Abstract
Angiostatin, a fragment of plasminogen, has been identified and characterized as an endogenous inhibitor of neovascularization. We show that angiostatin treatment of endothelial cells in the absence of growth factors results in an increased apoptotic index whereas the proliferation index is unchanged. Angiostatin also inhibits migration and tube formation of endothelial cells. Angiostatin treatment has no effect on growth factor-induced signal transduction but leads to an RGD-independent induction of the kinase activity of focal adhesion kinase, suggesting that the biological effects of angiostatin relate to subversion of adhesion plaque formation in endothelial cells.
Angiogenesis, formation of new vessels from preexisting microcapillaries, is recognized increasingly as a critical factor in a broad spectrum of diseases (1). The potential for therapeutic benefits from treatment of such diseases, e.g., tumors, with antiangiogenic substances therefore is very high. Angiostatin, a fragment encompassing the kringle region of plasminogen, has been identified and characterized as a potent inhibitor of neovascularization (2). Angiostatin has been shown to efficiently inhibit the growth of a broad spectrum of murine and human tumor models in mice (3). By inhibiting neovascularization of the tumor, angiostatin treatment leads to increased apoptosis of the tumor cells (4). In vitro, the proliferation of capillary endothelial cells is inhibited by angiostatin (2). Its mechanism of action has, however, remained unclear.
Because growth factors such as fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF) have been shown to induce angiogenesis in several experimental situations, the antiangiogenic effect of angiostatin could result from interference at any level with growth factor-induced signal transduction in endothelial cells. Growth factors bind with high affinity to cell surface-expressed receptors, which dimerize, thus leading to activation of the intrinsic tyrosine kinase, and to phosphorylation of receptor molecules. The phosphorylated receptors bind Src homology 2 or phosphotyrosine-binding domain-containing signal transduction molecules, whose intrinsic or associated activities are affected through different mechanisms, upon binding to the receptors (5). Such changes in enzymatic activities are propagated in signaling chains that ultimately are established as specific cellular responses, such as proliferation, death, migration, or differentiation.
In this work, we have analyzed the molecular mechanism whereby angiostatin inhibits endothelial cell function. Although a spectrum of endothelial cell responses were inhibited by angiostatin, we found that growth factor-induced signal transduction was not affected. Rather, angiostatin induced activation of focal adhesion kinase and apoptosis of endothelial cells.
MATERIALS AND METHODS
Cell Culture.
Human umbilical vein endothelial cells were a generous gift from Michael Gimbrone (Brigham and Women’s Hospital, Boston) and were grown on gelatinized plastics in medium M199 (JRH Biosciences, Lenexa, KS) with 20% heat-inactivated fetal bovine serum, 50 μg/ml of endothelial cell mitogen (Biomedical Technologies, Stoughton, MA) and 100 μg/ml heparin. MS-1 cells, murine pancreas endothelial cells immortalized by tsA58 simian virus 40 (SV40), was a kind gift from Jack Arbiser, Harvard Medical School, Boston. Murine brain endothelial cells (see ref. 6 for maintenance and characterization) were derived form transgenic mice carrying H-2 promoter-regulated tsA58 SV40 large T. Hollow tubes were formed in the presence of FGF-2 within 12 h, when these cells were cultured between two layers of collagen. Bovine adrenal cortex capillary endothelial cells (BCE; ref. 7) were cultured at 10% CO2 in DMEM, 10% newborn calf serum. Porcine aortic endothelial (PAE)/FGFR-1 cells have been characterized previously (8). Transfection of BCE cells with different vector, wild-type, and mutated cDNAs was performed by using Lipofectamine (GIBCO/BRL) according to the manufacturer’s recommendations. Angiostatin treatment of cells in general was performed by adding a final concentration of 2.5 μg/ml of angiostatin on cells in full growth medium, for time periods indicated in the figure legends. Angiostatin was purified from human elastase, as described before (3). Cells were treated as indicated with growth factors, either FGF-2 (human recombinant; Farmitalia) or VEGF165 (PeproTech, Boston).
Cell Migration.
Cell migration was measured in a modified Boyden chamber by using 8-μm Nucleopore polyvinylpyrrolidone-free polycarbonate filters coated with 13.4 μg/ml of fibronectin (Organon Taknika–Cappel). The filter was placed over a 48-well chamber (Neuroprobe, Cabin John, MD) containing either vehicle (control), FGF-2, or VEGF165 (10 ng/ml) in serum- and heparin-free M199 medium with 0.1% BSA (fraction V; Sigma). Human umbilical vein endothelial (HUVE) cells at passage 4 to 9 were pretreated with angiostatin for 30 min at the indicated concentrations and then added to the wells in the upper chamber (100,000 cells/50 μl of serum-free medium). After incubation at 37°C for 5 h, the filters were analyzed for number of cells that had migrated through the filter, as described previously (9).
Immunoprecipitation and SDS/PAGE.
Cells were lysed in 50 mM Tris⋅HCl, pH 7.5/150 mM NaCl/0.5% Triton X-100/1 mM phenylmethylsulfonyl fluoride/10 mM NaF/1 mM sodium orthovanadate on ice, the nuclei were pelleted, and supernatants were either subjected to SDS/PAGE or used for immunoprecipitation with antibodies against phosphotyrosine (PY20; Transduction Laboratories, Lexington, KY) or focal adhesion kinase (FAK; Transduction Laboratories). For Shb immunoprecipitations, the supernatants from pelleting of the nuclei were boiled for 2 min in 1% SDS and diluted 10-fold in lysis buffer before immunoprecipitation with 5 μg/ml affinity-purified Shb antibody (ref. 10). When indicated, the immunoprecipitates were incubated in kinase buffer (10 mM Tris⋅HCl, pH 7.5/0.01% Triton X-100/10 mM MnCl2) in the presence of [γ-32P]ATP (immune complex kinase assay). SDS/PAGE was performed in 7% or 10% linear polyacrylamide gels. The gels were used as indicated for wet transfer to membrane, followed by immunoblotting of the filter. Blotting was performed by using antibodies specifically recognizing activated mitogen-activated protein kinase (New England Biolabs), or with antiphosphotyrosine antibodies. Immunoreactive material was detected by using ECL. When analyzing 32P-labeled material, the gel was treated for autoradiography, by incubation in 2.5% glutaraldehyde, to fix the gels, followed by incubation in 0.5 M KOH, at 55°C for 45 min, to hydrolyze serine phosphorylation.
RESULTS AND DISCUSSION
Endothelial Cell Responses Are Inhibited by Angiostatin.
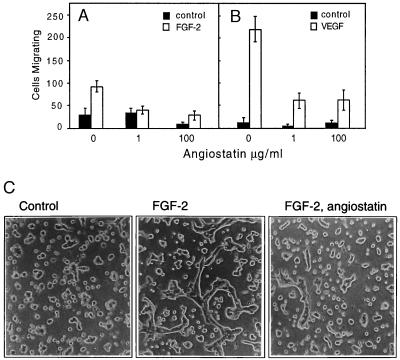
To understand the effect of angiostatin on growth factor-induced endothelial cell responses, we analyzed migration and tube formation in collagen gels of endothelial cells. Angiostatin treatment of HUVE cells inhibited migration of cells toward FGF-2 (Fig. 1A) and VEGF (Fig. 1B). HUVE cell migration, measured by using a modified Boyden chamber assay, was more efficient toward VEGF than toward FGF-2. Angiostatin at 1 μg/ml efficiently reduced migration of HUVE cells toward FGF-2, to the level of basal migration. At the same concentration of angiostatin, migration of HUVE cells toward VEGF was reduced to 25% of that seen in the absence of angiostatin. Increasing the concentration of angiostatin to 100 μg/ml had no further significant effects on the migration efficiency of growth factor-stimulated cells.
Figure 1.
(A and B) Angiostatin attenuates migration of HUVE cells toward FGF-2 (A) and VEGF (B). Cell migration was measured in a Boyden chamber, and the cells migrating through the filter during a 4-h incubation were stained and counted. (C) Angiostatin treatment of murine brain endothelial cells leads to diminished formation of tubes in three-dimensional collagen gels. Murine brain endothelial cells were seeded out on a solidified collagen gel and covered with a second layer of collagen. After 10 h of treatment in the presence or absence of growth factor and angiostatin, the cells were examined for formation of tube-like structures. The cells were treated as indicated with 10% fetal calf serum alone (Left), with 10% fetal calf serum and 5 ng/ml FGF-2 (Center), or pretreated for 72 h with 2.5 μg/ml of angiostatin in Ham’s F-12, 10% fetal bovine serum, followed by inclusion of angiostatin in the collagen gel and stimulation of tube formation by 5 ng/ml FGF-2 (Right). Angiostatin treatment led to 79% decreased formation of tubes, from 305 μm total tube length in the absence (−angiostatin) to 65 μm total tube length in the presence (+) of angiostatin. (×200.)
We have shown previously that FGF-2 treatment of murine brain endothelial cells in three-dimensional collagen gels leads to formation of hollow tube structures, which display tight junctions between cells, and apical pinocytosis (6, 11). These cells express VEGF receptor-1 (Flt-1) but not VEGF receptor-2 (KDR) and fail to respond to VEGF with tube formation. After 36 h of treatment of the cells with 2.5 μg/ml of angiostatin in the presence of 10% fetal calf serum, FGF-2-induced tube formation was reduced by 79%, as compared with FGF-2-treated control cultures (Fig. 1C). The presence of serum was necessary for angiostatin to exert an inhibitory effect (data not shown).
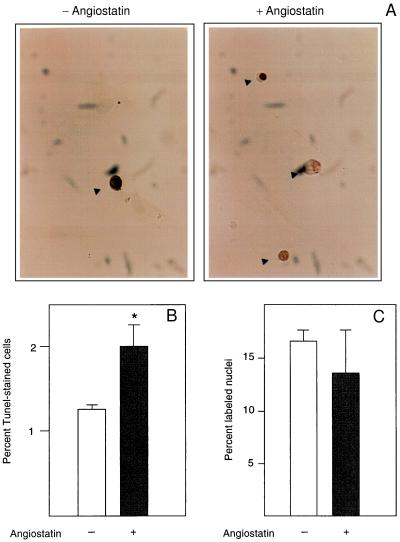
We further determined whether the angiostatin-induced inhibition of endothelial cell proliferation (2) is because of a decreased cell replication rate or an increase of programmed cell death, apoptosis. TUNEL staining (12), which visualizes nuclei containing fragmented DNA in cells undergoing apoptosis, was performed on primary bovine adrenal cortex capillary endothelial (BCE) cells treated with 2.5 μg/ml angiostatin for 3 days, in the presence of 10% calf serum (Fig. 2A). Angiostatin treatment led to a nearly doubled frequency of stained, pyknotic nuclei (Fig. 2B). Under these conditions, there was no effect of angiostatin on DNA synthesis (Fig. 2C), suggesting that decreased proliferation of angiostatin-treated BCE is caused by an increase in apoptosis.
Figure 2.
(A) Angiostatin induces apoptosis but has no effect on DNA synthesis in endothelial cells. BCE cells cultured in the presence of 10% serum on coverslips were kept in the absence (−) and presence (+) of 2.5 μg/ml angiostatin for 3 days, at which point the cells were fixed and processed for TUNEL staining (12), to detect cells undergoing programmed cells death. Cells stained positive in the TUNEL assay are indicated by arrowheads. (B) Number of TUNEL-positive cells in the untreated (open bar) and angiostatin-treated (solid bar) cultures. Values are percentage of positive cells ± SEM for four determinations. ∗ indicates P < 0.05. (C) Number of nuclei incorporating [3H]thymidine in the untreated (open bar) and angiostatin-treated (solid bar) samples. Cells were plated on coverslips and cultured in the presence of 10% newborn calf serum with or without 2.5 μg/ml angiostatin for 3 days. During the last 2 h of culture, 1 μCi/ml [3H]thymidine was present in the culture medium. The cells were washed, fixed, and processed for autoradiographic determination of [3H]thymidine incorporation. Values are percentage of labeled nuclei ± SEM for three determinations.
Growth Factor-Induced Signal Transduction Is Not Affected by Angiostatin.
We addressed the possibility that the biological effect of angiostatin is a result of an interference with binding of growth factors to their cell surface receptors or their downstream signal transduction. Iodinated VEGF was added to cultures of BCE cells in the presence and absence of 2 and 5 μg/ml angiostatin, followed by cross-linking to VEGF receptors expressed on these cells. Similar levels of ligand–receptor complexes were detected independently of whether or not angiostatin had been added (data not shown). To test the effect of angiostatin on signal transduction pathways downstream of the FGF receptor-1, tyrosine phosphorylation of the adapter protein Shb was studied. In untreated BCE cells, the Src homology 2- and Pro-rich-domain-containing protein Shb (14) is tyrosine phosphorylated at a basal level. Stimulation with FGF-2 for 10 min increased the level of tyrosine phosphorylation of Shb (Fig. 3 Upper). Pretreatment with 2.5 μg/ml angiostatin for 24 h had no effect on the FGF-2-stimulated Shb tyrosine phosphorylation, although the basal phosphorylation of Shb in the absence of FGF-2 was decreased. The decreased basal tyrosine phosphorylation of the Shb adapter protein could be of relevance for angiostatin-induced apoptosis, because Shb overexpression promotes apoptosis in NIH 3T3 fibroblasts in the absence of serum (10). In agreement, transient overexpression of Shb in BCE cells followed by angiostatin treatment leads to a marked increase in apoptotic index (see Fig. 5D). Furthermore, angiostatin did not inhibit the growth factor-stimulated Ras pathway, because FGF-2-induced activation of the p42 and p44 mitogen-activated protein kinases was not affected by angiostatin treatment of BCE cells for 3 days (Fig. 3 Lower).
Figure 3.
Tyrosine phosphorylation of the adapter protein Shb and activation of mitogen-activated protein (MAP) kinases in response to FGF-2 occur in the presence of angiostatin. BCE cells in DME/10% newborn calf serum were cultured for 24 h with or without 2.5 μg/ml angiostatin, and in some cultures this was followed by 10-min stimulation with 100 ng/ml FGF-2. SDS-denatured cell lysates were immunoprecipitated by using affinity-purified anti-Shb antibodies, followed by SDS/PAGE, transfer to filter, and immunoblotting with antiphosphotyrosine antibodies (PY20, Upper). Immunoblotting of cell extracts separated by SDS/PAGE was performed, followed by transfer to filter and incubation with antibodies specifically recognizing activated MAP kinases Erk 1 and 2 (Lower).
Figure 5.
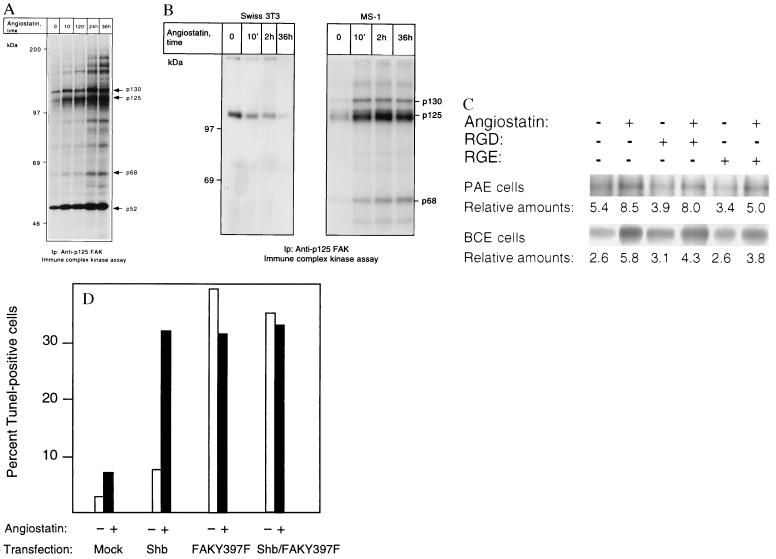
(A and B) p125 FAK is activated in response to angiostatin treatment only in endothelial cells. PAE/FGFR-1 cells (A) and Swiss 3T3 cells and murine pancreas endothelial cells (MS-1; B) were treated as indicated with 2.5 μg/ml of angiostatin for different time periods and processed for immunoprecipitation by using a mAb against p125 FAK. The immobilized immunoprecipitates were subjected to in vitro kinase assays in the presence of [γ-32P]ATP and analyzed by SDS/PAGE. The migration of 130-, 125-, and 68-kDa components that could correspond to Cas, FAK, and paxillin, respectively, as well as other angiostatin-induced phosphorylated components are indicated by arrows, and the relative masses of marker proteins run in parallel are indicated to the left. (C) Treatment with 1 mM RGD-containing peptide fails to block angiostatin-induced FAK activation. PAE/FGFR-1 cells or BCE cells were treated for 10 min with 2.5 μg/ml of angiostatin in the presence and absence of 1 mM peptide containing the RGD motif (Gly-Arg-Gly-Asp-Thr-Pro; Bachem) or with a control peptide containing RGE instead of RGD. The cells were lysed and processed for immunoprecipitation with anti-FAK mAb, followed by in vitro kinase assay. (D) Effects of angiostatin and dominant-negative FAK-Y397F on BCE cell survival. BCE cells were transfected by using the Lipofectamine procedure using lipofectamine alone (mock), Shb cDNA in pcDNA1, and FAK-Y397F in pCMV (a kind gift from J. T. Parsons, University of Virginia) as indicated. The transfected cells were cultured on coverslips in the absence or presence of 2.5 μg/ml angiostatin for 3 days, after which the cells were processed for TUNEL staining and the percentage of stained nuclei were counted.
Angiostatin Treatment Leads to Increased Tyrosine Phosphorylation.
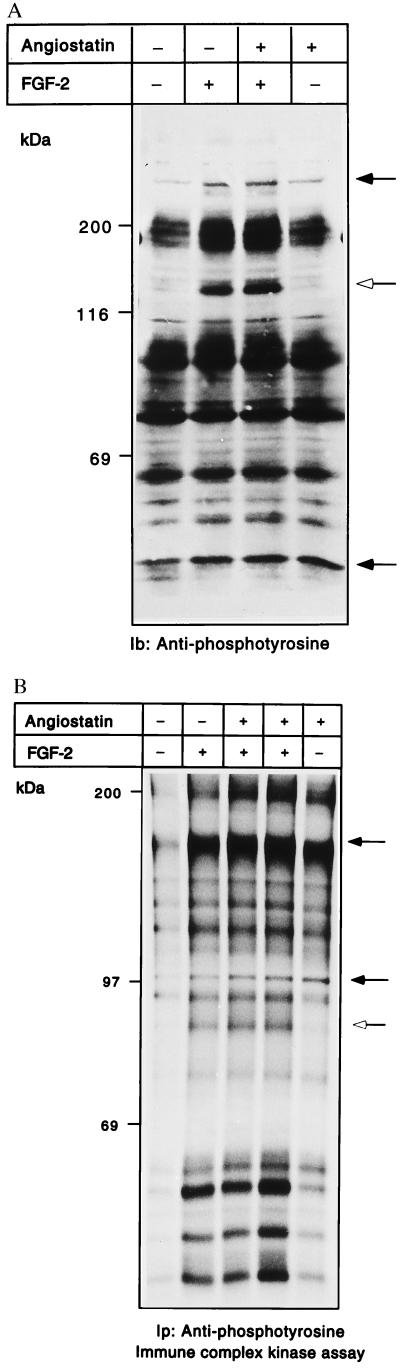
Because we were unable to detect marked effects on the signal transduction molecules described above, we analyzed total tyrosine phosphorylation in angiostatin-treated cells and compared samples with those from growth factor-stimulated cells. Fig. 4A shows an antiphosphotyrosine immunoblotting of whole cell extracts, from BCE cells treated or not with angiostatin for 24 h, and with FGF-2 included as indicated during the last 10 min of incubation. In FGF-2-stimulated cells, a major tyrosine-phosphorylated species of 140 kDa was induced, which was not affected by angiostatin (open arrow in Fig. 4A). However, angiostatin treatment alone did, in a reproducible manner, induce tyrosine phosphorylation of several molecules, of which the most abundant ones were 250 and 45 kDa (solid arrows in Fig. 4A). We further analyzed the total tyrosine kinase activity of porcine aortic endothelial (PAE) cells expressing FGF receptor-1, after treatment with 2.5 μg/ml angiostatin for 24 h, in the presence and absence of FGF-2, which was included during the last 10 min of the incubation. The PAE cells were chosen to allow increased responsiveness. Cells were lysed and immunoprecipitation was performed by using antiphosphotyrosine antibody. Kinase assays in the presence of [γ-32P]ATP were performed on the immobilized samples. As seen in Fig. 4B, addition of FGF-2 to the cultures resulted in the appearance of a number of 32P-labeled tyrosine-phosphorylated molecules, regardless of whether or not the cells had been pretreated with angiostatin. On the other hand, treatment with angiostatin alone for 24 h induced a spectrum of 32P-labeled tyrosine-phosphorylated components. By comparing the FGF-2-treated with the angiostatin-treated samples, it is obvious that certain, but not all, of the 32P-labeled molecules appearing in FGF-2-treated cells were detected, sometimes even more pronounced, in the angiostatin-treated cells (indicated by solid arrows in Fig. 4B).
Figure 4.
(A and B) Angiostatin treatment leads to induction of kinase activity and tyrosine phosphorylation. BCE cells were cultured in the absence or presence of 2.5 μg/ml angiostatin in the growth medium for 24 h; during the last 10 min, cultures were treated with 100 ng/ml FGF-2 as indicated. Samples of cell extracts were analyzed by immunoblotting by using antiphosphotyrosine antibodies (A). Open arrow indicates a 140-kDa tyrosine-phosphorylated species; solid arrows indicate increased tyrosine phosphorylation in response to angiostatin. PAE cells were similarly treated and cell lysates were used for immunoprecipitation with antiphosphotyrosine antibodies, followed by in vitro kinase assays on the immobilized beads in the presence of [γ-32P]ATP (B). The samples were separated by SDS/PAGE, and the gel was treated with 1 M KOH to reduce phosphorylation on Ser. Solid arrows indicate components with increased 32P-labeled radioactivity in response to angiostatin; open arrow indicates a component that is not affected by angiostatin.
Induction of Focal Adhesion Kinase by Angiostatin.
A possible interpretation of the data presented above is that angiostatin treatment leads to induction of growth factor-independent signal transduction resulting in apoptosis. One such growth factor-independent entry would be via interaction with integrins. Integrins are known to couple to tyrosine kinase activity localized in focal adhesions, e.g., focal adhesion kinase (FAK) (15). We analyzed whether angiostatin treatment affected the activity level of focal adhesion kinase by treating PAE cells for different time periods, from 10 min to 36 h with angiostatin, followed by immunoprecipitation with anti-FAK mAb and kinase assays on the immobilized precipitates. As seen in Fig. 5A, already 10 min of angiostatin treatment led to an induction of kinase activity. Notably, three components of 130, 125, and 68 kDa showed a time-dependent increase in labeling. Based on its predominance and migration, the 125-kDa band is likely to correspond to FAK. The identities of the other components are not known, although their migration rates indicate that they could correspond to Crk-associated substrate and paxillin, respectively. Similar results were obtained when using the immortalized murine pancreas endothelial cells (Fig. 5B). Again, 10-min treatment with angiostatin led to a marked induction of kinase activity in the FAK immunoprecipitates. However, unlike in the PAE cells, prolonged exposure to angiostatin did not further potentiate FAK kinase activity. Angiostatin treatment of Swiss 3T3 fibroblasts, on the other hand, failed to activate the FAK kinase (Fig. 5B). Because tyrosine kinases other than p125 FAK could be present in the FAK immunoprecipitates, by virtue of their ability to form complexes with FAK (16), we considered the possibility that the cytoplasmic tyrosine kinase Src could be responsible for the increased content of phosphotyrosine in the FAK immunoprecipitates from angiostatin-treated cells. We treated cells with angiostatin for increasing periods of time and used cell lysates for immunoprecipitation of Src, followed by kinase assays on the immobilized immunoprecipitate. Src kinase activity was not appreciably affected in repeated experiments (data not shown). FAK is activated in conjunction with extracellular matrix-induced clustering of β1 integrins (17). One binding motif for the β1 integrin is the tripeptide RGD (18), which is present in fibronectin and other extracellular matrix components. We tested the possibility that angiostatin might activate FAK or associated tyrosine kinases through RGD-dependent clustering of β1 integrins, by incubating PAE or BCE cells with angiostatin in the presence and absence of 1 mM RGD-containing peptide, for 10 min at 37°C. Neither the RGD peptide nor an RGE-control peptide was able to inhibit the angiostatin-induced tyrosine kinase activity in FAK immunocomplex assays in repeated experiments (Fig. 5C). To test the involvement of FAK in angiostatin-induced apoptosis, BCE cells were transiently transfected with a mutated FAK-Y397F, in which the major autophosphorylation site is removed, thus generating a dominant-negative FAK that is unable to protect cells from apoptosis (19). The cells were cultured in the absence or presence of 2.5 μg/ml angiostatin. Angiostatin caused an increase in the apoptotic index of mock-transfected cells to approximately 7% (Fig. 5D). Transfection with wild-type Shb cDNA followed by treatment with angiostatin increased the rate of apoptosis to more than 30% (Fig. 5D). When the cells were transfected with the mutated FAK-Y397F, alone or together with Shb, the rates of apoptosis were between 32 and 39%, and there was no additional effect of angiostatin (Fig. 5D).
Disturbance of FAK function, e.g., by inactivation of the FAK gene (20), by inhibiting targeting of FAK to focal adhesions (21, 22), or by treatment with antisense oligonucleotides (23), leads to decreased cell migration and increased apoptosis of cells. One possible interpretation of the findings in Fig. 5D is that FAK-Y397F and angiostatin are positioned in the same pathway leading to apoptosis in endothelial cells. To relate the angiostatin-induced increase in FAK kinase activity with inhibition of cell migration and increased apoptosis we suggest that angiostatin causes a flawed, integrin-independent activation of FAK that perturbs the ordered turnover of focal adhesion contacts induced by VEGF and FGF-2. This mechanism involves a rerouting of FAK from the integrin/focal adhesion complexes to other intracellular sites and thus functionally resembles the effects of FAK-Y397F (19) or FAK displaced from focal adhesion complexes by peptides (22) on cell viability. Experimental support for the inappropriate FAK activation hypothesis is provided by the fact that the angiostatin causes RGD-independent FAK activation, does not involve coactivation of Src, and thus is not likely to involve intergrin signaling.
Acknowledgments
We thank J. T. Parsons, University of Virginia, for his generous gift of the mutant p125FAK-Y397F and Andrius Kazlauskas for valuable discussions. We thank Ing-Marie Mörsare for skillful technical assistance. Funding for this study was provided in part by the Swedish Cancer Society (96 1168; 3820-B96–01XAB) to L.C.-W. and by Juvenile Diabetes Foundation International and The Swedish Medical Research Council (12X-10822) to M.W.
ABBREVIATIONS
- BCE
bovine adrenal cortex capillary endothelial
- FAK
focal adhesion kinase
- FGF
fibroblast growth factor
- VEGF
vascular endothelial growth factor
- PAE
porcine aortic endothelial
- HUVE
human umbilical vein endothelial
References
- 1.Folkman J. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 2.O’Reilly M S, Holmgren L, Shing Y, Chen C, Rosenthal R A, Moses M, Lane W S, Cao Y, Sage E H, Folkman J. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 3.O’Reilly M S, Holmgren L, Chen C, Folkman J. Nat Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 4.Holmgren L, O’Reilly M S, Folkman J. Nat Med. 1996;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 5.Pawson T. Nature (London) 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 6.Kanda S, Landgren E, Ljungström M, Claesson-Welsh L. Cell Growth Differ. 1996;7:383–395. [PubMed] [Google Scholar]
- 7.Folkman J, Haudenschild C C, Zetter B R. Proc Natl Acad Sci USA. 1979;76:5217–5221. doi: 10.1073/pnas.76.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wennström S, Landgren E, Blume-Jensen P, Claesson-Welsh L. J Biol Chem. 1992;267:13749–13756. [PubMed] [Google Scholar]
- 9.Kundra V, Escobedo J A, Kazlauskas A, Kim H K, Rhee S G, Williams L T, Zetter B R. Nature (London) 1994;367:474–476. doi: 10.1038/367474a0. [DOI] [PubMed] [Google Scholar]
- 10.Karlsson T, Welsh M. Oncogene. 1996;13:955–961. [PubMed] [Google Scholar]
- 11.Rahmanian M, Pertoft H, Kanda S, Christofferson R, Claesson-Welsh L, Heldin P. Exp Cell Res. 1997;237:223–230. doi: 10.1006/excr.1997.3792. [DOI] [PubMed] [Google Scholar]
- 12.Gavrieli Y, Sherman Y, Ben-Sasson S. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gitay-Goren H, Soker S, Vlodavsky I, Neufeld G. J Biol Chem. 1992;267:6093–6098. [PubMed] [Google Scholar]
- 14.Welsh M, Mares J, Karlsson T, Lavergne C, Breant B, Claesson-Welsh L. Oncogene. 1994;9:19–27. [PubMed] [Google Scholar]
- 15.Kanner S B, Reynolds A B, Vines R R, Parsons J T. Proc Natl Acad Sci USA. 1990;87:3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlaepfer D D, Broome M A, Hunter T. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaller M D, Otey C A, Hildebrand J D, Parsons J T. J Cell Biol. 1995;130:1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burridge K, Turner C E, Romer L H. J Cell Biol. 1992;118:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frisch S M, Vuori K, Ruoslathi E, Chan-Hui P Y. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Nature (London) 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 21.Gilmore A P, Romer L H. Mol Biol Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hungerford J E, Compton M T, Matter M L, Hoffstrom B G, Otey C A. J Cell Biol. 1996;135:1383–1390. doi: 10.1083/jcb.135.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L H, Owens L V, Sturge G C, Yang X, Liu E T, Craven R J, Cance W G. Cell Growth Differ. 1996;7:413–418. [PubMed] [Google Scholar]