Abstract
Objective
Patients presenting with an episode of preterm labor that subsides in response to tocolysis and who subsequently deliver at term are considered to have false preterm labor. However, the episode of “preterm labor” may represent the uterine response (i.e., uterine contractions) to an insult that was not severe enough to trigger preterm parturition but which may put the fetus at risk for additional pregnancy complications including growth restriction. The objective of this study was to compare the frequency of small for gestational age (SGA) neonates among patients with an episode of increased uterine contractility who delivered at term and those who delivered preterm.
Study design
This retrospective cohort study included 849 patients. Inclusion criteria were: 1) regular uterine contractions that required hospitalization; 2) intact membranes; 3) gestational age between 20 and 36 weeks. SGA was defined as a birth weight <10th percentile for gestational age. Placental pathology was reviewed and the results were used to classify patients into an inflammatory cluster, vascular cluster or both. Contingency tables, Mann-Whitney U test, and multivariate logistic regression were used for statistical analyses. A p-value of <0.05 was considered significant.
Results
1) The prevalence of SGA neonates in the study population was 16.1% (124/772); 2) patients who delivered at term had a significantly higher frequency of SGA neonates than those who delivered preterm [21.5% (64/298) vs. 12.7% (60/474); p=0.001]; 2) the results of placental pathology were available in 63.7% (492/772) of patients. Patients who delivered at term had a higher frequency of fetal or maternal vascular lesions without histologic evidence of inflammation than those who delivered preterm [29.1 % (43/148) vs. 18.9% (65/344); p=0.01]; and 3) term delivery after an episode of regular preterm uterine contractions was associated with an odds ratio of 2.22 (95% CI: 1.28-3.85) to deliver an SGA neonate after controlling for confounding variables. A sub-analysis limited to patients who received tocolysis showed similar results.
Conclusions
1) patients with an episode of increased uterine contractility that subsided and delivered at term are at risk for delivering an SGA neonate; 2) this suggests that an episode of false preterm labor is not a benign condition; and 3) we propose that insults to the feto-placental unit may be resolved by either irreversible preterm parturition or restricting fetal growth.
Keywords: increased uterine contractility, intact membranes, small for gestational age, placental pathology, vascular cluster, inflammatory cluster, term delivery
INTRODUCTION
Term delivery after hospitalization for spontaneous preterm labor occurs in 34-45% of patients with “idiopathic” preterm labor.1 These patients are often considered to have had an episode of “false preterm labor”.1 An alternative view is that symptoms of preterm labor, such as increased uterine contractility, may result from a pathologic insult whose nature and/or severity was not sufficient to induce irreversible spontaneous preterm parturition. If this is the case, neonates born to mothers with an episode of increased uterine contractility who required hospitalization may be at risk for neonatal complications not attributable to preterm birth.
Previous studies had demonstrated that spontaneous2 and indicated preterm labor2-4 are associated with an excess of SGA neonates and that a high proportion of fetuses destined to be delivered preterm do not reach their individual growth potential.5 Moreover, abnormalities of the supply line, such as maternal and/or fetal vascular pathology, have been implicated in the etiology of spontaneous preterm birth.6-8 Therefore, we propose that patients who have an episode of increased uterine contractility may be at risk for fetal growth deceleration and the delivery of a small for gestational age (SGA) neonate. If this were the case, an episode of spontaneous preterm labor that does not progress to preterm delivery may not be a benign event. Indeed, such an episode may serve to identify patients who require further surveillance, not only because of their risk for spontaneous preterm labor/delivery, but also for fetal growth disorders. Thus, the objective of this study was to determine the frequency of SGA neonates in women with an episode of increased uterine contractility that was severe enough to require hospitalization.
MATERIAL AND METHODS
Study design
This retrospective cohort study included patients enrolled in an observational study with the diagnosis of spontaneous preterm labor from February of 1992 until February of 2006 at Hutzel Women’s Hospital in Detroit, Michigan. Inclusion criteria were: 1) suspected preterm labor requiring hospitalization; 2) intact membranes; and 3) gestational age between 20 and 36 weeks; and 4) written informed consent for the collection of clinical information for research purposes. Patients with multiple pregnancies, fetal anomalies, diabetes mellitus, chronic hypertension, sickle cell disease and those who had a cerclage placed in the index pregnancy were excluded. Cervical dilation and effacement were determined by digital examination. SGA was defined as a birthweight (BW) <10th percentile for gestational age according a national birth weight distribution.9 Body mass index (BMI) was calculated as follows: BMI = [weight (in pounds) × 703)/height2 (in inches)].10 A low pre-pregnancy BMI was defined as <18.5.10 Beta-mimetic agents and/or magnesium sulfate were given intravenously for tocolysis. Steroids were administered between 24 and 34 weeks at the discretion of the attending physician. All women provided written informed consent for the collection of clinical data under protocols approved by the Institutional Review Boards of both Wayne State University and the National Institute of Child Health and Human Development of the National Institute of Health (NIH/DHHS).
Placental pathology
The indications for placental examination were those recommended by the guidelines of the American College of Pathologists.11 Placental tissues were fixed in 10% neutral formalin for 24 hours and then paraffin embedded. Hematoxylin-Eosin staining was performed on 5 micron-thick paraffin sections, and the slides were reviewed according to the diagnostic criteria described elsewhere.12 At least five sections from the placenta, three sections from the chorioamniotic membranes, and three sections from the umbilical cord were submitted for histological review. The results of the placental histology allowed classification of patients into: 1) an inflammatory cluster that included the maternal and fetal response (e.g., acute chorioamnionitis, funisitis, and chorionic vasculitis); and 2) a vascular cluster with findings consistent with maternal underperfusion (e.g., villous infarcts, increased syncitial knots, villous agglutination, increased intervillous fibrin, and absence of physiologic transformation of the decidual spiral arteries or atherosis of the decidual vessels) and/or fetal vascular thrombo-occlusive disease (e.g., villous stromal karyorrhexis, hyalinized avascular villi, large fetal vessel thrombosis, intimal fibrin cushions, fibromuscular sclerosis and chronic villitis with obliterative fetal vasculopathy).
Statistical analysis
Comparisons of proportions were performed using chi-square tests. Mann-Whitney U tests were used to contrast continuous variables which were non-normally distributed. Logistic regression analysis was used to explore the relationship between the occurrence of SGA and the following explanatory variables: term delivery after an episode of increased uterine contractility, maternal age ≥35 years,13 nulliparity,14 smoking status,15;16 ethnicity,13 and the administration of tocolysis, steroids, and antibiotics. Pre-pregnancy weight and height were available in 48.5% (374/772) of patients. Stepwise logistic regression analysis was performed to determine if a low pre-pregnancy BMI (<18.5) was an additional explanatory variable17;18 for the occurrence of SGA. A sub-analysis limited to patients who received tocolysis was performed. The risk to deliver an SGA neonate, derived from the logistic regression analysis, was plotted against gestational age at delivery, and was correlated with the time elapsed between the episode of increased uterine contractility and delivery. The statistical package used was SPSS v12.0 (SPSS Inc., Chicago, IL, USA), and a p-value of <0.05 was considered significant.
RESULTS
This study included 849 patients with suspected preterm labor requiring hospitalization, of which 9.1% (77/849) were lost to follow-up. Preterm delivery (defined as birth < 37 weeks) occurred in 61.4% (474/772) and term delivery in 38.6% (298/772) of patients. There was no significant difference in the neonatal gender between patients who delivered at term and those who delivered preterm. The demographic and clinical characteristics of the population are displayed in Tables I and II.
Table I.
Demographic and clinical characteristics of patients with increased uterine contractility according to term or preterm delivery
| Term delivery
(n=298) |
Preterm delivery
(n=474) |
p | |
|---|---|---|---|
| Maternal age (years) | 22
(14-40) |
23
(13-44) |
0.01 |
| Ethnic group (%) | |||
| African-American | 86.2 | 86.7 | |
| Caucasian | 10.4 | 10.5 | NS |
| Hispanic | 1.3 | 1.1 | |
| Asian | 0.3 | 0.6 | |
| Other | 1.7 | 1.1 | |
| Tocolysis | 76.2
(227/298) |
86.5
(410/474) |
<0.001 |
| Steroids | 47.7
(142/298) |
68.4
(324/474) |
<0.001 |
| Antibiotics | 41.3
(123/298) |
62.0
(294/474) |
<0.001 |
The results are expressed as percentage (proportions) and median (range).
Table II.
Clinical characteristics of the study population according to term or preterm delivery
| Term delivery
(n=298) |
Preterm delivery
(n=474) |
p | |
|---|---|---|---|
| Gestational age at enrollment (weeks) | 31.8
(20-36.4) |
30.2
(20.6-36.7) |
<0.001 |
| Gestational age at delivery (weeks) | 38.6
(37-41.8) |
33.1
(23-36.8) |
<0.001 |
| Birthweight (grams) | 2,992
(1,890-4,337) |
1,900
(426-3,520) |
<0.001 |
| Nulliparity (%) | 28.2
(84/298) |
33.8
(160/474) |
NS |
| Proportion of SGA neonates | 21.5
(64/298) |
12.7
(60/474) |
0.001 |
The results are expressed as percentage (proportions) and median (range). SGA: small for gestational age.
Intra-venous tocolysis was administered in 82.5% (637/772) of patients. Among patients who received tocolysis, the prevalence of preterm delivery was 64.4 (410/637) and that of term delivery was 35.6% (227/637). There was no significant difference in the rate of preterm delivery between the overall population and those who received tocolysis.
The results of the digital examination of the cervix was retrieved from 97.2% (750/772) of the medical records; 32.3% (144/446) of patients who met the conventional definition of preterm labor19;20 (cervical dilation ≥ 2cm and/or effacement ≥ 80%), delivered at term. The prevalence of preterm delivery in this subset of patients was 67.7% (302/446).
Prevalence of the study outcome
The prevalence of SGA neonates in the study population was 16.1% (124/772). Patients who delivered at term had a significantly higher frequency of SGA neonates than those who delivered preterm [21.5% (64/298) vs. 12.7% (60/474); p=0.001). A sub-analysis limited to patients who received intravenous tocolysis indicated that patients who delivered at term had a higher frequency of SGA neonates than those who delivered preterm [19.8% (45/227) vs. 11.2% (46/410); p=0.003]. Among patients who met the standard clinical criteria of preterm labor, the prevalence of SGA neonates in patients who had a term delivery was significantly higher than in those who delivered preterm [17.4% (25/144) vs. 9.9% (30/302); p=0.03]. A post-hoc power analysis indicated that this study had a 91% power to determine a 9% difference in the prevalence of SGA neonates among patients who delivered at term and those who delivered preterm.
Risk of SGA after adjustment for confounding factors
Term delivery after an episode of increased uterine contractility was associated with an odds ratio of 2.22 (95% CI: 1.28-3.85) to deliver an SGA neonate after controlling for maternal age ≥35 years, nulliparity, smoking status, ethnicity, and the administration of tocolysis, steroids, and antibiotics. A stepwise logistic regression analysis, which included pre-pregnancy BMI <18.5 as an additional covariate, indicated that term delivery after an episode of preterm labor was associated with and odds ratio of 3.1 (95% CI: 1.61-6.08) to deliver an SGA neonate. The risk of delivering an SGA neonate increased as a function of the gestational age at delivery (Figure 1). Moreover, there was a significant correlation between the risk of delivering an SGA neonate and the time elapsed from the episode of increased uterine contractility to delivery (Spearman’s rho correlation coefficient: 0.49; p<0.001).
Figure 1.
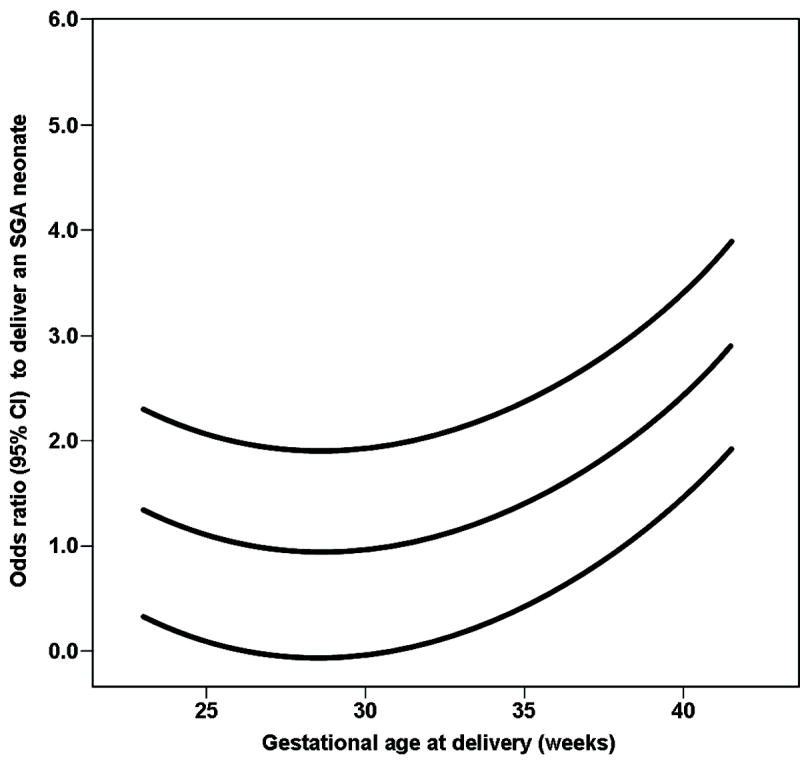
The risk to deliver an SGA neonate, after an episode of preterm labor, increasedwith gestational age at delivery. The lines represent the odds ratio and 95% confidenceinterval to deliver an SGA neonate derived from a multivariate logistic regression analysis.
Among patients who received tocolysis, term delivery after an episode of false preterm labor was associated with an odds ratio of 2.5 (95% CI: 1.34-4.79) to deliver an SGA neonate after controlling for the above mentioned covariates.
Placental pathology
The results of placental pathology were available in 63.7% (492/772) of patients. Patients who delivered at term had a higher frequency of fetal and/or maternal vascular lesions (without histologic evidence of inflammation) than those who delivered preterm [29.1 % (43/148) vs. 18.9% (65/344); p=0.01]. Table III displays the placental histologic findings according to the timing of delivery.
Table III.
Results of placental pathology according to term or preterm delivery
| Term delivery
(n=148) |
Preterm delivery
(n=344) |
p | |
|---|---|---|---|
| No vascular or inflammatory lesions | 50
(74/148) |
36
(124/344) |
0.01 |
| Vascular lesions only | 29.1
(43/148) |
18.9
(65/344) |
0.02 |
| Inflammatory lesions only | 13.5
(20/148) |
30.5
(105/344) |
<0.001 |
| Inflammatory and vascular lesions | 7.4
(11/148) |
14.5
(50/344) |
0.04 |
The results are expressed as percentage (proportions).
COMMENTS
Main findings of the study
Patients with an episode of increased uterine contractility requiring hospitalization who subsequently delivered at term, regardless of whether or not the criteria for the diagnosis of preterm labor is met, are at an increased risk of delivering an SGA neonate. This observation is novel and suggests that an episode of what is commonly called “false preterm labor” is not always a benign condition.
The reversible and irreversible phase of preterm parturition
Although myometrial activity occurs throughout pregnancy, labor is characterized by a dramatic change in the pattern of uterine contractility that evolves from “contractures” to “contractions.”21;22 The switch from a predominant “contracture” pattern to a predominant “contraction” pattern occurs physiologically during normal labor23 or can be induced by pathologic events such as food withdrawal,24-26 infection27 or maternal intra-abdominal surgery.28-30 Experimental studies have demonstrated that this switch can be a reversible phenomenon. For example, fasting in pregnant sheep can induce a “contracture” pattern that can be reversed by feeding.25 However, if the other components of the common pathway of human parturition [e.g., cervical ripening (dilation and effacement) and decidual/membrane activation] are recruited, preterm labor may reach an irreversible phase and parturition may occur. This outcome may depend on the nature, duration and intensity of the intrauterine insult as well as the fetal and maternal host responses to the insults.
One interpretation of our results is that an episode of increased uterine contractility that led to hospitalization may be the result of an insult that failed to induce the irreversible phase of preterm parturition. However, this insult may be caused by a compromised supply line (e.g., a vascular abnormality), which altered placental function with consequent fetal growth deceleration and delivery of an SGA neonate.
It is tempting to postulate that gene-environment interactions may partially account for this. For example, polymorphisms for anti-inflammatory cytokines have recently been associated with an increased risk to deliver an SGA neonate and spontaneous preterm delivery.31 Specifically, women who carry the “high producing” IL-4 (-589) T variant had an increased risk to deliver an SGA neonate, whereas patients carrying the IL-4 GCC haplotype had an increased risk for spontaneous preterm delivery [OR: 2.9 (95% CI: 1.2-7.4)].31 Therefore, maternal and/or fetal genetic factors may determine the response to an environmental insult.
What is the mechanism of disease whereby an episode of increased uterine contractility is associated with SGA?
Maternal and fetal vascular lesions in the placenta have been previously associated with fetal growth restriction32-35 and preterm labor and delivery.6-8 Indeed, Arias et al. reported that maternal vasculopathy (defined as the presence of failure of physiologic transformation of the decidual portion of the spiral arteries and organized thrombi, multiple placental infarcts, multiple syncitial knots and uneven accelerated villi maturation) was more frequent in placentas from women with preterm parturition than in those from normal patients.6 More recently, Kim et al. reported that failure of physiologic transformation of the myometrial portion of the spiral arteries, fetal thrombotic vasculopathy and decidual vessel thrombosis were more frequent in placental bed biopsies and placentas from patients with preterm labor and intact membranes who delivered preterm than those from normal patients.8 Since patients with an episode of “false preterm labor” had a higher frequency of maternal and/or fetal vascular lesions in the placenta (without histologic inflammation) than patients who delivered preterm, it is possible that vascular insults may have contributed to the occurrence of both the episode of preterm labor and the delivery of an SGA neonate.
Vascular lesions associated with histologic evidence of inflammation were not included in the analysis because between 7-20% of inflammatory lesions in the placenta are accompanied by vascular lesions in preterm parturition.6;7 Thus, it is difficult to determine if these vascular lesions were associated with the episode of increased uterine contractility or were secondary to the inflammatory process.
Strengths and weakness of the study
A major strength of the study is that the principal finding was observed with consistency among women who met the diagnostic criteria of preterm labor and those who were given intravenous tocolysis by the attending physicians. An important limitation of this study is its retrospective nature and that not all placentas were examined. Thus, prospective studies may be needed to confirm the results presented herein.
Clinical implications
Patients with symptoms of preterm labor may require further surveillance, not only because of their risk for spontaneous preterm delivery, but also because they are at an increased risk for delivering an SGA neonate.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grover CM, Posner S, Kupperman M, Washington EA. Term delivery after hospitalization for preterm labor: incidence and costs in california. Prim Care Update Ob Gyns. 1998;5:178. doi: 10.1016/s1068-607x(98)00086-9. [DOI] [PubMed] [Google Scholar]
- 2.Morken NH, Kallen K, Jacobsson B. Fetal growth and onset of delivery: a nationwide population-based study of preterm infants. Am J Obstet Gynecol. 2006;195:154–61. doi: 10.1016/j.ajog.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Ott WJ. Intrauterine growth retardation and preterm delivery. Am J Obstet Gynecol. 1993;168:1710–15. doi: 10.1016/0002-9378(93)90681-8. [DOI] [PubMed] [Google Scholar]
- 4.MacGregor SN, Sabbagha RE, Tamura RK, Pielet BW, Feigenbaum SL. Differing fetal growth patterns in pregnancies complicated by preterm labor. Obstet Gynecol. 1988;72:834–37. doi: 10.1097/00006250-198812000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Bukowski R, Gahn D, Denning J, Saade G. Impairment of growth in fetuses destined to deliver preterm. Am J Obstet Gynecol. 2001;185:463–67. doi: 10.1067/mob.2001.115865. [DOI] [PubMed] [Google Scholar]
- 6.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–91. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 7.Arias F, Victoria A, Cho K, Kraus F. Placental histology and clinical characteristics of patients with preterm premature rupture of membranes. Obstet Gynecol. 1997;89:265–71. doi: 10.1016/S0029-7844(96)00451-6. [DOI] [PubMed] [Google Scholar]
- 8.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–69. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 9.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–68. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 10.National Heart, Lung and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. NIH Publication No. 98-4083. 1998 [PubMed] [Google Scholar]
- 11.Altshuler G, Deppisch LM. College of American Pathologists Conference XIX on the Examination of the Placenta: report of the Working Group on Indications for Placental Examination. Arch Pathol Lab Med. 1991;115:701–03. [PubMed] [Google Scholar]
- 12.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation--a workshop report. Placenta. 2005;26(Suppl A):S114–S117. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Ananth CV, Balasubramanian B, Demissie K, Kinzler WL. Small-for-gestational-age births in the United States: an age-period-cohort analysis. Epidemiology. 2004;15:28–35. doi: 10.1097/01.ede.0000100288.37475.19. [DOI] [PubMed] [Google Scholar]
- 14.Yunis KA, Beydoun H, Tamim H. Maternal predictors of small-for-gestational age in uncomplicated births. Int J Gynaecol Obstet. 2002;79:33–35. doi: 10.1016/s0020-7292(02)00196-0. [DOI] [PubMed] [Google Scholar]
- 15.Ananth CV, Platt RW. Reexamining the effects of gestational age, fetal growth, and maternal smoking on neonatal mortality. BMC Pregnancy Childbirth. 2004;4:22. doi: 10.1186/1471-2393-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiolero A, Bovet P, Paccaud F. Association between maternal smoking and low birth weight in Switzerland: the EDEN study. Swiss Med Wkly. 2005;135:525–30. doi: 10.4414/smw.2005.11122. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe HM, Zador IE, Gross TL, Martier SS, Sokol RJ. The clinical utility of maternal body mass index in pregnancy. Am J Obstet Gynecol. 1991;164:1306–10. doi: 10.1016/0002-9378(91)90705-v. [DOI] [PubMed] [Google Scholar]
- 18.Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147–52. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 19.Creasy RK, Herron MA. Prevention of preterm birth. Semin Perinatol. 1981;5:295–302. [PubMed] [Google Scholar]
- 20.Gonik B, Creasy RK. Preterm labor: its diagnosis and management. Am J Obstet Gynecol. 1986;154:3–8. doi: 10.1016/0002-9378(86)90383-2. [DOI] [PubMed] [Google Scholar]
- 21.Hsu HW, Figueroa JP, Honnebier MB, Wentworth R, Nathanielsz PW. Power spectrum analysis of myometrial electromyogram and intrauterine pressure changes in the pregnant rhesus monkey in late gestation. Am J Obstet Gynecol. 1989;161:467–73. doi: 10.1016/0002-9378(89)90543-7. [DOI] [PubMed] [Google Scholar]
- 22.Nathanielsz P, Honnebier M. Myometrial function. In: Drife J, Calder A, editors. Prostaglandins and the Uterus. London: Springer-Verlag; 1992. p. 161. [Google Scholar]
- 23.Taylor NF, Martin MC, Nathanielsz PW, Seron-Ferre M. The fetus determines circadian oscillation of myometrial electromyographic activity in the pregnant rhesus monkey. Am J Obstet Gynecol. 1983;146:557–67. doi: 10.1016/0002-9378(83)90803-7. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan M, Eidelman AI, Aboulafia Y. Fasting and the precipitation of labor. The Yom Kippur effect. JAMA. 1983;250:1317–18. [PubMed] [Google Scholar]
- 25.Binienda Z, Rosen ED, Kelleman A, Sadowsky DW, Nathanielsz PW, Mitchell MD. Maintaining fetal normoglycemia prevents the increase in myometrial activity and uterine 13,14-dihydro-15-keto-prostaglandin F2 alpha production during food withdrawal in late pregnancy in the ewe. Endocrinology. 1990;127:3047–51. doi: 10.1210/endo-127-6-3047. [DOI] [PubMed] [Google Scholar]
- 26.Maymon E, Mazor M, Romero R, et al. The Yom Kippur effect on human parturition. Am J Obstet Gynecol. 1997;176:S115. [Google Scholar]
- 27.Romero R, Avila C, Sepulveda W, et al. The role of systemic and intrauterine infection in preterm labor. In: Fuchs A, Fuchs F, Stubblefield P, editors. Preterm Birth: Causes, Prevention, and Management. New York: McGraw-Hill; 1993. [Google Scholar]
- 28.Nathanielsz P, Poore E, Brodie A, et al. Update on molecular events of myometrial activity during pregnancy. In: Nathanielsz P, Parer J, editors. Research in Perinatal Medicine. Ithaca, NY: Perinatology; 1987. p. 111. [Google Scholar]
- 29.Allen JR, Helling TS, Langenfeld M. Intraabdominal surgery during pregnancy. Am J Surg. 1989;158:567–69. doi: 10.1016/0002-9610(89)90194-3. [DOI] [PubMed] [Google Scholar]
- 30.Kort B, Katz VL, Watson WJ. The effect of nonobstetric operation during pregnancy. Surg Gynecol Obstet. 1993;177:371–76. [PubMed] [Google Scholar]
- 31.Engel SA, Olshan AF, Savitz DA, Thorp J, Erichsen HC, Chanock SJ. Risk of small-for-gestational age is associated with common anti-inflammatory cytokine polymorphisms. Epidemiology. 2005;16:478–86. doi: 10.1097/01.ede.0000164535.36412.6b. [DOI] [PubMed] [Google Scholar]
- 32.Sheppard BL, Bonnar J. The ultrastructure of the arterial supply of the human placenta in pregnancy complicated by fetal growth retardation. Br J Obstet Gynaecol. 1976;83:948–59. doi: 10.1111/j.1471-0528.1976.tb00781.x. [DOI] [PubMed] [Google Scholar]
- 33.Sheppard BL, Bonnar J. An ultrastructural study of utero-placental spiral arteries in hypertensive and normotensive pregnancy and fetal growth retardation. Br J Obstet Gynaecol. 1981;88:695–705. doi: 10.1111/j.1471-0528.1981.tb01268.x. [DOI] [PubMed] [Google Scholar]
- 34.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. Br J Obstet Gynaecol. 1986;93:1049–59. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 35.Benirschke K, Kaufmann P. Classification of Villous Maldevelopment. In: Benirschke K, Kaufmann P, editors. Pathology of the Human Placenta. New York: Springer; 2000. pp. 437–58. [Google Scholar]


