head, trunk, and head and trunk perturbations (HP, TP, and HTP, respectively). Positive values in panel B indicate an earlier EMG burst in the neck extensor as compared to the neck flexor. * means p<0.05. Lines connecting the mean bars indicate the differences found.
The average across subjects time lag (Δt) at the moment of R-peak is shown in Figure 7B. The positive time lag for the HP task indicates an earlier burst of the neck extensor activity as compared to the neck flexor burst. The average time lag for TP and HTP was negative indicating an earlier burst of neck flexor activity about 10 ms before the neck extensor burst. On average, there was a difference of about 40 ms in Δt between HP and the other two tasks (TP and HTP). Despite its relatively small magnitude, this difference was statistically significant. The relatively low average value of Δt in the HP task was partly due to the fact that some subjects showed clear time-shifted (reciprocal) patterns of activation in the neck flexors and extensors while others showed nearly simultaneous (co-activation) patterns. The small number of subjects in each subgroup did not allow us to test these differences statistically; hence, this remains a qualitative observation.
One-way ANOVAs confirmed the significant effect of Perturbation on both z-scores (F[2,81]=17.89, p<0.01) and Δt (F[2,81]=3.54, p<0.05). Tukey’s HSD tests confirmed higher z-scores of the R-peak and smaller Δt for the TP and HTP tasks as compared to HP task (p<0.05).
3.4. Muscle activation during APAs: Leg and trunk muscles
The actions used in our experiments produced relatively mild perturbations for the trunk. As a result, a few muscles such as gastrocnemius lateralis, tibialis anterior, and rectus femoris did not show reproducible APAs that would differ across the tasks and conditions. However, APAs in biceps femoris (BF), erector spinae (ES), and rectus abdominis (RA) were reproducible and showed task dependence.
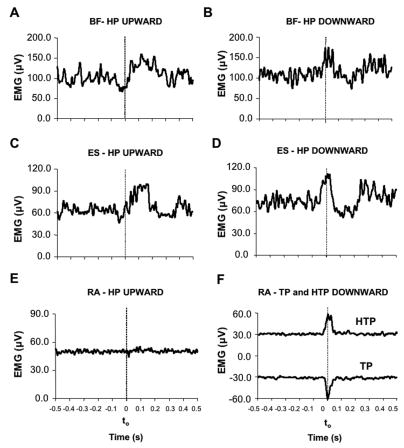
Figure 8 illustrates typical EMG patterns in BF, ES, and RA for a representative subject (subject #4). The upper four panels show the EMGs of BF and ES during upward (panels A and C) and downward (panels B and D) arm movements in the HP task. Prior to head unloading, there was a reduction in the BF and ES activity, while prior to head loading these muscles showed an increase in the activity. Similar pattern of changes in the activity of these muscles were also observed in the TP and HTP tasks (not illustrated).
Figure 8.
Panels A, B, C, and D: Averaged across seven trials EMGs of biceps femoris (BF) and erector spinae (ES) muscles are shown for a representative subject (subject #4) who performed the HP task. Data for upward arm movements are shown in panels A and C, and data for downward arm movements are shown in panels B and D. Note the opposite changes in muscle activity during APAs for different arm movement directions. Panels E and F: rectus abdominis (RA) EMG during upward arm movements in the HP task (panel E) and TP and HTP tasks (panel F). The vertical dashed line indicates the moment of the movement initiation (t0). Note: RA time profile for the TP condition (panel F) was inverted (turned into negative values) to avoid superposition of lines; negative values of larger magnitude indicate increased muscle activation.
Panels E and F (Figure 8) shows the RA activity during upward arm movements under the HP (panel E) and TP and HTP (panel F) tasks. No visible APAs were seen in the HP task, while there was an increase in the RA activity in the TP and HTP tasks. Similarly, during downward arm movements, APAs in RA were absent in the HP task and present in the TP an HTP tasks (not illustrated, see Figure 9).
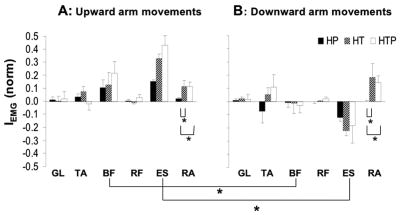
Figure 9.
Integrated EMG index (IEMG) of gastrocnemius lateralis (GL), tibialis anterior (TA), biceps femoris (BF), rectus femoris (RF), lumbar erector spinae (ES), and rectus abdominis (RA) during upward and downward upper limb movements (panels A and B, respectively) for the three tasks, head perturbations, trunk perturbation, and head-and-trunk perturbation (HP, TP, and HTP, respectively). Average data across subjects with standard error bars are shown; * means p<0.05. Lines connecting the mean bars and { signs indicate significant differences.
APAs in the leg and trunk muscles were quantified using an index of integrated EMG activity (IEMG) over the period from − 100 ms to + 50 ms with respect to t0 (see Methods). Figure 9 shows IEMG (averages across subjects with standard error bars) for all the leg and trunk muscles during upward (panel A) and downward (panel B) arm movements in the HP, TP, and HTP tasks. Note the effect of hand movement direction on IEMG for BF and ES, and the effect of task on IEMG for RA.
A two-way MANOVA (Direction × Perturbation) was used to test these differences. It showed a significant effect of Direction (F[6,31]=2.83, Wilks’ Lambda p<0.05) on IEMG, while the effect of Perturbation was just under the level of significance (F[12,62]=1.75, Wilks’ Lambda p=0.077). We explored both effects using ANOVAs. Six ANOVAs (Direction as the factor) were used as post-hocs on IEMG for each muscle. There was no difference between upward and downward arm movements for TA, GL, RF, and RA. There were significant differences for both BF and ES (F[2,36]=7.70, p<0.01; and F[2,36]=13.65, p<0.01, respectively). As illustrated in Figure 9, there were larger (positive) IEMG for BF and ES muscles during upward arm movements as compared to the downward movements. Tukey’s HSD tests confirmed this result (p<0.05). One-way ANOVA (Perturbation as the factor) revealed differences in IEMG for RA (F=3.76, p<0.05). Tukey’s HSD test confirmed larger IEMG for RA in the TP and HTP tasks as compared to the HP task (p<0.05).
4. Discussion
The main hypothesis tested in the study suggests the existence of two patterns of APA in neck muscles, reciprocal and co-activation, used in a task-specific way. The main findings of the experiments related to this hypothesis can be summarized as follows. When perturbations were applied directly to the head, APAs in the neck muscles showed a reciprocal pattern that is an increase in the activity of one muscle of a flexor-extensor pair without an increase in the activity or its antagonist (Figures 5–7, similar to results described by Gurfinkel et al. 1988). When perturbations were applied to the trunk, APAs represented predominantly unidirectional changes (co-contraction) of the activity in both neck flexor and neck extensor muscles (Figures 2, 5–7, similar to the report by Van der Fits et al. 1998). Taken together, these observations support our first specific hypothesis formulated in the Introduction.
Note that any muscle activation pattern during fast actions has elements of both co-activation and reciprocal muscle activation. Even the famous tri-phasic EMG pattern during single-joint fast movements may be viewed as a combination of time-shifted (reciprocal) bursts of activation in the agonist and antagonist muscles superimposed on their co-activation (e.g., Gottlieb et al. 1989). In our study, we found a shift from nearly perfectly simultaneous bursts of muscle activity in the neck flexor-extensor pair, which we address as “co-activation”, to a pattern characterized by a significantly larger time delay between the two EMG bursts, which we address as “reciprocal”.
The remaining two specific hypotheses were falsified. In particular, the results showed similar APAs when perturbations were applied to the trunk and to the trunk and the head simultaneously, but APAs were much weaker or even absent when perturbations were applied to the head (Figures 6 and 9).
4.1. APAs in neck muscles: Two patterns for two purposes?
Two APA patterns were observed in our experiments. One represented time-shifted (reciprocal) activation of the neck flexors and extensors while the other consisted in nearly simultaneous unidirectional changes in the activity of both muscle groups (co-activation). The first pattern was more frequently observed when perturbations were applied directly to the head (HP series) while the second pattern dominated in trials with perturbations involving the trunk (TP and HTP). Another potentially important observation is the high magnitude of APAs in the neck muscles in the series with perturbation applied to the trunk (TP condition), at least as high as in the HP condition. Taken together, these observations allow to offer the following interpretation.
A predictable perturbation applied to the head may be expected to be associated with APAs organized optimally to minimize the effects of the perturbation on the head posture. A reciprocal pattern of changes in muscle activation leads to a time-varying net torque either in flexion or in extension to counteract the expected direction of the perturbation. Reciprocal APA patterns have been indeed reported in many studies of vertical posture (Cordo and Nashner 1982; Horak et al 1984; Bouisset and Zattara 1987; Aruin and Latash 1995, 1996).
However, all APAs are based on prediction and are expected to lead to under-compensation in some trials and over-compensation in others (reviewed in Massion 1992). Hence, the net result of a combined action of an APA and an external perturbation on the trunk may be hard to predict. For example, the COM can in one trial deviate forward and in the next trial, under seemingly identical conditions – backward. This may be the reason for much more reproducible EMG indices during APAs as compared to mechanical indices such as COP shifts (Massion 1992; Aruin and Latash 1995, 1996; Shiratori and Latash 2000). Because of the mechanical coupling across the body segments, perturbations applied to the trunk and leg/trunk APAs are both sources of head perturbation. The direction of this net perturbation may be poorly predictable. Co-contraction of neck flexors and extensors may be viewed as a method of increasing the apparent neck stiffness to a perturbation irrespective of its direction – a method of alleviating effects of perturbations whose direction is poorly predictable. This interpretation remains speculative since we did not manipulate predictability of perturbations and have no independent measure of how the subjects perceived predictability of the direction of the perturbations.
As suggested in the Introduction, APAs may be defined by two groups of factors, local and global. The former represent effects of perturbations acting directly on a particular segment. The latter reflect perturbations that are secondary to a perturbation acting on the COM of the whole body. Earlier publications (reviewed in Massion 1992) and the current results suggest that a default APA pattern to deal with local perturbations represents time-shifted (reciprocal) changes in activation of agonist-antagonist muscle pairs. Perturbations from the second group may be associated with reciprocal or co-contraction patterns depending on predictability of direction of their net effects.
Co-contraction patterns of changes in muscle activation during APAs have been described in a number of studies. In particular, they are more common in persons whose postural control system may be challenged such as elderly (Woollacott et al. 1988) and persons with Down syndrome (Aruin and Almeida 1997). They can also be seen in young control subjects in challenging conditions such as standing on roller-skates (Shiratori and Latash 2000) or on a surface with decreased support area (Aruin et al 1998; Slijper and Latash 2000). In all these studies, co-contraction patterns have been interpreted as reflecting a trade-off between efficacy and safety, which is ensured by increased joint apparent stiffness that counteracts any perturbation, very much in line with the interpretation offered in this study.
4.2. Do APAs in the leg and trunk muscle help stabilize the head?
APAs in trunk and leg muscles produce joint torques that act on the head because of the mechanical coupling among the body segments. These effects can help stabilize head posture or produce additional perturbations for the head. Based on previous studies that have documented head stabilization during a variety of actions (Berthoz and Pozzo 1994; Pozzo et al. 2001), we hypothesized that leg/trunk APAs might contribute to head stabilization. This is an attractive hypothesis because of two reasons. First, the functional importance of head stability during standing is obvious. The sensitivity of posture to visual and vestibular information (Lestienne et al 1977; Horstmann and Dietz 1988; Buchanan and Horak 1999; Maurer et al. 2006) makes it imperative for the central nervous system to ensure stability of sensory signals of those modalities during standing. Second, APAs are typically associated with rather small net changes in such variables as COP location and shear forces (e.g., Massion 1992; Aruin and Latash 1995). For example, COP shifts during APAs are typically of the order of 1 mm. Mechanical effects of such small shifts on vertical posture seem unlikely, particularly if one considers the much higher amplitude of typical spontaneous COP shifts during quiet standing (e.g., Winter et al. 1996).
However, in our experiments, perturbations applied only to the trunk led to APAs that were not different from perturbations applied to the trunk and the head. In other words, when the same action led to larger head perturbation (in addition to the trunk perturbation), the controller did not use larger APAs. Moreover, a perturbation applied to the head only induced minimal APAs in the leg and trunk muscles. Taken together, these results fail to support the hypothesis on the importance of APAs in leg/trunk muscle for head stability.
If APAs in the leg/trunk muscles are not generated by the central nervous system to ensure head stability, they represent an additional source of head perturbation. The idea that APAs may be viewed by the central nervous system as perturbing factors is not novel; it was invoked to interpret APA changes during standing on surfaces with a decreased support area (Aruin et al. 1998).
Our results suggest that the main purpose of APAs in postural muscles is to ensure stability of a body segment, which is directly under the control of these muscles. In particular, APAs in leg/trunk muscles try to alleviate mechanical effects of expected perturbations on vertical posture, while APAs in neck muscles try to ensure head stability under perturbations coming from the trunk as well as from the environment. This is the most straightforward interpretation on our results as well as of many earlier studies (reviewed in Massion 1992).
One of the limitations of the current study is the use of relatively unusual tasks and perturbations. As in most studies with relatively artificial (but easy to standardize) tests, we have assumed that the behaviors observed in our experiments reflected adjustments of previously learned APAs based on the variety of everyday actions associated with perturbations to the trunk and to the head. We have also assumed that APAs developed over the lifetime can be scaled and adjusted to new tasks based on a couple of practice trials. Unfortunately, using artificial laboratory tests remains an unavoidable component of movement studies that allows to separate and explore specific factors that affect movement patterns (although see Cordo et al. 2006). Within this study, we did not explore learning effects over the short series of trials and hope to address this issue in future.
Acknowledgments
The study was in part supported by NIH grants AG-018751 and NS-035032, and by CAPES 2105039.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aruin AS, Almeida GL. A coactivation strategy in anticipatory postural adjustments in persons with Down syndrome. Motor Control. 1997;1:178–191. [Google Scholar]
- Aruin AS, Forrest WR, Latash ML. Anticipatory postural adjustments in conditions of postural instability. Electroencephalogr Clin Neurophysiol. 1998;109:350–359. doi: 10.1016/s0924-980x(98)00029-0. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. Directional specificity of postural muscles in feed-forward postural reactions during fast voluntary arm movements. Exp Brain Res. 1995;103:323–332. doi: 10.1007/BF00231718. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Latash ML. Anticipatory postural adjustments during self-initiated perturbations of different magnitude triggered by a standard motor action. Electroencephalogr Clin Neurophysiol. 1996;101:497–503. doi: 10.1016/s0013-4694(96)95219-4. [DOI] [PubMed] [Google Scholar]
- Belen’kii V, Gurfinkel VS, Pal’tsev YI. Elements of control of voluntary movements. Biofizika. 1967;10:135–141. [PubMed] [Google Scholar]
- Berthoz A, Pozzo T. Head and body coordination during locomotion and complex movements. In: Swinnen S, Heuer H, Massion J, Casaer P, editors. Interlimb coordination: neural, dynamical, and cognitive constraints. San Diego: Academic Press; 1994. pp. 147–165. [Google Scholar]
- Bouisset S, Zattara M. Biomechanical study of the programming of anticipatory postural adjustments associated with voluntary movement. J Biomech. 1987;20:735–742. doi: 10.1016/0021-9290(87)90052-2. [DOI] [PubMed] [Google Scholar]
- Buchanan JJ, Horak FB. Emergence of postural patterns as a function of vision and translation frequency. J Neurophysiol. 1999;81:2325–2339. doi: 10.1152/jn.1999.81.5.2325. [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Hodges PW, Smith TC, Brumagne S, Gurfinkel VS. Scaling and non-scaling of muscle activity, kinematics, and dynamics in sit-ups with different degrees of difficulty. J Electromyogr Kinesiol. 2006;16:506–521. doi: 10.1016/j.jelekin.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Cordo PJ, Nashner LM. Properties of postural adjustments associated with rapid arm movements. J Neurophysiol. 1982;47:287–302. doi: 10.1152/jn.1982.47.2.287. [DOI] [PubMed] [Google Scholar]
- Freitas SMSF, Duarte M, Latash ML. Two kinematic synergies in voluntary whole-body movements during standing. J Neurophysiol. 2006;95:636–645. doi: 10.1152/jn.00482.2005. [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, Corcos DM, Agarwal GC. Strategies for the control of voluntary movements with one mechanical degree of freedom. Behav Brain Sci. 1989;12:189–250. [Google Scholar]
- Gurfinkel VS, Lipshits MI, Lestienne FG. Anticipatory neck muscle activity associated with rapid arm movements. Neurosc Lett. 1988;94:104–108. doi: 10.1016/0304-3940(88)90278-9. [DOI] [PubMed] [Google Scholar]
- Horak FB, Esselman P, Anderson ME, Lynch MK. The effects of movement velocity, mass displaced, and task certainty on associated postural adjustments made by normal and hemiplegic individuals. J Neurol Neurosurg Psychiatry. 1984;47:1020–1028. doi: 10.1136/jnnp.47.9.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann GA, Dietz V. The contribution of vestibular input to the stabilization of human posture: a new experimental approach. Neurosci Lett. 1988;19:179–184. doi: 10.1016/0304-3940(88)90653-2. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy V, Latash ML, Scholz JP, Zatsiorsky VM. Muscle synergies during shifts of the center of pressure by standing persons. Exp Brain Res. 2003;152:281–292. doi: 10.1007/s00221-003-1574-6. [DOI] [PubMed] [Google Scholar]
- Lee WA. Anticipatory control of postural and task muscles during rapid arm flexion. J Mot Behav. 1980;12:185–196. doi: 10.1080/00222895.1980.10735219. [DOI] [PubMed] [Google Scholar]
- Lestienne F, Soechting JF, Berthoz A. Postural readjustments induced by linear motion of visual scenes. Exp Brain Res. 1977;28:363–384. doi: 10.1007/BF00235717. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Anticipatory postural responses in the human subject. J Physiol. 1978;275:47–48. [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Maurer C, Mergner T, Peterka RJ. Multisensory control of human upright stance. Exp Brain Res. 2006;171:231–250. doi: 10.1007/s00221-005-0256-y. [DOI] [PubMed] [Google Scholar]
- Oliveira LF, Simpson DM, Nadal J. Calculation of area of stabilometric signals using principal component analysis. Physiol Measures. 1996;17:305–312. doi: 10.1088/0967-3334/17/4/008. [DOI] [PubMed] [Google Scholar]
- Pozzo T, Ouamer M, Gentil C. Simulating mechanical consequences of voluntary movement upon whole-body equilibrium: the arm-raising paradigm revisited. Biol Cybern. 2001;85:39–49. doi: 10.1007/PL00007995. [DOI] [PubMed] [Google Scholar]
- Shiratori T, Aruin AS. Anticipatory postural adjustments associated with rotational perturbations while standing on fixed and free-rotating supports. Clin Neurophysiol. 2004;115:797–806. doi: 10.1016/j.clinph.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Shiratori T, Latash ML. The roles of proximal and distal muscles in anticipatory postural adjustments under asymmetrical perturbations and during standing on rollerskates. Clin Neurophysiol. 2000;111:613–623. doi: 10.1016/s1388-2457(99)00300-4. [DOI] [PubMed] [Google Scholar]
- Slijper H, Latash ML, Mordkoff JT. Anticipatory postural adjustments under simple and choice reaction time conditions. Brain Res. 2002;924:184–197. doi: 10.1016/s0006-8993(01)03233-4. [DOI] [PubMed] [Google Scholar]
- Slijper H, Latash ML. The effects of instability and additional hand support on anticipatory postural adjustments in leg, trunk, and arm muscles during standing. Exp Brain Res. 2000;135:81–93. doi: 10.1007/s002210000492. [DOI] [PubMed] [Google Scholar]
- Van der Fits IBM, Klip AWJ, Van Eykern LA, Hadders-Algra M. Postural adjustments accompanying fast pointing movements in standing sitting and lying adults. Exp Brain Res. 1998;120:202–216. doi: 10.1007/s002210050394. [DOI] [PubMed] [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol. 1996;75:2334–2343. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Inglin B, Manchester D. Response preparation and posture control. Neuromuscular changes in the older adult. Ann New York Acad Sci. 1988;515:42–53. doi: 10.1111/j.1749-6632.1988.tb32964.x. [DOI] [PubMed] [Google Scholar]