Abstract
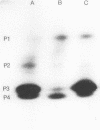
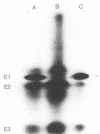
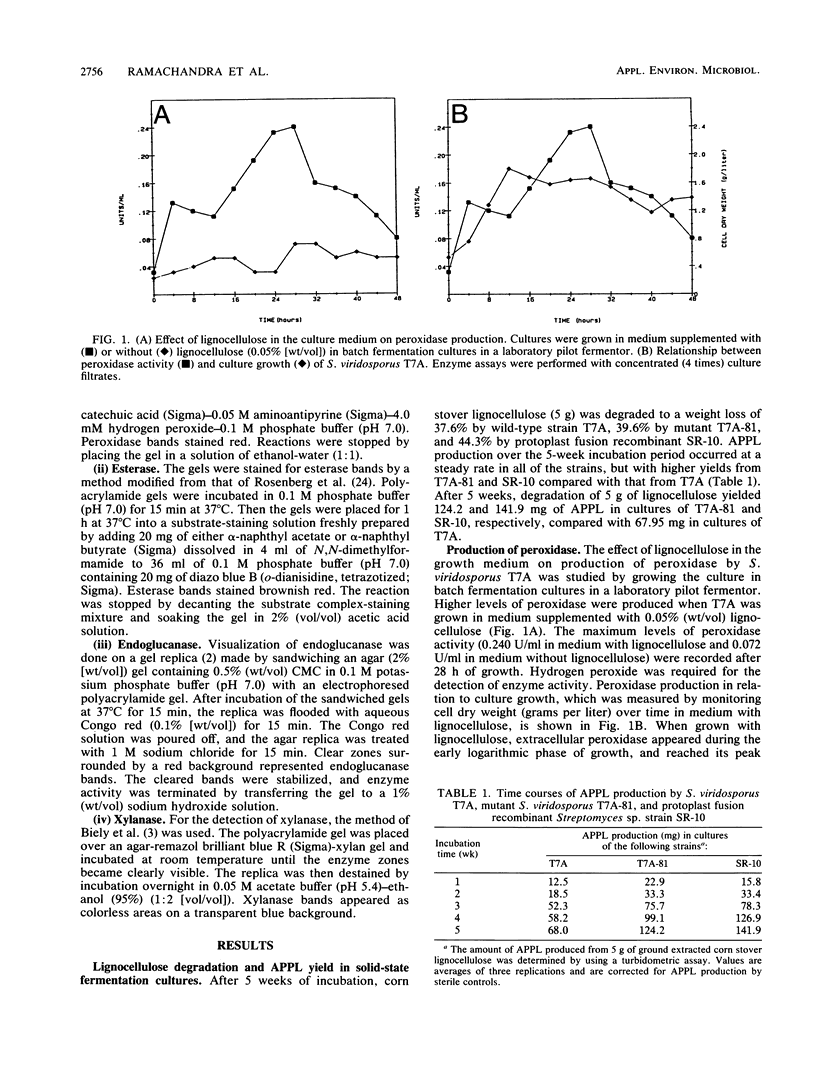
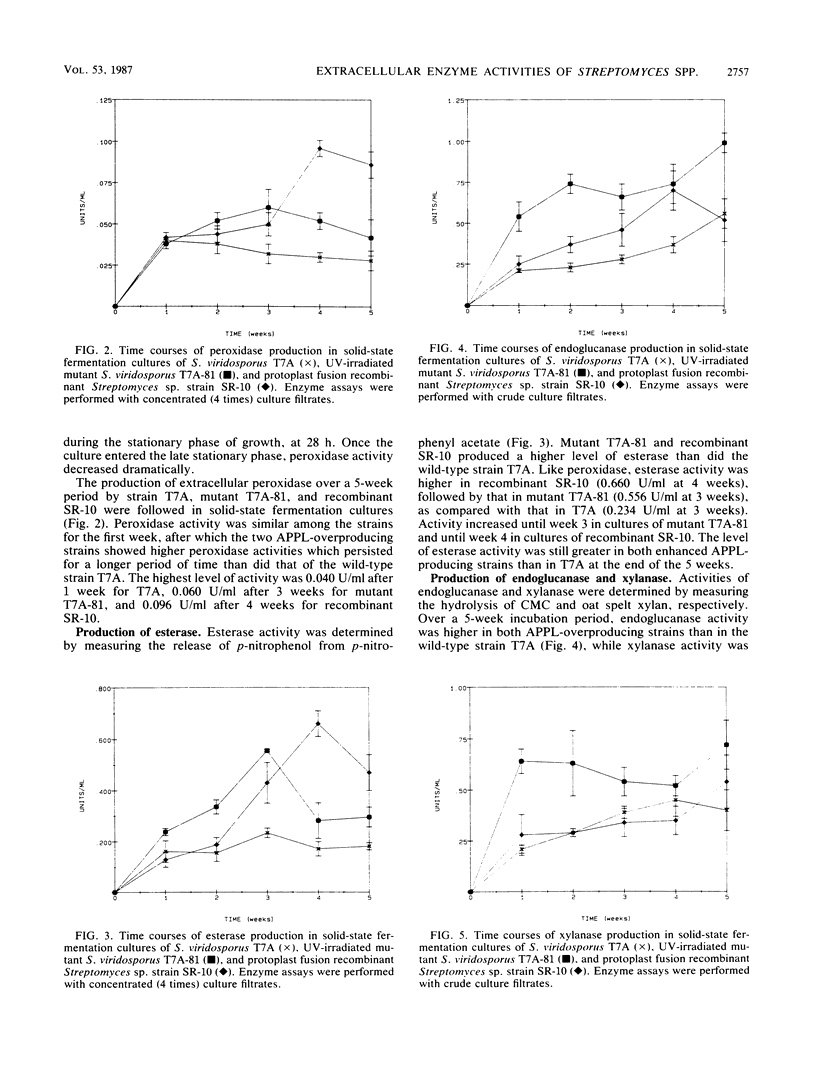
The wild-type ligninolytic actinomycete Streptomyces viridosporus T7A and two genetically manipulated strains with enhanced abilities to produce a water-soluble lignin degradation intermediate, an acid-precipitable polymeric lignin (APPL), were grown on lignocellulose in solid-state fermentation cultures. Culture filtrates were periodically collected, analyzed for APPL, and assayed for extracellular lignocellulose-catabolizing enzyme activities. Isoenzymes were analyzed by polyacrylamide gel electrophoresis and activity staining on the gels. Two APPL-overproducing strains, UV irradiation mutant T7A-81 and protoplast fusion recombinant SR-10, had higher and longer persisting peroxidase, esterase, and endoglucanase activities than did the wild-type strain T7A. Results implicated one or more of these enzymes in lignin solubilization. Only mutant T7A-81 had higher xylanase activity than the wild type. The peroxidase was induced by both lignocellulose and APPL. This extracellular enzyme has some similarities to previously described ligninases in fungi. This is the first report of such an enzyme in Streptomyces spp. Four peroxidase isozymes were present, and all catalyzed the oxidation of 3,4-dihydroxyphenylalanine, while one also catalyzed hydrogen peroxide-dependent oxidation of homoprotocatechuic acid and caffeic acid. Three constitutive esterase isozymes were produced which differed in substrate specificity toward α-naphthyl acetate and α-naphthyl butyrate. Three endoglucanase bands, which also exhibited a low level of xylanase activity, were identified on polyacrylamide gels as was one xylanase-specific band. There were no major differences in the isoenzymes produced by the different strains. The probable role of each enzyme in lignocellulose degradation is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biely P., Markovic O., Mislovicová D. Sensitive detection of endo-1,4-beta-glucanases and endo-1,4-beta-xylanases in gels. Anal Biochem. 1985 Jan;144(1):147–151. doi: 10.1016/0003-2697(85)90096-x. [DOI] [PubMed] [Google Scholar]
- Borgmeyer J. R., Crawford D. L. Production and Characterization of Polymeric Lignin Degradation Intermediates from Two Different Streptomyces spp. Appl Environ Microbiol. 1985 Feb;49(2):273–278. doi: 10.1128/aem.49.2.273-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béguin P. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal Biochem. 1983 Jun;131(2):333–336. doi: 10.1016/0003-2697(83)90178-1. [DOI] [PubMed] [Google Scholar]
- Crawford D. L. Lignocellulose decomposition by selected streptomyces strains. Appl Environ Microbiol. 1978 Jun;35(6):1041–1045. doi: 10.1128/aem.35.6.1041-1045.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford D. L., Pometto A. L., 3rd, Crawford R. L. Production of useful modified lignin polymers by bioconversion of lignocellulose with Streptomyces. Biotechnol Adv. 1984;2(2):217–232. doi: 10.1016/0734-9750(84)90006-5. [DOI] [PubMed] [Google Scholar]
- Crawford D. L., Pometto A. L., Crawford R. L. Lignin Degradation by Streptomyces viridosporus: Isolation and Characterization of a New Polymeric Lignin Degradation Intermediate. Appl Environ Microbiol. 1983 Mar;45(3):898–904. doi: 10.1128/aem.45.3.898-904.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLING M., HOROWITZ N. H., HEINEMANN S. F. The isolation and properties of crystalline tyrosinase from Neurospora. J Biol Chem. 1963 Jun;238:2045–2053. [PubMed] [Google Scholar]
- Kirk T. K., Tien M., Kersten P. J., Mozuch M. D., Kalyanaraman B. Ligninase of Phanerochaete chrysosporium. Mechanism of its degradation of the non-phenolic arylglycerol beta-aryl ether substructure of lignin. Biochem J. 1986 May 15;236(1):279–287. doi: 10.1042/bj2360279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszczyński A., Huynh V. B., Crawford R. Comparison of ligninase-I and peroxidase-M2 from the white-rot fungus Phanerochaete chrysosporium. Arch Biochem Biophys. 1986 Feb 1;244(2):750–765. doi: 10.1016/0003-9861(86)90644-2. [DOI] [PubMed] [Google Scholar]
- Pettey T. M., Crawford D. L. Enhancement of Lignin Degradation in Streptomyces spp. by Protoplast Fusion. Appl Environ Microbiol. 1984 Feb;47(2):439–440. doi: 10.1128/aem.47.2.439-440.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy R. E., Kolattukudy P. E. Depolymerization of a hydroxy fatty acid biopolymer, cutin, by an extracellular enzyme from Fusarium solani f. pisi: isolation and some properties of the enzyme. Arch Biochem Biophys. 1973 Nov;159(1):61–69. doi: 10.1016/0003-9861(73)90429-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Roegner V., Becker F. F. The quantitation of rat serum esterases by densitometry of acrylamide gels stained for enzyme activity. Anal Biochem. 1975 May 26;66(1):206–212. doi: 10.1016/0003-2697(75)90738-1. [DOI] [PubMed] [Google Scholar]
- Sichak S. P., Dounce A. L. Analysis of the peroxidatic mode of action of catalase. Arch Biochem Biophys. 1986 Sep;249(2):286–295. doi: 10.1016/0003-9861(86)90004-4. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Wynne E. C., Pemberton J. M. Cloning of a Gene Cluster from Cellvibrio mixtus which Codes for Cellulase, Chitinase, Amylase, and Pectinase. Appl Environ Microbiol. 1986 Dec;52(6):1362–1367. doi: 10.1128/aem.52.6.1362-1367.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]