Abstract
Neoplastic cells are generally poor immunogens. Transfection of the murine tumor CT-26 with β-galactosidase (β-gal), a proteinfrom Escherichia coli, did not alter its growth rate in vivo, or its lethality, and did not elicit a measurable anti-β-gal immune response. Immunization with β-gal-expressing recombinant vaccinia viruses (rVV) elicited specific anti-β-gal cytolytic T lymphocytes, but rVV-β-gal was only marginally therapeutic when given to tumor-bearing mice. With the aim of expanding the immune response against β-gal, used here as a model tumor Ag, we gave mice exogenous IL-2 starting 12 h after the poxvirus. The therapeutic effectiveness of the combination of poxvirus and IL-2 was far greater than either of these treatments alone. When the cDNA for IL-2 was inserted into the viral genome of the rVV construct to make a double recombinant (drVV), antitumor activity was further augmented. One mechanism of action may be the enhanced activation or expansion of cytotoxicT cells, because a marked increase in primary cytotoxic responses against vaccinia determinants was observed. Interestingly, other cytokines (mGM-CSF, mTNF-α, and mIFN-γ) inserted into the rVV genome did not modify the efficacy of the rVV constructs. The increase in specific CTL responses against β-gal by drVV expressing the tumor-associated Ags (TAA) and IL-2 was morepronounced inmice bearing the lacZ-transduced tumor than in those bearing the parental cell line, suggesting that the TAA presented by growing tumor cells can either pre-activate or otherwise amplify the immune response induced by the rVV. Unfortunately, in several long-term surviving mice, tumor recurred that no longer expressed β-gal. These results indicate that treatment of disseminated tumors by using recombinant viruses expressing TAA can be enhanced by IL-2 provided exogenously, or encoded within the recombinant virus.
Tumor-associated Ags (TAA)3 cells have been recently identified that are recognized by CD8+ T cells in both murine and human tumor cells (1-6). In some cases, CTL cell lines specifically recognizing these TAA are able to induce cancer regression in patients with disseminated metastases (4, 6, 7). With genes encoding relevant TAA now in hand, targeted, Ag-specific vaccines for cancer therapy are being developed. Expression of TAA by recombinant viruses scan elicit specific immune responses that have been used to protect mice from subsequent tumor challenge inmurine models, and may one day be used in patients at high risk of developing particular cancers, because of either genetic predisposition or environmental exposure to toxic or infectious agents. The use of recombinant vaccines to treat disease, not merely to prevent it, would be of enormous utility.
Among the different viruses that may provide a means of overcoming the limited natural responsiveness to TAA are poxviruses. They can express a large amount of heterologous genetic material and have not been shown to possess oncogenic potential. Protein transcription and translation, as well as viral DNA replication, is achieved by poxviruses entirely within the cytoplasm of the infected cell, without the need for cellular transcription factors. Recombinant poxviruses, including vaccinia viruses (rVV), and more recently fowlpox viruses (rFPV), can induce strong and long-lasting CTL responses (8-11), and can protect animals from a challenge with infectious agents or tumor cell lines (12-15). rFPV are safe vaccine candidates because they are replication defective in mammalian cells, their productive cycle being limited to some avian species (16, 17).
Other weapons in the immunotherapists’ armamentarium are lymphokines, administered exogenously or secreted by genetically modified tumor cells (reviewed in Refs. 18 and 19). Particularly promising have been IL-2, IFN-γ, and TNF-α, which are all produced by the Th1 subset of CD4+ lymphocytes and are key regulators of the cell-mediated response against intracellular parasites and neoplastic cells. These lymphokines influence several aspects of the immune response, from Tcell growth and differentiation to the regulation of important steps in Ag processing and presentation (20-22). GM-CSF, a multi-lineage stimulating factor for bone marrow progenitors, promotes differentiation of hematopoietic precursors to dendritic cells that are “professional” APCs capable of priming naïve lymphocytes (23, 24).
The present studies evaluate the influence of murine cytokines on the immunogenicity of heterologous proteins expressed by recombinant poxviruses, and their abilities to function therapeutically in vivo as antitumor reagents. We hypothesized that lymphokines like IFN-γ, TNF-α, IL-2, and GM-CSF may enhance the immunogenicity of Ags expressed by recombinant poxviruses. We also recognized that cytokines could also affect viral replication and have potentially negative effects, such as the more rapid resolution of viral infection with a concomitant shorter and less complete exposure to heterologous protein (25, 26). Indeed, no clear evidence exists for a cytokine-dependent enhancement of cell-mediated responses against the recombinant protein (reviewed in Ref. 27).
In this report, we show that exogenously administered IL-2 can enhance the immunogenicity of a model TAA encoded in poxviruses, resulting in enhanced therapeutic effectiveness of established pulmonary metastases. Furthermore, a drVV expressing both IL-2 as well as a model TAA β-gal) can further improve this therapeutic response.
Materials and Methods
Cell lines
CT26 is an N-nitroso-N-methylurethane-induced BALB/c (H-2d) undifferentiated colon carcinoma generously supplied by D. Pardoll (Baltimore, MD). CT26 was cloned to produce a wild-type parental tumor line, CT26.WT. The gene for lacZ was stably transfected into CT26.WT as described previously (27a). Briefly, a plasmid donated from A. D. Miller (Fred Hutchinson Cancer Research Center, Seattle, WA) containing the gene for β-gal and a neo-resistance marker were used to construct an ecotropic producer cell line secreting the LZSN retroviral construct. This retroviral supernatant was used to transduce the CT26.WT cell line. Transductants were selected in a G418 media and then subcloned by limiting dilution analysis at 0.3 cells/well. Subclones that expressed β-gal were screened by X-gal staining and in 51Cr release assays with anti-β-gal effectors. The subclone CT26.CL25 was selected for use in all studies because of its stable expression of both β-gal and the class I molecule H-2 Ld. A clone of the mouse thymoma EL4 (H-2b) stably transfected with β-gal, termed E22 (provided by Y. Paterson, (Department of Microbiology, University of Pennsylvania, Philadelphia)) was used as a negative control in 51Cr release assays. BSC-1 cells (American Type Culture Collection (ATCC), Rockville, MD; CCL 26) were used to expand and titer all VV. Cell lines were maintained in RPMI 1640, 10% heat-inactivated FCS (Biofluids, Rockville, MD), 0.03% l-glutamine, 100 μg/ml streptomycin, 100 μg/ml penicillin, and 50 μg/ml gentamicin sulfate (National Institutes of Health Media Center, Bethesda, MD). CT26.CL25 and E22 were maintained in media containing 400 μg/ml G418 (Life Technologies, Inc., Grand Island, NY).
rVV and rFPV
All the rVV used in this study were originated by insertion of the foreign genes into the VV thymidine kinase (TK) gene by homologous recombination, resulting in the generation of TK-negative progeny as described (28). The recombinant stocks were produced by using the TK-human osteosarcoma 143/B cell line (ATCC, CRL 8303). From these stocks, rVV were propagated in BSC-1 cells and used as crude cell lysate. The BSC-1 cell line was also utilized to determine virus concentration by plaque titration. The rVV used in a single experiment were titered together to maximize the accuracy of the relative titers. Preparation of rVV expressing the influenza A/PR/8/34 nucleoprotein (NP) was previously described (29). In the HPV16-E6Vac, Escherichiu coli lacZ was under the control of the early promoter element of the VVP, p7.5 promoter from plasmid pSC65 (S. Chakrabarti, J. Sisler, and B. Moss, NIAID, NIH, Bethesda, MD); this construct was named VJS6 for simplicity. Control CR19 VV (wild-type vaccinia) was kindly provided by J. Yewdell and J. Bennink (NIAID, NIH, Bethesda, MD). Murine IL-2 cDNA was amplified by PCR from pBMGNeomIL-2 and ligated into the SmaI-BamHI site of a vaccinia expression vector, pMJ601 (a gift from B. Moss), which contains the β-gal gene under the control of the p7.5 early vaccinia promoter (30). The other cytokines (GM-CSF, IFN-γ, and TNF-α) were inserted into the wtVV genome with a similar procedure. The cytokines produced after infection with the rVV have been confirmed for their bioactivity.
The POXVAX-TC strain of FPV was used in these studies and is designated FPV.wt. Workers at Therion Biologics (Cambridge, MA) inserted foreign sequences into FPV by homologous recombination as described elsewhere (31). FPV.bg40K is a recombinant that contains the E. coli lacZ gene under the control of the VV 40k promoter, placed in the BamHI J region of the FPV genome.
Peptides
The synthetic peptide, TPHPARIGL, spanning amino acids 876–884 of β-gal, the naturally processed H-2 Ld-restricted epitope (32), was synthesized by Peptide Technologies (Washington, DC) to a purity of greater than 99% as determined by HPLC and amino acid analysis.
Evaluation of primary response
Primary lymphocyte populations were obtained by injecting 8- to 12-wk-old female BALB/c mice (Animal Production Colonies, Frederick Cancer Research Facility, National Institutes of Health, Frederick, MD) i.v. with varying PFUso of recombinant viruses. The spleens were taken on day 6 after immunization, separated into a single cell suspension and tested for their ability to lyse β-gal-positive targets in a 6-h 51Cr release assay. Splenocytes were resuspended in complete media including RPMI 1640 with 10% FCS (Biofluids, Rockville, MD), 0.1 mM nonessential amino acids, 1 mM sodium pyruvate (Biofluids) and 5 × 10−51 μM 2-ME (Life Technologies, Inc., Rockville, MD), and used in 6-h 51Cr release assays as described elsewhere (33). To summarize, 2 × 106 target cells were treated with 200 mCi Na51CrO4, (51Cr) for 90 min. Peptide-pulsed CT26.WT were incubated with 1 μg/ml of peptide during labeling. Target and effector cells were mixed at appropriate ratios for 6 h. The amount of 51Cr released was determined by gamma-counting and the percent specific lysis was calculated from triplicate samples according to the following formula:
Detection of cytokine production
Cytokine production was determined by ELISA. The ELISA kits for detection of murine GM-CSF, IFN-γ, TNF-α, and IL-2 from Endogen (Endogen, Boston, MA) were used according to the manufacturer’s instructions. IL-2 concentrations were sometimes given in Cetus units, 1 Cetus unit/ml corresponding to 600 pg/ml of IL-2.
In vivo treatment studies
BALB/c mice were immunized with virus (5 × 106 to 107 PFUs) 3 or 6 days after i.v. challenge with tumor cells (105 to 5 × 105) to establish pulmonary metastases. All animals were randomized before receiving virus. Treatment with IL-2 was initiated 12 h after immunization; 6 doses of high dose IL-2 (100,000 Cetus units/injection) or 10 doses of low dose IL-2 (15,000 Cetus units/injection) were administered to selected groups of mice. Mice were killed on day 12 and lung metastases were enumerated in a blind fashion. Identically treated groups of mice were followed for survival.
Whole organ X-gal staining
Lungs from mice treated with i.v. tumor challenge as described above were inflated with PBS (Biofluids) before X-gal staining. Lungs were fixed in a solution containing 2% formaldehyde (v/v), 0.2% glutaraldehyde (v/v) in PBS for 45 min, washed in PBS three times, and stained in X-gal solution for 12 h at 37°C. X-gal solution for whole organs was prepared by combining the following: 0.02% (v/v) Nonidet P-40, 0.01% (w/v) sodium deoxycholate, 1 mg/ml X-gal, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, in PBS. After staining in X-gal solution, the lungs were rinsed briefly with 3% (v/v) dimethyl sulfate, and then with PBS. The stained lungs were stored at 4°C in 0.02% (w/v) sodium azide in PBS (34).
Statistical analysis
The Wilcoxon-Mann-Whitney U test was used to examine the null hypothesis of identity of ranks between two sets of data. Kaplan-Meier plots and Mantel-Haenszel test were used to compare survival of mice belonging to different treatment groups.
Results
Administration of exogenous IL-2 with rVV encoding the model TAA reduces the number of pulmonary metastases and prolongs survival
When inoculated i.v. at adose of 5 × 105 cells, both the parental line CT26.WT and the subclone CT26.CL25, transduced with a retrovirus encoding the model TAA β-gal, grew progressively and killed the animals in 11 to 15 days. At the time of death, these mice had greater than 500 pulmonary metastases. In the experiment presented in Table I, control mice received no treatment and had pulmonary metastases that were too numerous to count. Neither the inoculation of moderate doses of rIL-2 for 5 days nor one i.v. injection of 5 × 106 PFU/mouse of a rVV-encoding β-gal (VJS6) induced a significant reduction of the number of pulmonary metastases counted 12 days after tumor inoculation. Immunization with VJS6 3 days after tumor injection combined with rIL-2 administration induced a significant reduction in CT26.CL25 pulmonary metastases (p = 0.005) whereas no significant decrease in the number of pulmonary metastases of the TAA-negative parental line was seen.
Table I.
Treatment of established pulmonary metastases with rVV and exogenous IL-2a
| Mice inoculated with | ||||
|---|---|---|---|---|
| CT26.WT | CT26.Ct25 | |||
| rvv Treatment | Average no
metases |
Metatses/Mouse | Average no
metatses |
Metases/Mouse |
| Noneb | >500 | >500 × 5 | >500c | >500 × 5 |
| VJS6 | >500 | >500 × 5 | >405.2 | >500 × 3,267, 259 |
| Exogeneous rIL-2 | >500 | >500 × 5 | >500 | >500 × 5 |
| Exogeneous rIL-2 + VJS6 | 406.4 | >500 × 3, 298, 234 | 20.8 | 43, 6, 0, 52, 3 |
Five BALB/c mice per each treatment group were injected i.v. with 0.5 ml of HBSS containing 5 × 105 tumor cells of either CT26.WT or CT26.CL25. Three days later they received a single i.v. injection of 5 × 106 PFU of β-gal expressing rVV, VJS6. Treatment with exgoneous rIL-2 (15,000 Cetus U, twice a day, i.p.) was started 6 h after rVV inoculation and continued for 5 days. Lungs were harvested on day 12 after tumor inoculation and pulmonary metastases were counted in a blind fashion.
Control mice were injected with HBSS alone.
All the mice in this group died between days 11 and 12 (before lung harvest).
Active immunotherapy with the combination of exogenous rIL-2 and rVV also prolonged the survival of mice bearing 3-day-old pulmonary metastases (Fig. 1). Two administration regimens for IL-2 were chosen: 100,000 U rIL-2 for 3 days (high dose) and 15,000 U for 5 days (low dose) were administered i.p. following a single i.v. injection of VJS6. As an additional negative control, a group of mice was treated with the highest dose of exogenous rIL-2 together with a TK-disrupted rVV expressing a protein different from β-gal, the influenza virus NP (V69; Fig. 1). VJS6 exerted a marginal effect on mouse survival, consistent with a partial reduction of the number of metastases observed in some experiments (see below). A clear improvement of survival was obtained when exogenous rIL-2 was administered with the β-gal-encoding rVV but not with the NP-expressing rVV (Fig. 1, lower panel). There was no significant difference in the survival of mice receiving the “high dose” or “low dose” rIL-2 regimens (p = 0.231). Improved survival of mice bearing the parental cell line was not observed in any treatment group (Fig. 1, upper panel).
FIGURE 1.

Active immunotherapy is enhanced when exogenous rIL-2 and rVV are given in concert. BALB/c mice (five per group) were challenged i.v. with 5 × 105 CT26.WT or CT26.CL25 tumor cells. After 3 days they received a single i.v. injection of medium alone (HBSS) or medium containing 5 × 106 PFU of different TK− rVV either expressing (VJS6) or not expressing (V69) β-gal. Two different regimens of rIL-2 administration were started 12 h after rVV injection: high dose (HD, 100,000 Cetus U, twice a day, i.p. for 3 days) or low dose (LD, 15,000 Cetus U, twice a day, i.p. for 5 days). Mice were checked twice a day for survival.
The therapeutic advantage of exogenous rIL-2 was not limited to fully replication-competent viruses because a prolongation of survival was also observed with rFPV encoding β-gal but not with the wild-type virus (Fig. 2). The effect of a single i.v. injection of 107 PFU of the recombinant FPV.bg40k, expressing β-gal under the control of the 40k vaccinia promoter, on the survival of mice bearing the β-gal-positive tumor was limited and consistent with a partial reduction in the number of pulmonary metastases. However, daily inoculation of high IL-2 doses significantly increased the therapeutic effect, resulting in 40% of mice still surviving 2 mo after treatment.
FIGURE 2.
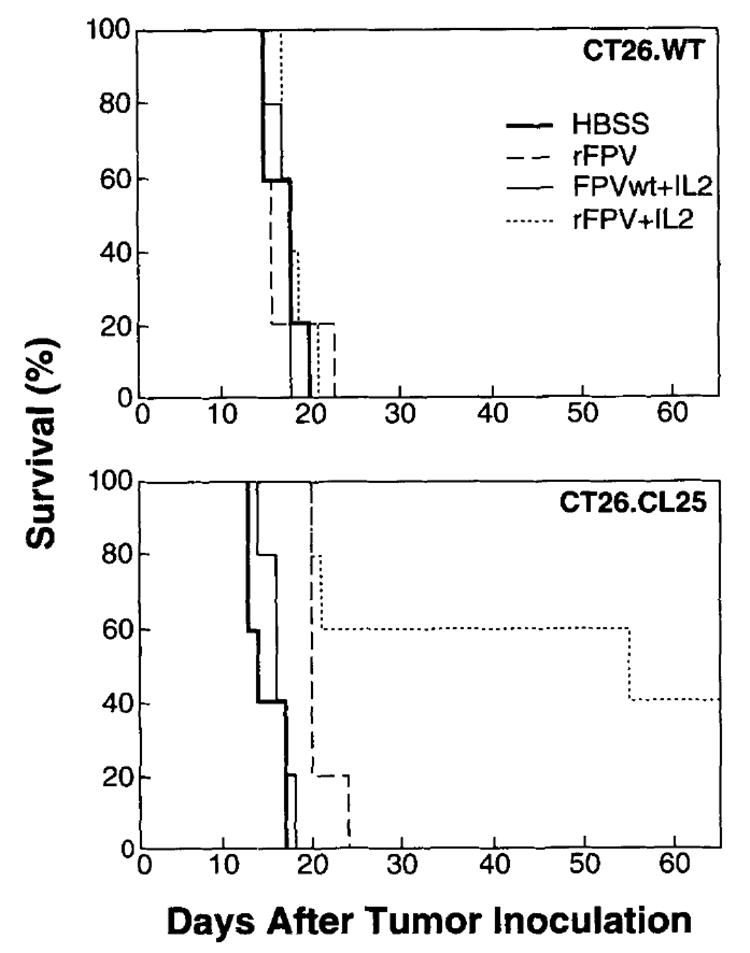
Exogenous rIL-2 enhances the function of rFPV. BALB/c mice (five per group) were inoculated i.v. with 5 × 105 CT26.WT or CT26.CL25 tumor cells. On day 3 after tumor injection, they received a single i.v. injection of the following viruses: no virus (HBSS alone), 107 PFU of FPV.bg40k (rFPV), or FPV wild-type (FPVwt). rIL-2 (100,000 Cetus U, twice a day) was administered i.p. starting 12 h after FPV injection and continued for 3 days. Mice were checked twice a day for survival.
To examine a more advanced disease model, mice were injected with 105 tumor cells and treated on day 6. Following the i.v. inoculation of 105 CT26.CL25 tumor cells, all the untreated mice died by day 22 (Fig. 3). Lungs examined after 6 days of tumor growth revealed the presence of more than 100 macroscopically visible nodules (not shown). At this time point, a single inoculation of VJS6 was able to slightly increase survival, but all the mice died within 24 days. Addition of high rIL-2 dose treatment resulted in a significant survival benefit (p = 0.005) also in this advanced disease model, with two of five mice surviving until day 35. Once again, the response was specific and limited to the combination of β-gal-positive tumor and rVV; the survival of CT26.WT tumor-bearing mice was not affected (data not shown).
FIGURE 3.
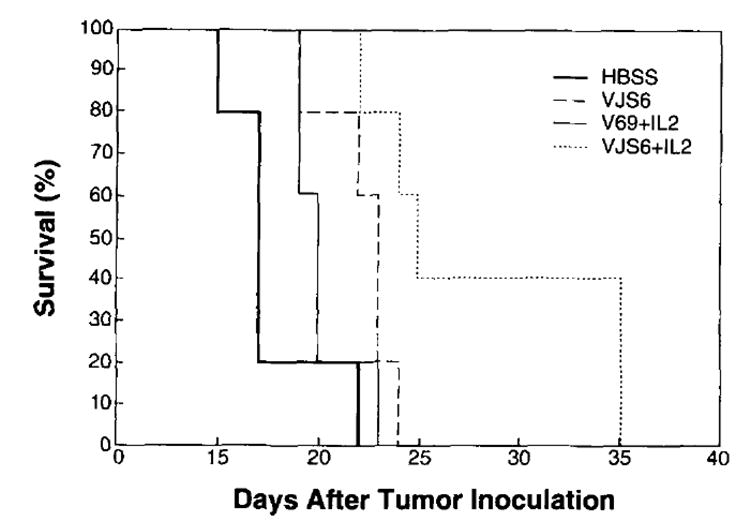
Exogenous rIL-2 plus rVV is therapeutic in the more advanced 6-day tumor model. BALB/c mice (five per group) were inoculated i.v. with 105 CT26.CL25 tumor cells. Six days after tumor injection, they received the same treatments described in Figure 1 with the exception that only the highest dose of rIL-2 was administered. Mice were checked daily for survival. No prolongation of survival was obtained by the various treatments in mice bearing 6-day-old pulmonary metastases of CT26.WT tumor (data not shown).
Therapeutic efficacy of IL-2 produced from drVV also expressing the model TAA
The large vaccinia genome can host up to 25 kb of exogenous DNA, allowing the expression of different heterologous proteins in addition to the model TAA (9, 10). The results obtained with exogenous rIL-2 prompted us to investigate the effect of cytokine production at the site of virus infection and replication. A drVV co-encoding the model TAA β-gal and IL-2 was previously described (25). On the basis of the above results with passively administered IL-2, we constructed a new set of drVV in which cytokine production was driven by a powerful synthetic promoter (35). The murine cytokines included in this study were the following: IL-2, GM-CSF, IFN-γ, and TNF-α. Because the same plasmid was used to construct the various TK− drVV, a similar level of β-gal enzymatic activity was detected after infection of the BSC-1 cell line (data not shown). Supernatants from the same infected cultures were harvested at various times and tested for the presence of the different cytokines. Table II shows that specific and elevated cytokine production was detected only in supernatants from BSC-1 cells infected with the relevant viruses. For example, approximately 40,000 U IL-2/ml were released during 36 h in supernatant from BSC-1 at a moi of 1. IL-2 was barely detectable in all the other supernatants. Comparable levels of IL-2 production in murine and human tumor cell lines infected with the same rVV were recently reported (30). No cytokine was present in supernatants from cell cultures infected with the VJS6 virus expressing the E6 protein from HPV16 in addition to β-gal.
Table II.
Defection of cytokine production following in vitro infection of BSC-1 cells with 1:1 moi of different rVVa
| Cytokine production after 12 h | ||||
|---|---|---|---|---|
| rVV | IL-2 | GM-CSF | IFN-γ | TNF-α |
| VJS6 | <34 | <15 | <47 | <30 |
| IL-2 | 2 × 106 | <15 | <47 | <30 |
| GM-CSF | <34 | 5.1 × 105 | <47 | <30 |
| IFN-γ | <34 | <15 | 5.1 × 104 | <30 |
| TNF-α | <34 | <15 | <47 | 2.05 × 103 |
| Cytokine production after 36 h | ||||
| VJS6 | <34 | <15 | <47 | <30 |
| IL-2 | 3.9 × 106 | <15 | <47 | <30 |
| GM-CSF | 54 | 4.2 × 106 | <47 | <30 |
| IFN-γ | <94 | <15 | 6.7 × 104 | <30 |
| TNF-α | <34 | 2.2 × 103b | <47 | 1.35 × 105 |
Duplicate wells of 105 BSC-1 cells were infected with different rVV at a multiplicity of infection (moi) of 1 PFU/cell. At different intervals, supernatants were removed, centrifuged to eliminate cellular debris, serially diluted, and used to estimate cytokine production by using an ELlSA assay specific for the murine cytokines indicated in the table. Values are expressed as pg/ml of supernatant.
Only one of the two wells was positive. No positivity was detected in subsequent determinations.
When a single i.v. injection of 5 × 106 PFU/mouse was used to treat mice bearing 3-day-old pulmonary metastases, only the IL-2 drVV was able to significantly reduce the number of pulmonary nodules in mice inoculated with the β-gal-positive tumor cell line (p = 0.005) (Fig. 4). A partial decrease was obtained with the other cytokine-encoding viruses and with VJS6. No appreciable change was observed in the number of metastases of the parental tumor cell line. The effect on pulmonary metastases also correlated with an increased survival (see below).
FIGURE 4.
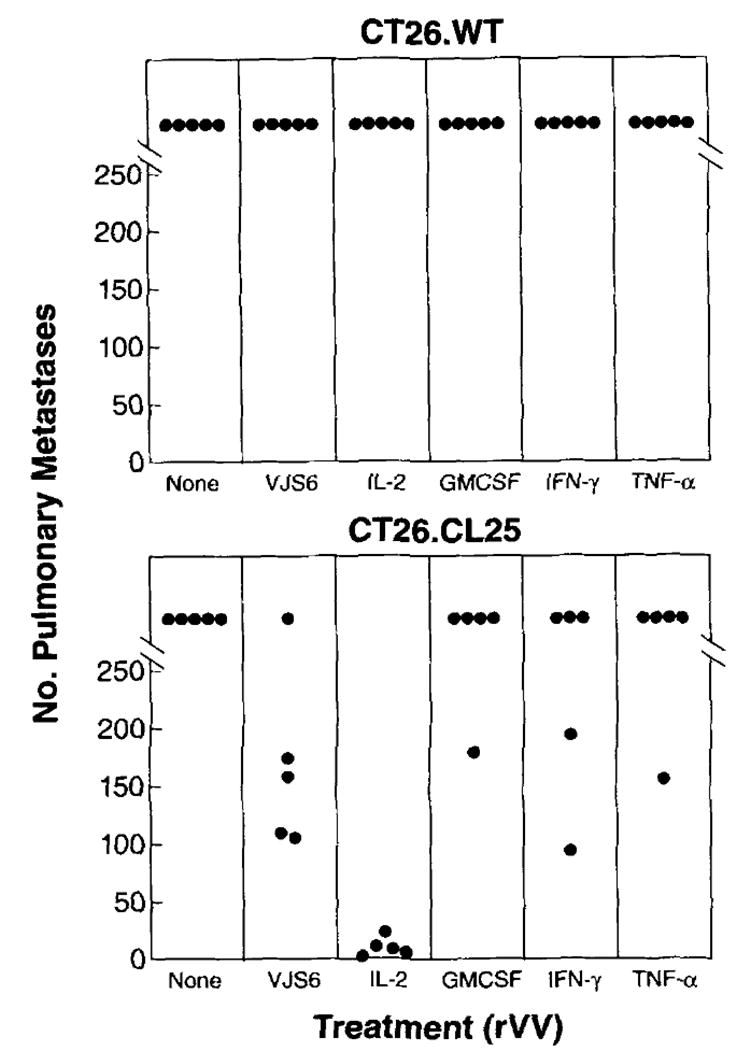
A drVV expressing IL-2,. but not GM-CSF, IFN-γ, or TNF-α, significantly reduces the number of pulmonary metastases in a 3-day model. Five BALB/c mice per group were injected i.v. with 5 × 105 tumor cells of either CT26.WT or CT26.CL25 cell lines. Three days later they received a single i.v. injection of HBSS alone (none) or containing 5 × 106 PFU/mouse of different rVV, as indicated. On day 12 post tumor challenge, lungs were harvested and pulmonary nodules were enumerated in a blind fashion. An independent repeat of this experiment gave identical results.
These results were particularly interesting because the drVV expressed 20- to 50-fold less β-gal enzyme activity than the VJS6 rVV, as detected after infection of BSC-1 cells with an equivalent moi (data not shown) and also suggested that the production of IL-2 could overcome the lower production of the model TAA. When compared under the same experimental conditions, IL-2 drVV together with exogenous IL-2 gave the best results in terms of prolongation of survival (p = 0.002), whereas the survival in the group treated with IL-2 rVV or the combination VJS6 plus exogenous IL-2 was similar (Fig. 5).
FIGURE 5.

The function of a drVV expressing IL-2 is further enhanced when additional exogenous IL-2 is provided. BALB/c mice (five per group) were challenged i.v. with 5 × 105 CT26.WT or CT26.CL25 tumor cells. After 3 days they received a single i.v. injection of plain medium (HBSS) or medium containing 5 × 106 PFU of rVV encoding β-gal alone (VJS6) or together with IL-2 (IL-2 rVV). Twelve hours after rVV, 100,000 rIL-2 U were inoculated, according to the regimen described in Figure 1. Mice were checked twice a day for survival.
IL-2 enhances the primary CTL response against VV and heterologously expressed β-gal
Because the presence of CD8+ lymphocytes recognizing tumor determinants has been associated with an antitumor effect (36), we studied the generation of effector CTL against β-gal in the spleens of mice 6 days after immunization. This time point represents the peak of the CD8+ CTL anti-vaccinia response (37). BALB/c mice were immunized with different rVV indicated in Figure 6, and the spleens of two mice, removed 6 days after immunization, were pooled and tested directly in a short-term 51Cr release assay. The response of VJS6-immunized splenocytes against the CT26.WT target cells infected with the crude 19 VV can be assumed to be the baseline for the primary response elicited by a TK− VV, because the viruses presented in Figure 6 were all generated through recombination in the TK region of the vaccinia genome. The virus-driven cytokine production resulted in either an increase (IL-2 and GM-CSF rVV) or in a suppression (TNF-α and IFN-γ) of the primary cytotoxic response to vaccinia Ags. This effect was even more pronounced when a comparison between the lytic units in each spleen was performed because of the difference in spleen size among the different groups: in fact, the number of lytic units in GM-CSF and IL-2 groups were increased 8.3- and 6.4-fold, whereas TNF-α and IFN-γ were reduced 5.1- and 2.9-fold, respectively, compared with the control cytokine-negative VJS6 (Fig. 6). In the same experiments, the primary response against β-gal-positive target cells was evaluated but no lysis was detected, with the exception of a small response (5 to 10% specific 51Cr release) in mice immunized with IL-2 drVV (see below). After in vitro restimulation with the antigenic peptide from β-gal (TPHPARIGL), splenocytes of BALB/c mice immunized 14 days before with 5 × 106 PFU of different drVV generated effector CTL able to specifically recognize the β-gal-positive clone CT26.CL25 or the parental cell line pulsed with the minimal determinant antigenic peptide. Despite the clear effect of IL-2, either exogenously administered or endogenously produced by rVV, in the treatment of tumor-bearing mice, no difference in the cytolytic activity against the specific target cells was observed in secondary cultures from animals immunized with different rVV co-expressing the TAA with the different murine cytokines (data not shown).
FIGURE 6.
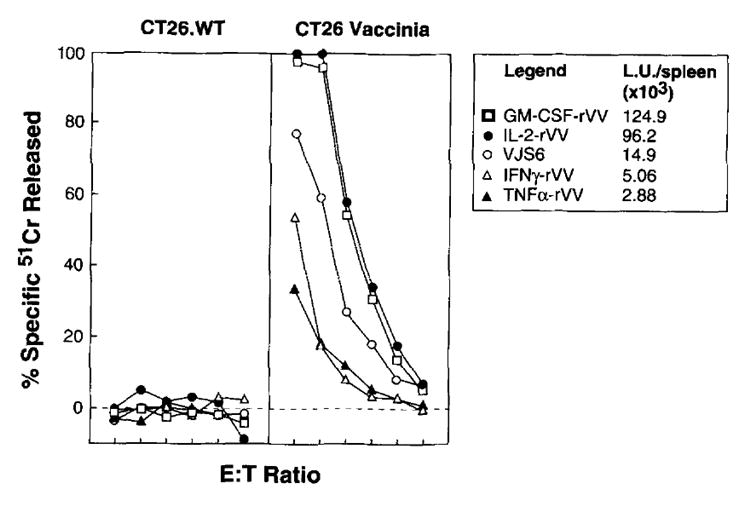
Expression of IL-2 by drVV enhances the antivaccinia CTL response. Two BALB/c mice were immunized with 5 × 106 PFU/mouse of different rVV. After 6 days the spleens were aseptically removed, mixed together, and tested directly in a 6-h 51Cr release assay against CT26.WT tumor cell line, either infected (CT26 vaccinia) or noninfected (CT26.WT) during the isotope labeling with more than 10:1 moi of crude 19 VV preparation. Spontaneous release of target cells never exceeded 20%. E:T cell ratio was 100:1 and then 1:3 dilutions. Lytic units 30% (L.U. 30%) indicate the number of effector cells required to obtain 30% lysis of 10,000 target cells. L.U. 30% were normalized for the total number of cells recovered for each spleen and expressed as total L.U./spleen.
Because analysis of primary and secondary responses did not show any clear difference in reactivity against β-gal in normal mice inoculated with different rVV, we investigated the possibility that the in vivo response could be influenced by the presence of growing tumor by comparing the primary cytotoxic activity in normal and in tumor-bearing mice. Groups of three mice were mock inoculated (HBSS) or injected with different doses (5 × 105, 105, 5 × 104) of either the parental CT26.WT or the β-gal-positive CT26.CL25 cell line, After 3 days mice received a single i.v. dose of different rVV, and their spleens, collected after 6 days from virus immunization, were tested for primary CTL activity. Figure 7 summarizes the results of one experiment representative of three others. As previously indicated, immunization of normal BALB/c mice with IL-2 drVV produced a small but consistent primary response against the β-gal peptide-pulsed CT26.WT and the transduced CT26.CL25 target cells; this response was augmented in a dose-dependent fashion in mice bearing an increasing number of β-gal-positive pulmonary metastases as a consequence of an increased inoculum of tumor cells. Conversely, the specific anti-β-gal response in mice injected with the highest dose of CT26.WT tumor cells was not comparably increased (in Fig. 7 the mice inoculated with the highest number of CT26.WT cells were shown) and was characterized by an elevated level of nonspecific cytotoxicity against the E22 target cells, a β-gal-positive line expressing a different restriction element (H-2b). No response was observed in mice inoculated with 5 × 105 CT26.CL25 cells and treated with VJS6 (Fig. 7) or drVV expressing GM-CSF, IFN-γ, or TNF-α (not shown). The same enhancement of primary response was obtained with exogenous IL-2 and VJS6 (data not shown).
FIGURE 7.
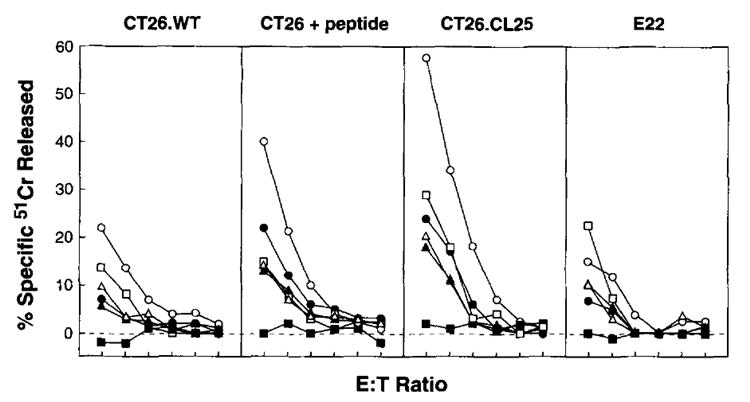
The presence of tumor cells specifically enhance the CTL response elicited by IL-2 rVV in a dose-dependent manner. BALB/c mice were injected with HBSS alone or with varying doses of CT26.WT or CT26.C25 as specified. After 3 days, mice were immunized with 5 × 106 PFU/mouse of either VJS6 or IL-2 rVV. O n day 9 after tumor challenge (day 6 after vaccination) the primary cytotoxic response was evaluated in a 6-h 51Cr release assay against CT26.WT, CT26.WT pulsed with the β-gal Ld-restricted peptide (CT26T + peptide), CT26.CL25, and the irrelevant target cells E22 as shown. The effects of escalating doses of tumor on the generation of the primary cytotoxic response is shown for the immunization with IL-2 rVV in nontumor-bearing mice (▲), or in mice bearing 5 × 104 CT26.CL25 (△), 1 × 105 CT26.CL25 (•), 5 × 105 CT26.CL25 (○), or the highest dose, 5 × 105 of the parental (non-β-gal-expressing) CT26.WT cell line (□). An additional control is the non-IL-2-expressing VJS6 virus injected in mice bearing 5 × 105 CT26.CL25 (▪).
Pulmonary metastases in mice dead after a prolonged survival do not present detectable levels of the model TAA
Tumor cells devoid of the β-gal marker protein are easily detected by a simple assay, facilitating the recognition of antigenic modulation occurring in vivo. Lungs from mice found dead during the survival follow-up were inflated, fixed, and exposed to an X-gal solution allowing the staining of the whole organ (34). Lungs from untreated mice, dead after 12 to 16 days from the i.v. injection of 5 × 105 CT26.CL25 tumor cells, showed an intense, dark-blue staining. Lungs removed from mice treated with the combination high dose exogenous rIL-2 and rVV and found dead after 50 days of survival in the experiment described in Figure 1 were completely negative for X-gal staining as were the mice inoculated with the β-gal-negative parental cell line(data not shown). The only slight positivity detected in one sample was associated with the mouse liver, strongly adherent to the lung through a β-gal-negative invasive metastasis. This positivity is probably related to the β-gal-like activity present in lysosomal compartment (38). A similar negativity to X-gal staining was observed in pulmonary metastases from mice treated with the combination IL-2-rVV and exogenous rIL-2 in the survival study presented in Figure 5 (not shown).
Discussion
In this communication we describe the use of recombinant viruses and cytokines in the treatment of established pulmonary metastases from a tumor cell line expressing a model TAA. The β-gal tumor model offers the advantage of a known peptide (TPHPARIGL) presented by Ld and recognized by specific CD8+ CTL (32). β-gal also offers the easy detection of the presence of the model TAA by X-gal staining. Finally, the construction of recombinant viruses expressing the lacZ gene is convenient because this has been used as a reporter gene in a number of different plasmids (34).
The expression of foreign proteins in tumor cell lines is not necessarily associated with increased immunogenicity or with an in vivo change in lethality or growth rate of tumor cells. Transduction of murine tumor cell lines with exogenous genes like the NP of vesicular stomatitis virus (39), human carcinoembryonic Ag (CEA) (40), or β-gal did not lead to rejection of transplanted tumors; and although the corresponding proteins are known targets of humoral and cell-mediated responses, they were not able to prime an immune response in syngeneic, immunocompetent mice when presented by tumor cells. CTL secondary cultures were not generated after in vitro restimulation of splenocytes from mice bearing 9-day-old pulmonary metastases of the CT26.CL25, β-gal-positive cell line (V. Bronte, unpublished observations).
Despite the presence of TAA, the immunogenicity of many tumors is low. An appropriate example is the parental cell line, the murine adenocarcinoma CT26, that is able to generate a CTL population and protect from the tumor challenge only when transduced with the IL-2 gene, a modification that is supposed to provide the necessary helper signal(41). We were surprised that GM-CSF had no effect on our system when encoded within the drVV, particularly because experiments have shown that GM-CSF greatly enhances the immunogenicity of this particular tumor (24). This finding may imply that APC are not limiting when Ag is supplied by rVV.
The failure of the model TAA to induce an effector T cell population in vivo when presented exclusively by the tumor cell line does not mean that the tumor is a mere bystander in relationship to the ongoing immune response. It is unclear whether tumor cells fail to activate lymphocytes, or whether lymphocytes are transiently activated and clonally expanded by tumor cells, then subsequently inactivated, perhaps because of lack of sufficient costimulation. Whatever the case may be, the effect of the stimulation of lymphocytes with the recombinant viruses, probably expressing the TAA in the proper immunogenic form, can be enhanced by the presence of tumor cells expressing the TAA as indicated by the increase of primary cytolytic activity in tumor-bearing mice. This increase is dependent on the number of tumor cells because the highest response was observed in animals with the largest tumor burden and is also specific because it was dictated by the presence of the model TAA within the tumor cells (Fig. 7). These results are similar to the data published by Kündig et al. (42), who described the ability to maintain a specific DTH response against VSV-NP peptide in immune mice challenged periodically with a NP-transduced EL4 tumor cell line but not with an EL4neo control cell line.
Several considerations arise from our results. The first deals with the relationship between the accessibility of tumor cells to the circulating CTL and the amplification of the immune response observed in the present study. The second, related and more important, consideration is the prediction that a low tumor burden, not capable of affecting the immune response against the model TAA, might require a higher production of TAA via recombinant viruses or its prolonged persistence over time for a full therapeutic effect. A first suggestion that this is indeed the case comes from preliminary experiments in which a low number of tumor cells was injected. In this case, IL-2 was able to increase the survival of mice treated with the rVV expressing high levels of β-gal but not that of mice treated with rVV expressing low levels(data not shown). However, a complete answer will come from the comparison of the immune response in mice presenting different burdens and/or localizations of the experimental tumor treated with rVV producing variable amount of TAA as a consequence of the use of different vaccinia promoters(V. Bronte, M. Wang, M. Carroll, B. Moss, S. A. Rosenberg, and N. P. Restifo, manuscript in preparation).
Any therapeutic approach relying exclusively on the host immune defense to eradicate a rapidly growing tumor must be designed to elicit an adequate number of effector cells specifically recognizing and eliminating the tumor cells before the tumor kills the host (36). Several cytokines can be helpful in this regard because it is known that many of them are able to enhance the immune response. However, among the different cytokines tested in this study, only IL-2 seemed to fulfill the requisites indicated above. Compared with the other drVV, lower levels of IFN-γ and TNF-α were produced after in vitro infection, a possible consequence of the direct antiviral effects of these two cytokines. However, unlike IL-2, exogenous administration of TNF-α, GM-CSF, and IFN-γ doses that were previously shown to exert antitumor activity did not change the in vivo therapeutic efficacy of rVV-expressing β-gal (J. B. Rao, unpublished observations), which makes it difficult to explain our findings on the sole basis of the amount of cytokines released during infection.
It is commonly believed that IL-2 rVV does not alter the magnitude of the antivaccinia or antirecombinant protein immune response, either humoral or cellular, in immune competent mice (43-45). This is in contrast with the increase in primary cytotoxic activity against vaccinia determinants detected in spleens of mice immunized with IL-2 drVV (Fig. 6). Nonetheless, the previous IL-2 drVV constructs markedly differ from IL-2-rVV used in this study (hemoagglutinin and TK disruption vs TK alone; p7.5 promoter driving the IL-2 production vs psynthetic late; see Refs. 25 and 46). Interestingly, when a drVV producing about 100-fold less IL-2 but a higher amount of model TAA than our drVV was used to treat the 3-day-old pulmonary metastases, no significant survival benefit was observed above the rVV only expressing the β-gal (data not shown), suggesting that the quantity of cytokine released could be important for successful treatment of established disease.
Expression of IL-2 in a rVV construct resulted in the resolution of viral infection in athymic nude mice that normally succumb to the exposure of the same rVV without the IL-2 gene. IL-2 production at the site of viral replication was found to be essential because these results could not be reproduced with passively administered IL-2 (27, 47). Our results indicate that exogenous IL-2 increases the therapeutic effect of rVV, but local production may also play a role because, when compared directly, the treatment regimen including exogenous rIL-2 administered with IL-2-secreting drVV was more effective than exogenous rIL-2 in combination with a rVV only expressing the model TAA (Fig. 4). The explanation for this is not known and we can only hypothesize that this effect is dependent on IL-2 released in the lung, one of the earliest sites of viral replication after i.v. injection of TK− viruses (48).
With few exceptions, all the mice successfully treated with the combination of IL-2 and rVV died. But surprisingly, in all the cases examined (Fig. 1, group treated with high rIL-2 dose and VJS6; Fig. 5, group treated with exogenous rIL-2 and IL-2 rVV), lung metastases did not stain or stained poorly after exposure to X-gal solution, indicating that, in the presence of a powerful immune response, escape variants are selected that are responsible for the death of mice. The reasons for this down-regulation of β-gal production are currently being investigated but it is possible to exclude a genetic loss because after in vitro culture the tumor cells can recover β-gal positivity; moreover, concurrent defects in H-2 class I levels can be ruled out, as freshly isolated β-gal-negative metastases are fully recognized by specific CTL when pulsed with the β-gal nonamer peptide (V. Bronte, unpublished observations). These preliminary results suggest that: 1) virtually all the tumor cells expressing the model TAA were eliminated by the host immune response; 2) even after prolonged in vivo growth the tumor cell line is still not able to elicit a therapeutic immune response against TAA different from β-gal.
Descriptions of rVV-induced regression of established tumors are sporadic despite an ample literature showing the efficacy of rVV immunization to protect against a subsequent tumor challenge(12, 14, 48). Reduced tumor growth was observed in mice inoculated s.c. with 5 × 105 CEA-transduced MC-38 mouse colon adenocarcinoma cells and vaccinated with rVV-CEA by tail scarification at days 7, 14, and 21. Among these mice, 30% failed to develop tumor for 4 mo. No effect was obtained with the nontransduced tumors or with acontrol rVV lacking the CEA gene (40). Fewer examples are available concerning the therapeutic efficacy of rVV on established metastases. In a previous report, vaccination was performed by tail scarification with a rVV that expressed the human melanoma-associated glycoprotein p97, 2 days after i.v. injection of 5 × 104 K1735 murine melanoma cells transfected with a p97 expression plasmid. The p97-rVV-immunized mice lived a mean of 14 days longer than control-infected mice and 20% of them survived for 3 mo (49). Multiple virus injections were used in previous studies. When we compared two injections of IL-2 rVV or VJS6 vs a single virus injection, no significant increase in survival was observed (data not shown). The reason for this discrepancy is not known, but it might be dependent on the fast growth rate of the CT26 cell line, limiting the number and the interval between subsequent injections.
The majority of the world’s population was vaccinated with VV before 1980, during the WHO smallpox eradication program. Thus, a large portion of possible candidates for treatment could present an impaired response following vaccination because previous exposure to VV strongly reduces the generation of Abs and CTL against recombinant proteins expressed by rVV (11, 50). These findings, together with the severe complications occurring after immunization with VV, particularly in immunocompromised individuals (51), may limit the use of rVV as a carrier of TAA. However, the present results indicate that the therapeutic enhancement achieved with passively administered rIL-2 is not restricted to rVV because it affected equally well treatment with rFPV expressing the model TAA. FPV may be an ideal vaccine candidate because it is replication defective in mammalian cells, assures high amounts of heterologous protein production, and exhibits a low if any cross-reactivity with VV determinants. However, a direct comparison between the relative efficacy of rVV vs rFPV was not conducted in the present study and will be addressed in future experiments also involving the use of double recombinant FPV co-expressing IL-2.
In conclusion, the present report describes a treatment protocol performed on the basis of the coordinate administration of recombinant viruses and IL-2 that could be useful for the treatment of pathologic conditions in which a rapid and massive generation of effector CTL is required to control the disease.
Acknowledgments
The authors thank K. Irvine for the expert advice, P. Spiess and D. Jones for help with the animal experiments, and B. Moss and M. Carroll for critical reagents and helpful discussion.
Abbreviations used in this paper
- TAA
tumor-associated Ags
- VV
vaccinia virus
- FPV
fowlpox virus
- GM-CSF
granulocyte-macrophage CSF
- drVV
double recombinant vaccinia virus
- TK
thymidine kinase
- β-gal
β-galactosidase
- NP
nucleoprotein
- PFU
plaque-forming unit
References
- 1.De Plaen E, Lurquin C, Van Pel A, Mariamé B, Szikora JP, Wölfel T, Sibille C, Chomez P, Boon T. Immunogenic (tum−) variants of mouse tumor P815: cloning of the gene of tum− antigen P91A and identification of the tum− mutation. Proc Natl Acad Sci USA. 1988;85:2274. doi: 10.1073/pnas.85.7.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brichard V, Van Pel A, Wolfel T, Wolfel C, De Plaen E, Lethe B, Coulie P, Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox AL, Skipper J, Chen Y, Henderson RA, Darrow TL, Shabanowitz J, Engelhard VH, Hunt DF, Slingluff CL., Jr Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994;264:716. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E, Yannelli JR, Adema GJ, Miki T, Rosenberg SA. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci USA. 1994;91:6458. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Rivoltini L, Topalian SL, Miki T, Rosenberg SA. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci USA. 1994;91:3515. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins PF, El-Gamil M, Kawakami Y, Stevens E, Yannelli JR, Rosenberg SA. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res. 1994;54:3124. published erratum appears in Cancer Res. 1994 Jul 15; 54(14):3952. [PubMed] [Google Scholar]
- 7.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, Simpson C, Carter C, Bock S, Schwartzentruber D, Wei JP, White DE. Use of tumor infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma: preliminary report. N Engl J Med. 1988;319:1676. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 8.Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991;252:1662. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- 9.Moss B. Poxvirus vectors: cytoplasmic expression of transferred genes. Curr Opin Genet Dev. 1993;3:86. doi: 10.1016/s0959-437x(05)80346-6. [DOI] [PubMed] [Google Scholar]
- 10.Mackett M, Smith GL, Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci USA. 1982;79:7415. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rooney JF, Wohlenberg C, Cremer KJ, Moss B, Notkins AL. Immunization with a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: long-term protection and effect of revaccination. J Virol. 1988;62:1530. doi: 10.1128/jvi.62.5.1530-1534.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lathe R, Kieny MP, Gerlinger P, Clertant P, Guizani I, Cuzin F, Chambon P. Tumour prevention and rejection with recombinant vaccinia. Nature. 1987;326:878. doi: 10.1038/326878a0. [DOI] [PubMed] [Google Scholar]
- 13.Blancou J, Kieny MP, Lathe R, Lecocq JP, Pastoret PP, Soulebot JP, Desmettre P. Oral vaccination of the fox against rabies using a live recombinant vaccinia virus. Nature. 1986;322:373. doi: 10.1038/322373a0. [DOI] [PubMed] [Google Scholar]
- 14.Meneguzzi G, Kieny MP, Lecocq JP, Chambon P, Cuzin F, Lathe R. Vaccinia recombinants expressing early bovine papilloma virus (BPV1) proteins: retardation of BPV1 tumour development. Vaccine. 1990;8:199. doi: 10.1016/0264-410x(90)90045-n. [DOI] [PubMed] [Google Scholar]
- 15.Taylor J, Weinberg R, Languet B, Desmettre P, Paoletti E. Recombinant fowlpox virus inducing protective immunity in non-avian species. Vaccine. 1988;6:497. doi: 10.1016/0264-410x(88)90100-4. [DOI] [PubMed] [Google Scholar]
- 16.Baxby D, Paoletti E. Potential useof non-replicating vectors as recombinant vaccines. Vaccine. 1992;10:8. doi: 10.1016/0264-410x(92)90411-c. [DOI] [PubMed] [Google Scholar]
- 17.Somogyi P, Frazier J, Skinner MA. Fowlpox virus host range restriction: gene expression, DNA replication, and morphogenesis in nonpermissive mammalian cells. Virology. 1993;197:439. doi: 10.1006/viro.1993.1608. [DOI] [PubMed] [Google Scholar]
- 18.Heaton KM, Grimm EA. Cytokine combinations in immunotherapy for solid tumors: a review. Cancer Immunol Immunother. 1993;37:213. doi: 10.1007/BF01518513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardoll DM. Cancer vaccines. Immunol Today. 1993;14:310. doi: 10.1016/0167-5699(93)90051-L. [DOI] [PubMed] [Google Scholar]
- 20.Fitch FW, McKisic MD, Lancki DW, Gajewski TF. Differential regulation of murine T lymphocyte subsets. Annu Rev Immunol. 1993;11:29. doi: 10.1146/annurev.iy.11.040193.000333. [DOI] [PubMed] [Google Scholar]
- 21.Kawamura H, Rosenberg SA, Berzofsky JA. Immunization with antigen and interleukin 2 in vivo overcomes Ir gene low responsiveness. J Exp Med. 1985;162:381. doi: 10.1084/jem.162.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Restifo NP, Kawakami Y, Marincola F, Shamamian P, Taggarse A, Esquivel F, Rosenberg SA. Molecular mechanisms usedby tumors to escape immune recognition: immunogene-therapy and the cell biology of major histocompatibility complex class I. J Immunother. 1993;14:182. doi: 10.1097/00002371-199310000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flexner C, Moss B, London WT, Murphy R. Attenuation and immunogenicity in primates of vaccinia virus recombinants expressing human interleukin-2. Vaccine. 1990;8:17. doi: 10.1016/0264-410x(90)90171-h. [DOI] [PubMed] [Google Scholar]
- 26.Sambhi SK, Kohonen-Corish MR, Ramshaw IA. Local production of tumor necrosis factor encoded by recombinant vaccinia virus is effective in controlling viral replication in vivo. Proc Natl Acad Sci USA. 1991;88:4025. doi: 10.1073/pnas.88.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramshaw I, Ruby J, Ramsay A, Ada G, Karupiah G. Expression of cytokines by recombinant vaccinia viruses: a model for studying cytokines in virus infections in vivo. Immunol Rev. 1992;127:157. doi: 10.1111/j.1600-065x.1992.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang M, Bronte V, Chen PW, Gritz L, Pameali D, Rosenberg SA, Restifo NP. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J Immunol. 1995;154:4685. [PMC free article] [PubMed] [Google Scholar]
- 28.Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith GL, Levin JZ, Palese P, Moss B. Synthesis and cellular location of the ten influenza polypeptides individually expressed by recombinant vaccinia viruses. Virology. 1987;160:336. doi: 10.1016/0042-6822(87)90004-3. [DOI] [PubMed] [Google Scholar]
- 30.Whitman ED, Tsung K, Paxson J, Norton JA. In vitro and in vivo kinetics of recombinant vaccinia virus cancer-gene therapy. Surgery. 1994;116:183. [PubMed] [Google Scholar]
- 31.Jenkins S, Gritz L, Fedor CH, O’Neill EM, Cohen LK, Panicali DL. Formation of lentivirus particles by mammalian cells infected with recombinant fowlpox virus. AIDS Res Hum Retroviruses. 1991;7:991. doi: 10.1089/aid.1991.7.991. [DOI] [PubMed] [Google Scholar]
- 32.Gavin MA, Gilbert MJ, Riddell SR, Greenberg PD, Bevan MJ. Alkali hydrolysis of recombinant proteins allows for the rapid identification of class I MHC-restricted CTL epitopes. J Immunol. 1993;151:3971. [PubMed] [Google Scholar]
- 33.Restifo NP, Esquivel F, Asher AL, Stötter H, Barth RJ, Bennink JR, Mulé JJ, Yewdell JW, Rosenberg SA. Defective presentation of endogenous antigens by a murine sarcoma: implications for the failure ofan anti-tumor immune response. J Immunol. 1991;147:1453. [PMC free article] [PubMed] [Google Scholar]
- 34.Lin WC, Pretlow TP, Pretlow TG, Culp LA. Bacterial lacZ gene as a highly sensitive marker to detect micrometastasis formation during tumor progression. Cancer Res. 1990;50:2808. [PubMed] [Google Scholar]
- 35.Davison AJ, Moss B. New vaccinia virus recombination plasmids incorporating a synthetic late promoter for high level expression of foreign proteins. Nucleic Acids Res. 1990;18:4285. doi: 10.1093/nar/18.14.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- 37.Bennink JR, Yewdell JW. Recombinant vaccinia viruses as vectors for studying T lymphocyte specificity and function. Curr Top Microbiol Immunol. 1990;163:153. doi: 10.1007/978-3-642-75605-4_6. [DOI] [PubMed] [Google Scholar]
- 38.Sanes JR, Rubenstein JL, Nicolas JF. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986;5:3133. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kundig TM, Bachmann MF, Lefrancois L, Puddington L, Hengartner H, Zinkernagel RM. Nonimmunogenic tumor cells may efficiently restimulate tumor antigen-specific cytotoxic T cells. J Immunol. 1993;150:4450. [PubMed] [Google Scholar]
- 40.Kantor J, Irvine K, Abrams S, Kaufman H, DiPietro J, Schlom J. Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen-vaccinia virus vaccine. J Natl Cancer Inst. 1992;84:1084. doi: 10.1093/jnci/84.14.1084. [DOI] [PubMed] [Google Scholar]
- 41.Fearon ER, Pardoll DM, Itaya T, Golumbek P, Levitsky HI, Simons JW, Karasuyama H, Vogelstein B, Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990;60:397. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- 42.Kundig TM, Althage A, Hengartner H, Zinkernagel RM. Skin test toassess virus-specific cytotoxic T-cell activity. Proc Natl Acad Sci USA. 1992;89:7757. doi: 10.1073/pnas.89.16.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullbacher A, Ramshaw IA, Coupar BE. Vaccinia-interleukin 2 recombinant virus or exogenous interleukin 2 does not alter the magnitude or immune response gene defects of the cytotoxic T-cell response to vaccinia virus in vivo. Scand J Immunol. 1989:29–1. doi: 10.1111/j.1365-3083.1989.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 44.Karupiah G, Woodhams CE, Blanden RV, Ramshaw IA. Immunobiology of infection with recombinant vaccinia virus encoding murine IL-2: mechanisms of rapid viral clearance in immunocompetent mice. J Immunol. 1991;147:4327. [PubMed] [Google Scholar]
- 45.Ruby J, Brinkman C, Jones S, Ramshaw I. Response of monkeys to vaccination with recombinant vaccinia virus which coexpress HIV gp160 and human interleukin-2. Immunol Cell Biol. 1990;68:113. doi: 10.1038/icb.1990.16. [DOI] [PubMed] [Google Scholar]
- 46.Ramshaw IA, Andrew ME, Phillips SM, Boyle DB, Coupar BE. Recovery of immunodeficient mice from a vaccinia virus/IL-2 recombinant infection. Nature. 1987;329:545. doi: 10.1038/329545a0. [DOI] [PubMed] [Google Scholar]
- 47.Ramsay AJ, Ruby J, Ramshaw IA. A case for cytokines as effector molecules in the resolution of virus infection. Immunol Today. 1993;14:155. doi: 10.1016/0167-5699(93)90277-R. [DOI] [PubMed] [Google Scholar]
- 48.Elkins KL, Ennist DL, Winegar RK, Weir JP. In vivo delivery of interleukin-4 by a recombinant vaccinia virus prevents tumor development in mice. Hum Gene Ther. 1994;5:809. doi: 10.1089/hum.1994.5.7-809. [DOI] [PubMed] [Google Scholar]
- 49.Estin CD, Stevenson US, Plowman GD, Hu SL, Sridhar P, Hellstrom I, Brown JP, Hellstrom KE. Recombinant vaccinia virus vaccine against the human melanoma antigen p97 for use in immunotherapy. Proc Natl Acad Sci USA. 1988;85:1052. doi: 10.1073/pnas.85.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kundig TM, Kalberer CP, Hengartner H, Zinkernagel RM. Vaccination with two different vaccinia recombinant viruses: long-term inhibition of secondary vaccination. Vaccine. 1993;11:1154. doi: 10.1016/0264-410x(93)90079-d. [DOI] [PubMed] [Google Scholar]
- 51.Redfield RR, Wright DC, James WD, Jones TS, Brown C, Burke DS. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987;316:673. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]


