Abstract
Epidemiological studies link arsenic exposure to increased risks of cancers of the skin, kidney, lung, bladder and liver. Additionally, a variety of non-cancerous conditions such as diabetes mellitus, hypertension, and cardiovascular disease have been associated with chronic ingestion of low levels of arsenic. However, the biological and molecular mechanisms by which arsenic exerts its effects remain elusive. Here we report increased renal hexokinase II (HKII) expression in response to arsenic exposure both in vivo and in vitro. In our model, HKII was up-regulated in the renal glomeruli of mice exposed to low levels of arsenic (10 ppb or 50 ppb) via their drinking water for up to 21 days. Additionally, a similar effect was observed in cultured renal mesangial cells exposed to arsenic. This correlation between our in vivo and in vitro data provides further evidence for a direct link between altered renal HKII expression and arsenic exposure. Thus, our data suggest that alterations in renal HKII expression may be involved in arsenic-induced pathological conditions involving the kidney. More importantly, these results were obtained using environmentally relevant arsenic concentrations.
Keywords: Arsenic, Arsenite, Hexokinase
Introduction
Millions worldwide suffer from the effects of chronic arsenic exposure, which typically occurs via ingestion of contaminated drinking water or agricultural products. Efforts to address this dilemma continue to be complicated by the fact that some of the most severely affected regions are in lesser developed areas of the world, such as Bangladesh and West Bengal, where approximately 79.9 million and 42.7 million people respectively are exposed to ground water with arsenic levels above the 50 ppb limit set by the World Health Organization (Ratnaike, 2003). Epidemiological studies in these and other affected regions link arsenic exposure with increased risks of cancers of the skin, kidney, lung, bladder and liver (Hopenhayn-Rich et al., 1998; Frumkin and Thun, 2001; Lu et al., 2001; Centeno et al., 2002; Bates et al., 2004; Schoen et al., 2004; Sun, 2004; Yoshida et al., 2004). Additionally, a variety of non-cancerous conditions such as diabetes mellitus, hypertension and cardiovascular disease have been associated with chronic ingestion of low levels of arsenic (Tseng et al., 2000a; Tseng et al., 2000b; Simeonova and Luster, 2004; Sun, 2004; Tseng, 2004). Various possible modes of carcinogenic action have been proposed including genotoxic or clastogenic effects, oxidative stress, altered expression of growth factors, and altered DNA repair mechanisms (Germolec et al., 1998; Goering et al., 1999; Simeonova et al., 2000; Kitchin, 2001; Mass et al., 2001; Rossman et al., 2001; Okoji et al., 2002; Styblo et al., 2002; Wauson et al., 2002; Rea et al., 2003; Burns et al., 2004; Cui et al., 2004; Schoen et al., 2004; Tseng, 2004). However, despite the well-documented role of arsenic as a carcinogen and tumor promoter, the biological and molecular mechanisms underlying its effects remain elusive.
The kidney is one organ that has proven particularly sensitive to arsenic exposure. In our recent in vivo studies in mice, we uncovered increased levels of hexokinase II (HKII) in the renal cortex in response to chronic ingestion of low levels of arsenic. The discovery of high levels of cortical HKII has significant biological implications. HKII is a key enzyme in cellular metabolism, growth and survival, with its primary function being the phosphorylation of glucose to yield glucose 6-phosphate (Glc 6-P). By catalyzing this first committed step in glucose uptake and cellular metabolism, HKII initiates and controls the metabolic flux of glucose and, ultimately, cellular metabolism and growth. Additionally, aberrant HKII expression has been linked to malignant transformation and tumor metastasis (Bustamante and Pedersen, 1977; Bustamante and Pedersen, 1980; Bustamante et al., 1981; Arora and Pedersen, 1988; Pedersen et al., 2002; Lee and Pedersen, 2003). In fact, a high rate of glycolysis and glucose catabolism is a common trait among many malignant tumor cells, and this highly glycolytic phenotype is frequently accompanied by increased HKII activity (Arora and Pedersen, 1988; Lee and Pedersen, 2003). Its enzymatic product, Glc 6-P, not only serves as a precursor for glycolysis, but also feeds into other important biological pathways leading to ATP generation, nucleic and fatty acid biosynthesis, and NADPH production. The discovery of high levels of HKII in the renal cortex in the present study may be particularly significant since altered HKII expression in the renal cortex has been associated with a variety of other pathological conditions including diabetes (Katzen et al., 1970; Anderson and Stowring, 1973; Steer et al., 1982).
In addition to supporting cellular growth and proliferation through the glycolytic and pentose phosphate pathways, enhanced HKII expression has been shown to block apoptosis, thus contributing to cellular immortalization and progression towards the neoplastic state (Gottlob et al., 2001; Bryson et al., 2002; Pastorino et al., 2002; Azoulay-Zohar et al., 2004; Majewski et al., 2004). HKII is known to exist in two states, one of which is associated with the mitochondria through binding with the voltage-dependent anion channel (VDAC) (Arora and Pedersen, 1988; Pastorino et al., 2002). The transmembrane porin VDAC mediates transport of metabolites including ATP and ADP, and is a constituent of the mitochondrial transition pore. The mitochondrial transition pore is involved in mitochondrial associated apoptotic signaling; its opening results in membrane depolarization and the release of proapoptotic intermembrane space proteins including cytochrome c. HKII, presumably through its association with VDAC, has been shown to interfere with signaling molecules such as Bax, which translocate to the mitochondria and relay apoptotic signals through binding with VDAC (Arora and Pedersen, 1988; Gottlob et al., 2001; Pastorino et al., 2002; Azoulay-Zohar et al., 2004; Majewski et al., 2004). Additionally, through this same VDAC association, HKII can gain preferential access to mitochondrial generated ATP, thus enabling an enhanced rate of glycolysis (Arora and Pedersen, 1988).
In the present study, we examine arsenic induced alterations in renal HKII expression both in vivo and in vitro. We demonstrate enhanced expression of HKII in the cortical glomeruli of mice exposed to either 10 ppb or 50 ppb arsenic via their drinking water. Furthermore, we show that exposure to arsenic results in the up-regulation of HKII in cultured renal mesangial cells. Overall, our findings suggest that, in the mouse model, alteration of renal HKII expression may be an important component of the biochemical pathways involved in arsenic-induced pathological conditions involving the kidney. Perhaps more significant is the fact that these observations were made using environmentally relevant concentrations of arsenic.
Methods
Reagents
Mouse renal mesangial cells (SV40 MES 13, passage 27, mycoplasm free) were obtained from American Type Culture Collection (Rockville, MD). Tissue culture media, fetal bovine serum (FBS), antibiotics, TRIzol, AlexaFuor fluorescent secondary antibodies, ProLong Gold antifade, and MitoTracker Deep Red were purchased from Invitrogen (Carlsbad, CA). Polyclonal HKII antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and HRP(horseradish peroxidase)-conjugated secondary antibodies from Cell Signaling Technology (Beverley, MA).
Animal Studies
Adult male C57BL6 mice 6-8 weeks old (Jackson Labs) were housed in a temperature and humidity controlled environment, with lights maintained on a 12:12-hour light:dark cycle at the University of Arizona Animal Care Facility. Upon arrival, the animals were housed two per cage and acclimated to the facility for approximately 1 week before use. During the treatment period, mice were given free access to rodent chow (Harlan Teklad Mouse/Rat Chow No. 7013, Madison, WI) and water. Control groups received Millipore ddH2O, while arsenic treated groups received Millipore ddH2O containing either 10 or 50 ppb arsenite (from addition of NaAsO2). The As3+ concentration of the water was verified (using HPLC/ICP-Mass Spec) by the Analytical Section of the Hazard Identification Core of the Southwest Environmental Health Sciences Center at the University of Arizona (concentrations of arsenate (As5+) and methylated arsenic species were negligible).
Animals were handled in accordance with the National Institutes of Health standards and Guide for Care and Use of Laboratory Animals. At the end of the treatment periods mice were euthanized by injection with approximately 200 μl of a ketamine:xylazine mix (anaesthetized) followed by cervical dislocation. These methods are consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Harvested tissues were either snap-frozen in liquid nitrogen (for RNA isolation) or fixed in 10% neutral buffered formalin (for immunohistochemistry). Paraffin embedding and sectioning was performed by the Histology Core Facility at the University of Arizona College of Medicine.
Analysis of Plasma and Urine
The urinary and plasma concentrations of arsenite [As(III)], arsenate [As(V)], and the mono- and dimethylated metabolites [MMA(V) and DMA(V)] were measured by HPLC/ICP-MS. These analyses were performed by Michael Kopplin at the Analytical Section of the Hazard Identification Core of the Superfund Basic Research Program.
Cell Culture
SV40 MES 13 cells were maintained in Dulbecco’s modified Eagles: Ham’s F12 medium (3:1) supplemented with 14 mM HEPES, 5% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cell monolayers were routinely grown on 10-cm tissue culture dishes in a humidified 37°C/5% CO2 incubator. Arsenic treatment was accomplished through addition of arsenite (As3+) to the media (from a concentrated stock solution of NaAsO2). After the specified treatment periods, the cells were rinsed with ice-cold PBS (phosphate buffered saline, pH 7.4) and lysed as described below.
RNA and Protein Isolation
Cultured mesangial cells were exposed to arsenic for the specified time periods, after which RNA and total protein were isolated by the TRIzol method according to the manufacturer’s recommended protocol. For microarray analysis, total RNA was isolated and purified from ~90 mg mouse kidney tissue using 1.0 ml Trizol reagent, following the recommended isolation procedure. The total RNA was quantified via UV spectrophotometry and ~100 μg of each sample was re-purified using Qiagen (Palo Alto, CA) RNA mini purification spin columns. RNA was then treated with Roche RNase free DNase I to remove residual DNA, then further purified via Qiagen RNA mini spin columns, followed by quantification via UV spectrophotometry. Quality and quantity of the total RNA was verified by electrophoretically resolving the samples on a 1% agarose formaldehyde gel.
Microarray Analysis
The University of Arizona Cancer Center Core facility performed the microarray gene expression analysis using Affymetrix GeneChip® Mouse Genome 430A 2.0 Arrays. Quantitative analysis was performed with Affymetrix GeneChip software utilizing the GC-RMA statistical algorithm. Data for HKII expression is presented as the mean ± SEM of 3 independent experiments. Significant differences (p < 0.05) were determined by ANOVA and Bonferroni’s multiple comparison tests.
PCR Gene Amplification
TRIzol isolated total RNA from the cultured mesangial cells was used to produce cDNA via reverse transcriptase utilizing Fermentas First Strand cDNA Synthesis kit (Hanover, MD). The RNA was quantified by UV spectrophotometry and 3 μg used for each RT-PCR reaction. Clonetech (Mountain View, CA) Advantage cDNA PCR kit was used to amplify the HKII gene transcript using gene specific primers, and the resulting PCR product electrophoretically separated on a 1% agarose gel. The ethidium bromide stained bands were visualized using a UV transilluminator. Optimal PCR conditions were optimized by systematically varying the number of amplification cycles. Bands were quantitated using Scion Image (Frederick, Maryland) Gel Plot 2 densitometry macro. Values for the fold increase compared to control are presented as the mean ± SEM of three independent experiments. Significant differences (p < 0.01) were determined by ANOVA and Bonferroni’s multiple comparison tests.
Immunoblot Analysis
Total protein and RNA were isolated simultaneously from the cell lysates using TRIzol. Isolation was performed according to the manufacturer’s written protocol. Protein concentrations were determined by the Bradford method using BSA as a standard (Bradford, 1976). Normalized samples (containing 100 μg total protein) were heated with Laemmli sample buffer for 1 minute, then separated on a 10% SDS-PAGE gel, after which the resolved proteins were electrophoretically transferred to nitrocellulose membranes. Membranes were blocked for 1 hour in TBS-T milk [0.05 M tris-buffered saline (0.138 M NaCl, 0.0027 M KCl) containing 0.15% Tween 20 and 5% (w/v) nonfat dry milk], incubated with primary antibody diluted in TBS-T milk (1:1000) for 1hour at room temperature, washed (3 X TBS-T), and incubated for 1 hour in HRP-conjugated secondary antibody diluted in TBS-T milk (1:10000). Protein expression was visualized using enhanced chemiluminesence substrate and exposure to Bio-max film. Membranes were routinely stripped and reprobed for actin or stained with Ponceau S to confirm even loading of samples. Western blots shown are representative of results obtained; all experiments were replicated a minimum of 3 times. Bands were quantitated using Scion Image (Frederick, Maryland) Gel Plot 2 densitometry macro. Values for the fold increase compared to control are presented as the mean ± SEM with statistical analysis performed as described earlier, with p < 0.05.
Immunohistochemistry
Sections cut from formalin-fixed, paraffin-embedded tissue blocks were deparrafinized, rehydrated through a graded series of ethanol, and endogenous peroxide activity blocked (30 min in 0.3% H2O2 in methanol). Antigens were unmasked by heating in 10 mM citrate buffer (pH 6.0) for 10 minutes (microwave), then non-specific binding sites blocked by incubating in 5% goat serum, 2% BSA in PBS for 1 hour at room temperature. The sections were then incubated with primary antibody (1:200 in 5% goat serum 2% BSA in PBS) overnight at 4°C in a humidified chamber. After which, sections were washed (3 X PBS), then incubated with HRP-conjugated secondary antibody (same dilution as primary) for 30 minutes at 37°C. Tissue sections were then developed with metal enhanced DAB (3,3’-Diaminobenzidine tetrachloride), counterstained with nuclear fast red, dehydrated through a graded series of ethanol and mounted with Cytoseal XYL. Relative staining intensity was compared by normalizing the backgrounds of images depicting similar regions in treated and control tissue, then using a 2-D density profile plot in Scion Image.
Immunofluorescence
Cell monolayers for immunofluorescence were grown on glass coverslips. After treatment, cells were fixed in 3.7% formaldehyde: 96.3% media for 15 minutes at 37°C, washed with PBS, permeabilized for 5 minute in ice cold acetone, washed again with PBS, blocked for 1 hour at room temperature with 5% goat serum 2% BSA in PBS, then incubated with primary antibody (1:200 in 5% goat serum 2% BSA in PBS) overnight at 4°C in a humidified chamber. After which, coverslips were washed and incubated with fluorescent secondary antibody (same dilution as primary) for 30 minutes at 37°C, washed with PBS, then mounted with ProLong Gold antifade reagent. Imaging was performed on a Zeiss LSM 510 confocal laser microscope at the Cellular Imaging Core Facility at The University of Arizona.
Hexokinase Activity Assay
Hexokinase activity was measured via a standard Glc 6-P dehydrogenase coupled spectrophotometric method as described previously (Robey et al., 2000; Robey et al., 2002). Briefly, hexokinase activity was determined as the total glucose phosphorylating capacity of whole cell lysates, and reported as U/mg total protein, where 1 unit (U) corresponds to the level of enzyme activity resulting in the phosphorylation of 1 μmol of glucose per minute. Data is presented as the mean ± SEM of three independent experiments, with statistical analysis performed as described earlier with p < 0.01.
Results
The HKII mRNA transcript is up-regulated in the kidneys of arsenic exposed mice
In our preliminary microarray studies, we found increased expression of the HKII mRNA transcript in the kidneys of mice that were exposed to low levels of arsenic in their drinking water. In these studies, mice were exposed to 50 ppb arsenic for 21 days, after which their kidneys were harvested for RNA isolation and microarray analysis. Gene expression patterns were analyzed using Affymetrix GeneChip 420 A mouse genome arrays and quantitative analysis performed using the GC-RMA statistical algorithm (n = 3 for each condition, p < 0.05). A 5.1 ± 0.8 fold increase in HKII mRNA levels relative to control was observed in the arsenic exposed mice. It should be noted that, since whole kidney tissue was utilized for RNA extraction, this value represents the average up-regulation of HKII within the entire kidney, and is probably an underestimation of HKII in the glomerular regions, which occupy a relatively small volume of the kidney.
Arsenic induced HKII protein expression is localized in the cortical glomeruli
To confirm the up-regulation of HKII protein and localize its expression, immunohistochemical staining was performed on formalin-fixed, paraffin-embedded kidney sections. In this study, mice were exposed to either 10 ppb or 50 ppb arsenic via their drinking water for 21 days. Kidneys were then harvested, fixed and embedded in paraffin. Tissue sections were probed using a HKII isoform specific primary antibody and matched HRP-conjugated secondary antibody, and metal enhanced DAB used as the chromogen for colorimetric detection. In agreement with our microarray data, substantial increases in HKII were observed in both of the arsenic exposed groups, with the most significant increases noted in the cortical glomeruli.
Representative images of renal cortexes from both arsenic exposed and control mice are shown in figure 1. In the normal kidneys, very light, diffuse staining was noted in the glomeruli, indicative of the low levels of basal HKII present within these structures (Fig. 1A). In contrast, the expression levels observed in the arsenic exposed mice were markedly different (Fig. 1B & C). In the kidneys of the exposed mice, heavy staining was seen within the glomeruli, demonstrating substantially increased HKII expression within these structures. As shown, the enhanced glomerular HKII expression was a widespread phenomenon, occurring throughout the renal cortex in the arsenic exposed mice.
Fig. 1.
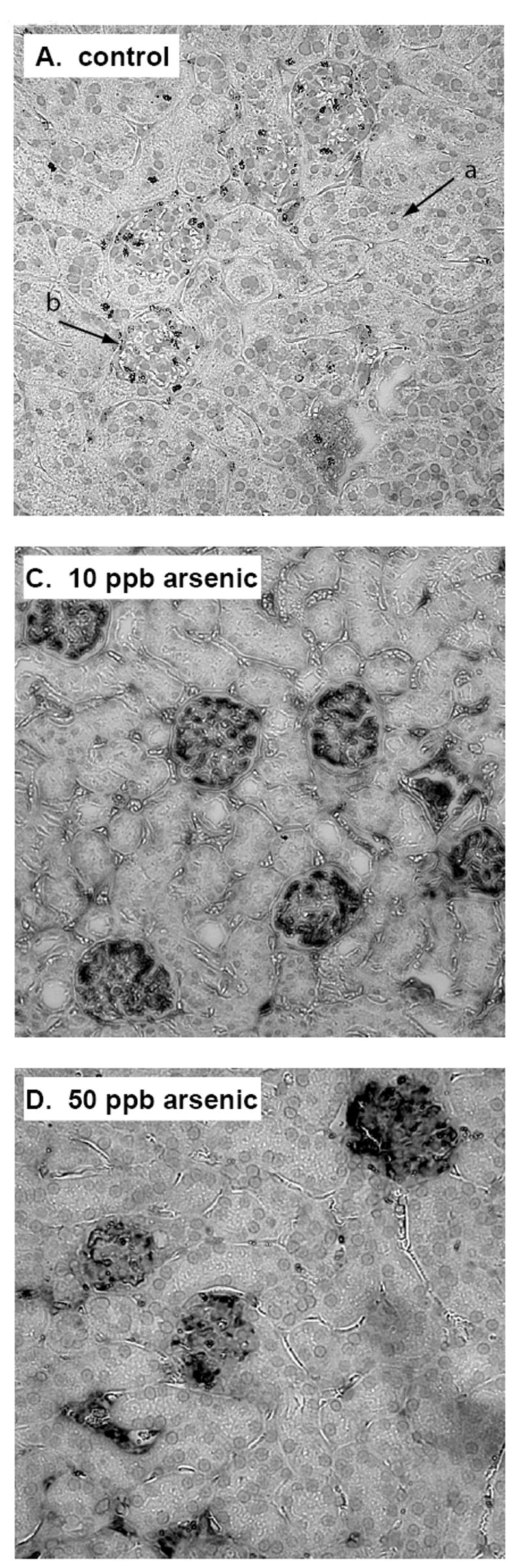
Immunostaining for HKII expression in the kidneys of control mice (A & B), and mice exposed to 10 ppb arsenic (C), and 50 ppb arsenic for 21 days. Tissue sections were probed with a HKII isoform specific antibody and matched HRP-linked secondary antibody, then developed with metal enhanced DAB and counterstained with nuclear fast red. HKII appears as dark grey to black staining within the glomeruli. The cytoplasm appears light grey, and the counterstained nuclei as the slightly darker round inclusions (arrow a). Arrow b indicates the location of an individual glomerulus for reference.
The increase in staining intensity is also evident on comparison of a single glomerulus in an exposed tissue sample to one in a control tissue sample (Fig. 2). Images of glomeruli of similar shape and size are shown along with 2-D densitometry plots created in Scion Image. The Y-axis is a measure of mean staining intensity in arbitrary units assigned during analysis. At the time of analysis each was an 8-bit, 256-grayscale image 400 pixels wide by 366 pixels in height. Each Y-value represents the mean staining intensity of the column of pixels directly beneath it in the relevant image, which has been normalized to the average background value to aid in comparison. Therefore, the representative glomerulus from the arsenic exposed mouse shown exhibits an approximate 3-fold increase in the mean staining intensity compared to the glomerulus from the control mouse.
Fig. 2.
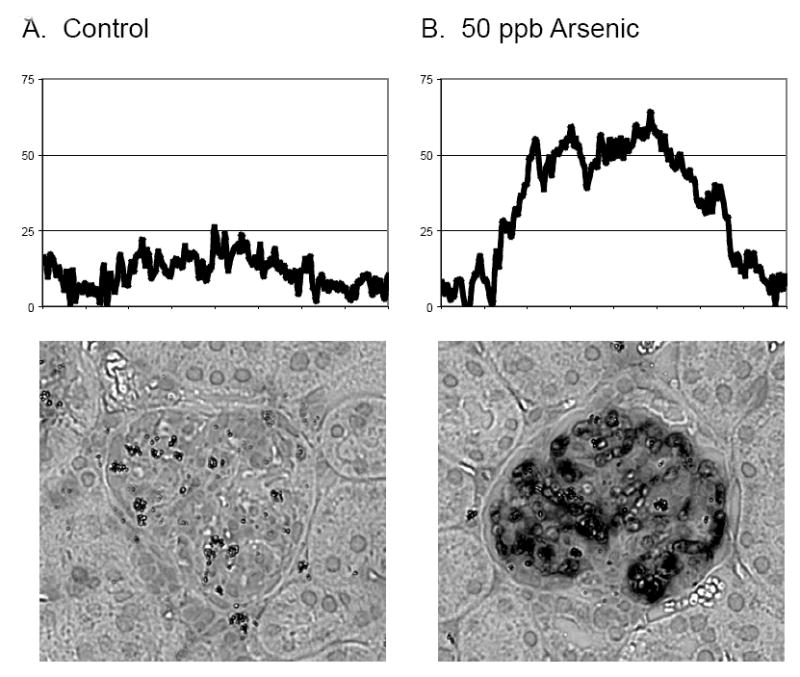
Comparison of staining intensity, and corresponding HKII II levels, in representative glomeruli from control (A) and 50 ppb arsenic exposed mice (B). Tissue sections were stained as described in figure 1. The relative intensity of staining was compared by creating 2-D densitometry plots in Scion Image. Each 8-bit, 256-grayscale image (400 pixels wide by 366 pixels in height) was first normalized to their background signal intensities. In the densitometry plots, each Y-value represents the mean staining intensity of the column of pixels directly beneath it in the corresponding image. The mean staining intensity values on the Y-axis are arbitrary units based on the numerical intensity values to each pixel according to the 256-greyscale convention of shading.
Concomitant increase in HKII levels in the urine of arsenic exposed mice
To evaluate the potential use of HKII as a biomarker for arsenic exposure, we measured urinary HKII levels by SDS-PAGE and Western blot. In this study, mice were exposed to either 10 ppb or 50 ppb arsenic via their drinking water for up to 21 days, and urine collected at the end of the exposure periods. Equivalent amounts of urine (40 μl) were separated on 10% polyacrylamide gels, transferred to nitrocellulose membranes, and the membranes probed for HKII. Surprisingly, we found that the amount of HKII in the urine was significantly increased after only 3 days of exposure to 10 ppb arsenic (Fig. 3). Urine was collected from the mice at the time of euthanasia, and each sample represents a different animal within the study. Quantitative analysis by densitometry shows approximate fold increases of 13 and 31 on day 3, 6 and 8 on day 7, 23 and 35 on day 14, and 12 and 12 on day 21 for the 10 ppb and 50 ppb arsenic exposed groups respectively compared to controls, with p < 0.10 for the 10 ppb groups and p < 0.05 for the 50 ppb groups on days 3 and 7 and p < 0.01 for all other values. Statistical analysis confirms that the large and seemingly widely varying values obtained result from comparison to the very low, or undetectable, levels of HKII in the urine of the control mice. As shown, the mice in both exposure groups continued to exhibit increased urinary output of HKII throughout the study.
Fig. 3.
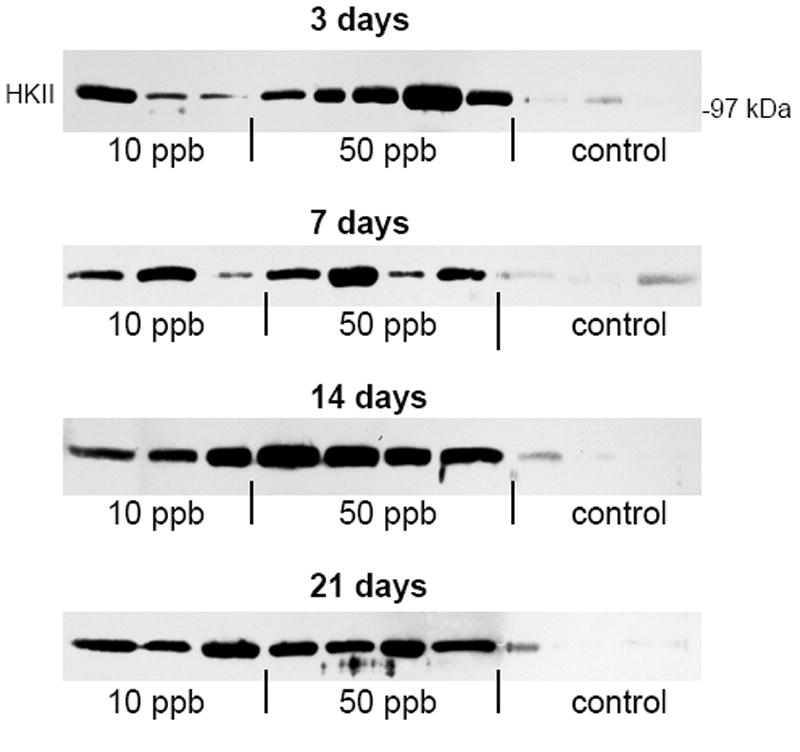
Levels of urinary HKII in control, 10 ppb arsenic, and 50 ppb arsenic exposed mice. Mouse urine was collected after 3, 7, 14, and 21 days of arsenic exposure. Equivalent volumes (40 μL) of each sample were resolved by SDS-PAGE and HKII levels measured by Western blot.
As mentioned, each sample was normalized by volume before loading and analysis. The total amount of urinary protein typically varies between tests samples, depending on such factors as liquid intake, total urinary output, and disease state. The low solute concentrations investigated here would not typically effect water consumption or total urinary output. While water consumption was not specifically measured during this study, signs of polydipsia (excessive thirst) or polyuria (excessive urine) were not observed. These findings are further substantiated by the levels of arsenic and arsenic metabolites observed in the plasma and urine of the mice. Levels of arsenite [As(III)], arsenate [As(V)], and the mono- and dimethylated metabolites [MMA(V) and DMA(V)] in plasma and urine were measured by HPLC/ICP-MS and statistically significant differences between the treated and control groups assessed by ANOVA and Bonferroni’s multiple comparison tests. After 21 days of exposure to either 10 or 50 ppb arsenite, there was no statistically significant difference in the urinary or plasma levels of As(III), which further supports our observations regarding no noticeable change in liquid intake, or urinary output. A statistically significant increase in plasma DMA(V), and urinary As(V), MMA(V) and DMA(V) was observed in the 50 ppb exposure group, and urinary As(V) and DMA(V) in the 10 ppb exposure group compared to control (p < 0.10 for urinary As(V) in the 10 ppb group, p < 0.05 for all others). The increased levels of metabolites confirm that significant arsenic exposure occurred in the mice that were given drinking water containing 10 ppb or 50 ppb arsenic.
Proteinuria is typically associated with chronic conditions in which the glomerular basement membrane becomes compromised, thus allowing extravasation of serum proteins into Bowman’s capsule. As the membrane progressively deteriorates, it becomes increasingly leaky, allowing passage of increasingly larger proteins. However, this is not the most likely explanation for our observations. HKII is a relatively large protein (100 kDa), which would indicate significant damage to the basement membrane. In the present study, substantial increases in urinary HKII were observed after as little as 3 days of arsenic exposure. It is unlikely that the glomerular basement membrane would sustain such damage in this short of a time frame. Instead, it is more likely that the HKII originated in renal cells and was directly deposited into the glomerular filtrate. This suggests that urinary HKII level may be a useful indicator of early renal response to arsenic exposure.
Arsenic induces up-regulation of the HKII mRNA transcript in cultured mesangial cells
To further characterize the effects of arsenic exposure on renal cells, in vitro studies were performed using renal mesangial cells as a model system. The use of an in vitro model eliminates potential confounding effects from other tissues, making it easier to differentiate between direct and indirect effects of arsenic on the kidney. In this study, we used SV40 MES 13 murine mesangial cells, which exhibit normal biochemical and morphological features in culture (Robey et al., 2000; Robey et al., 2002). Cultured mesangial cells were exposed to arsenic for the specified time periods, after which RNA and total cellular protein were isolated using TRIzol. The extracted RNA was used to produce cDNA by reverse transcriptase, and gene specific primers used to amplify the HKII mRNA transcript. The amplified PCR product was then electrophoretically resolved on a 1% agarose gel for analysis. As shown in figure 4, exposure to either 10 ppb or 50 ppb arsenic resulted in a substantial increase in HKII mRNA transcript levels within 12 hours (1.87 ± 0.08 and 2.85 ± 0.25 fold for 10 ppb and 50 ppb treated, respectively), and these levels remained elevated after 24 hours of exposure (2.55 ± 0.15 and 2.56 ± 0.13 fold for 10 ppb and 50 ppb treated, respectively). Fold-increase values are presented as the mean ± SEM of three replicate experiments as quantitated by densitometry followed by statistical analysis showing significant differences (p < 0.01) for each treatment group compared to control. Moreover, these results demonstrate that arsenic has a direct effect on mesangial cells, thus suggesting that increased HKII expression in the mouse kidney is due to a direct effect of arsenic on the kidney, rather than a systemic alteration that is manifested in the kidney.
Fig. 4.
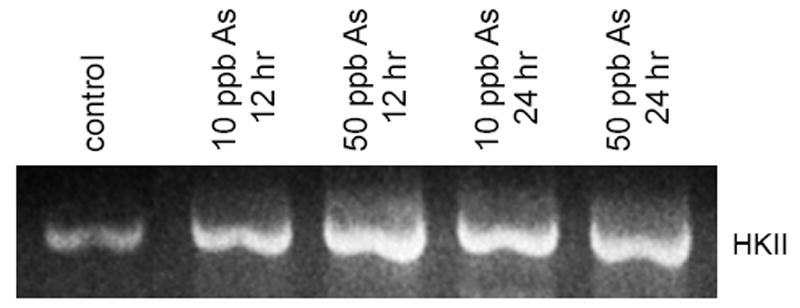
Expression of the HKII mRNA transcript in cultured mesangial cells. SV40 MES 13 mesangial cells were treated with either 0, 10 ppb, or 50 ppb arsenic for 12 or 24 hours. Following treatment, RNA was extracted and used as a template for production cDNA via reverse transcriptase. Gene specific primers were used to amplify the HKII transcript, and the PCR product electrophoretically resolved on a 1% agarose gel for semi-quantitative analysis.
Arsenic induces increased HKII protein expression in cultured mesangial cells
Although mRNA transcript levels would typically reflect alterations in transcriptional regulation under the conditions investigated here, they are not always indicative of protein translation and expression levels. To confirm the up-regulation of HKII at the protein level, the total protein extracts from cell lysates were analyzed by SDS-PAGE and Western blot. Cultured mesangial cells were exposed to arsenic, then total cellular protein isolated as described above. Equivalent amounts of total protein were separated on a 10% polyacrylamide gel, transferred to nitrocellulose membrane, and the membranes probed for HKII. Consistent with our PCR data, increased expression of HKII was evident as early as 12 hours after arsenic exposure was initiated and continued to be elevated throughout the time course of the study. Figure 5 shows a representative blot of lysates from mesangial cells that were untreated (lane 1), treated for 12 hours with 10 ppb (lane 2) and 50 ppb (lane 3) arsenic, and treated for 24 hours with 10 ppb (lane 4) and 50 ppb (lane 5) arsenic. As can be seen, the increased expression of HKII appears to be both time and dose dependent in the mesangial cells. Exposure to 10 ppb and 50 ppb arsenic led to a 3.03 ± 0.04 and 4.15 ± 0.06 fold increase after 12 hours, and a 4.33 ± 0.10 and 5.39 ± 0.07 fold increase in HKII protein levels after 24 hours, respectively. Fold-increase values are presented as the mean ± SEM for three replicate experiments as quntitated by densitometry, with statistical analysis showing significant differences (p < 0.05) for each treatment group compared to control.
Fig. 5.
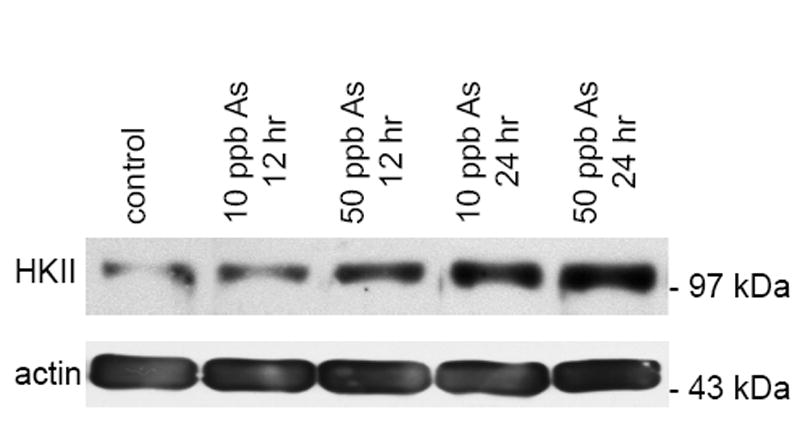
Western blot analysis of HKII protein expression in cultured mesangial cells. SV40 MES 13 mesangial cells were treated with either 0, 10 ppb, or 50 ppb arsenic for 12 or 24 hours. Total protein was isolated from whole cell lysates according to the TRIzol method. Equivalent amounts of total protein (100 μg) were analyzed by SDS-PAGE and Western blot with actin as a loading control.
The expressed HKII is highly co-localized with the mitochondria in arsenic treated mesangial cells
As previously mentioned, mitochondrial associated HKII has been linked to both malignant transformation and evasion of apoptosis. Therefore, it was important to determine the location of the expressed HKII within the arsenic treated cells. To accomplish this task, we used immunofluorescence staining techniques. Mesangial cells were exposed to arsenic for 24 hours then fixed, permeablized and stained with fluorescently labeled antibodies. The mitochondria were stained with MitoTracker Deep Red, HKII was stained with an isoform specific primary antibody and matched fluorescent secondary antibody, and the nuclei counterstained with DAPI. The cells were then imaged on a Zeiss LSM 510 confocal laser microscope (Fig. 6). As shown, the expressed HKII protein was highly co-localized with the mitochondria in the arsenic treated cells.
Fig. 6.
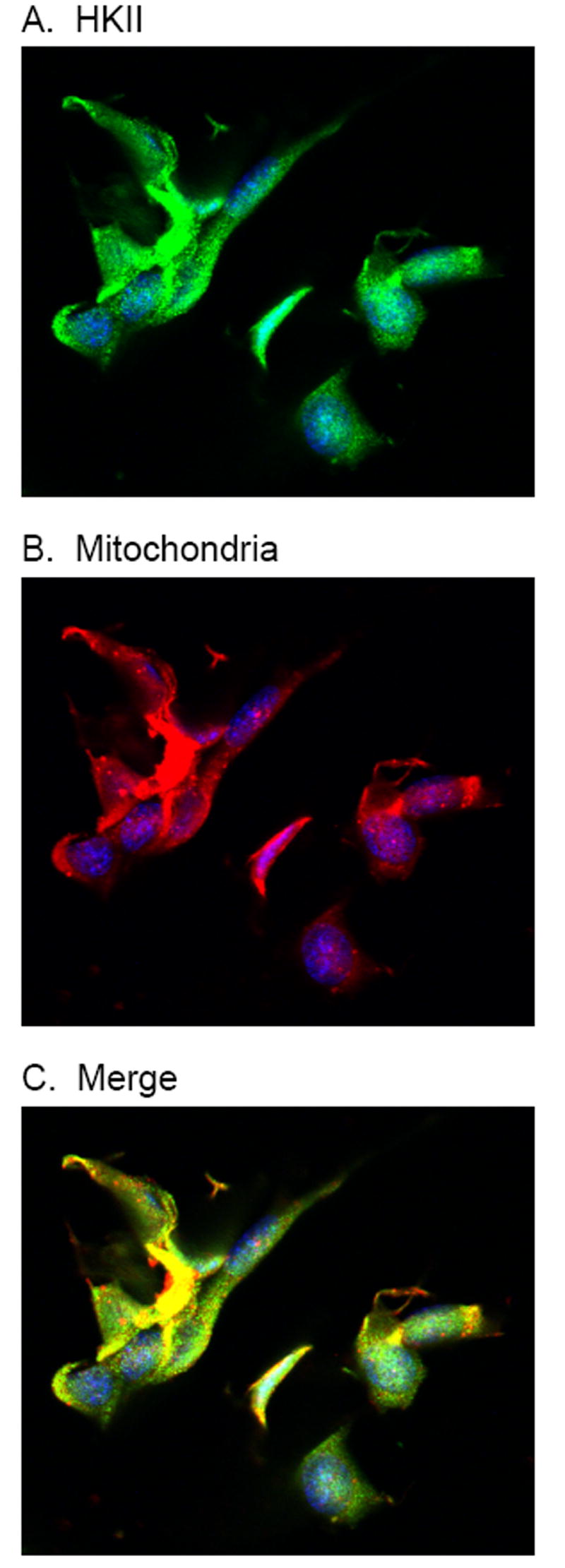
Immunofluorescence imaging of arsenic treated mesangial cells. Cultured mesangial cells were treated with 50 ppb arsenic for 24 hours, then fixed and stained. HKII was with an isoform specific primary and matched fluorescent secondary antibody (A), mitochondria were stained with MitoTracker Deep Red (B), and nuclei counterstained with DAPI (blue). The merged image (C) shows the co-localization of HKII with the mitochondria.
The expressed HKII is catalytically active
Because increased protein expression does not necessarily correlate with increased activity, it was important to also confirm that the arsenic-induced increases in HKII expression corresponded with increased levels of enzymatic activity. To evaluate the enzymatic activity of the HKII, the total glucose phosphorylating capacity of cell lysates was measured using a standard spectrophotometric Glc 6-P dehydrogenase-coupled assay (Robey et al., 2000; Robey et al., 2002). The measured hexokinase activity was 10.15 U/mg +/- 0.39, 15.43 U/mg +/- 0.35, and 36.35 U/mg +/- 2.66 for the control, 10 ppb arsenic treated, and 50 ppb arsenic treated cells respectively (reported as mean ± SEM, n = 3, p < 0.01), where activity is reported as units of glucose phosphorylated per mg of total cellular protein. Thus, the up-regulation of HKII in mesangial cells was accompanied by a corresponding increase in enzymatic activity.
Discussion
In this study, we demonstrate the strong up-regulation of renal HKII in mice exposed to low levels of arsenic via their drinking water. Immunohistochemical staining localized HKII expression primarily within the cortical glomeruli (Fig. 1). Additionally, in vitro studies in cultured renal mesangial cells showed enhanced expression of HKII in response to arsenic treatment. This correlation between our in vivo and in vitro data provides further evidence for a direct link between altered glomerular HKII expression and arsenic exposure. More importantly, these results were obtained using environmentally relevant arsenic concentrations. In fact, the concentrations used (50 ppb and 10 ppb) represent the old and new standards for maximal levels of arsenic in drinking water.
The principal functional unit of the kidney is the nephron, which consists of the renal corpuscle, the glomerulus and its associated tubule. In the process of plasma filtration and urine formation, the blood circulates through specialized glomerular capillaries, which have a fenestrated endothelium, immediately outside of which lies the filtration, or basement membrane. In a normally functioning kidney, the fenestrations are small enough to retain cells and serum proteins within the capillaries while allowing water and small molecules to pass freely through the filtration membrane and into Bowman’s space where they subsequently drain into the proximal tubule. Once in the tubule, the filtrate is further processed into urine through the selective reabsorption of components such as water, ions and small proteins. Because the components of the nephron are intricately tied to one another, disruptions in any portion of the system can adversely affect all other components. For instance, uncontrolled proliferation of cells within the glomerulus can constrict capillaries, alter glomerular blood pressure and rate of filtration, and compromise the integrity of the basement membrane (Schlondorff, 1987).
Mesangial cells, modified smooth muscle cells located primarily within the glomeruli, are the primary renal cell type affected by a variety of pathological conditions such as diabetes and glomerulonephritis (Whiteside and Dlugosz, 2002; Haneda et al., 2003; Mason and Wahab, 2003). Mesangial cells can both produce and respond to a wide variety of signaling molecules including prostaglandins, cytokines, regulators of proliferation, vasorelaxants and constrictors. Through their contractility, they are capable of regulating the glomerular filtration rate and, consequently, intrarenal blood pressure (Schlondorff, 1987). Additionally, they produce extracellular proteins including collagen, laminin, and fibronectin, which are components of both the glomerular mesangial matrix and basement membrane. Composition and turnover of these components are important factors in the maintenance of homeostasis and filtration membrane integrity. Degradation, accumulation, or post-translational modification of these proteins can affect the structural integrity of the basement membrane, leading to the leakage of serum proteins into the filtrate and a variety of pathological conditions such as necrosis and proteinuria (Whiteside and Dlugosz, 2002; Haneda et al., 2003; Mason and Wahab, 2003). In addition, uncontrolled mesangial cell proliferation can lead to decreased filtration rates and renal hypertension (Whiteside and Dlugosz, 2002; Haneda et al., 2003; Mason and Wahab, 2003). Thus, mesangial cells provide a highly suitable model for studying the effects of renal toxicants.
The discovery of high levels of HKII in the renal cortex of arsenic exposed mice has considerable biological implications. Its enzymatic product, Glc 6-P, not only serves as a precursor for glycolysis, but also feeds into cellular growth and survival pathways leading to ATP generation, nucleic and fatty acid biosynthesis, and NADPH production. Thus, in general, enhanced expression of HKII dysregulates cellular metabolism and promotes cellular proliferation. In fact, this increased rate of metabolism and biosynthesis is believed to be the primary mechanism by which the high growth rate of many rapidly proliferating tumor cells is supported (Bustamante and Pedersen, 1977; Bustamante and Pedersen, 1980; Bustamante et al., 1981; Arora and Pedersen, 1988; Pedersen et al., 2002; Lee and Pedersen, 2003). Accordingly, it may also be an important contributor to the pathological process involved in arsenic-related cancerous and non-cancerous conditions involving the kidney. As previously mentioned, overexpression of HKII has been linked to malignant transformation and tumor metastasis. Thus, it is conceivable that enhanced expression of HKII may also represent an early stage in arsenic induced dysregulation of the cell cycle and progression towards a neoplastic phenotype.
In addition to the abovementioned associations, alterations in HKII expression have been implicated in non-cancerous pathological disorders such as diabetes mellitus (Katzen et al., 1970; Anderson and Stowring, 1973). Of particular interest with respect to mesangial cells is the fact that while diabetes is typically associated with a state of glucose underutilization in insulin sensitive tissues, a state of glucose overutilization occurs in the renal cortex (Robey et al., 2000). This may be explained, in part, by the differential expression patterns of glucose transporters (GLUTs) within these tissues. The primary glucose transporter expressed in renal mesangial cells, GLUT 1, is a non-insulin dependent transporter, as opposed to the insulin dependent GLUT 4, which is the most abundant transporter found in many insulin sensitive tissues, such as adipose. Therefore, in mesangial cells glucose uptake and intracellular availability for phosphorylation by HKII does not rely on systemic or local insulin levels or activity. Instead, increases in blood glucose levels will result in increased intracellular glucose levels and, consequently, greater substrate availability (Haneda et al., 2003).
The intracellular location of HKII has also been shown to be an important factor in enzymatic activity, structural stability, and evasion of apoptosis. HKII is known to exist in both a cytoplasmic and mitochondrial associated state, each having differing catalytic efficiencies (Bustamante and Pedersen, 1980; Parry and Pedersen, 1984; Arora and Pedersen, 1988). The mitochondrial associated state exhibits greater enzymatic efficiency, presumably through its binding with VDAC. HKII mediated phosphorylation of glucose proceeds via an ATP dependent pathway and, through its association with VDAC, HKII is able to gain preferential access to mitochondrial generated ATP, thus enabling a greater sustained rate of activity (Arora and Pedersen, 1988). This bound form has also been shown to be less susceptible to inhibition by Glc 6-P. Additionally, mitochondrial associated HKII has been shown to block Bax mediated apoptosis via this same VDAC association (Gottlob et al., 2001; Pastorino et al., 2002; Azoulay-Zohar et al., 2004). In agreement with previous studies in tumor cell lines, we found HKII expression in arsenic treated mesangial cells to be highly co-localized with the mitochondria. Furthermore, increased hexokinase enzymatic activity was also observed in conjunction with the increases in HKII expression, confirming that the over expressed HKII was catalytically active.
In summary, doses of arsenic in the parts per billion range in the drinking water of mice increase the expression of HKII in the kidney. To better understand the significance of elevated HKII expression, future studies will need to determine how arsenic regulates HKII transcription or protein turnover. The data presented in this study demonstrate that arsenic has profound effects on the mouse kidney. The molecular effects of arsenic on the human kidney are not known, however it is known that reducing arsenic exposure in humans reduces renal disease mortality in exposed populations (Chiu and Yang, 2005). In future studies, it would be useful to correlate HKII expression in exposed populations to determine whether decreased renal disease mortality correlates with decreased HKII expression.
Acknowledgments
This work was supported, in part, by National Institutes of Health Superfund Basic Research Grant ES04940 and NIH grants ES12007, AG19710, and ES06694 (R.R.V), and HL67067 (J.B.H). M.D.P was supported in part by a post-doctoral fellowship from the Interdisciplinary Physiological Sciences Program at the University of Arizona (National Institutes of Health Training Grant HL07249).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JW, Stowring L. Glycolytic And Gluconeogenic Enzyme-Activities In Renal Cortex Of Diabetic Rats. Am J Physiol. 1973;224:930–936. doi: 10.1152/ajplegacy.1973.224.4.930. [DOI] [PubMed] [Google Scholar]
- Arora KK, Pedersen PL. Functional-Significance Of Mitochondrial Bound Hexokinase In Tumor-Cell Metabolism - Evidence For Preferential Phosphorylation Of Glucose By Intramitochondrially Generated Atp. J Biol Chem. 1988;263:17422–17428. [PubMed] [Google Scholar]
- Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. In self-defence: Hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem J. 2004;377:347–355. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates MN, Rey OA, Biggs ML, Hopenhayn C, Moore LE, Kalman D, Steinmaus C, Smith AH. Case-control study of bladder cancer and exposure to arsenic in Argentina. Am J Epidemiol. 2004;159:381–389. doi: 10.1093/aje/kwh054. [DOI] [PubMed] [Google Scholar]
- Bradford MM. Rapid And Sensitive Method For Quantitation Of Microgram Quantities Of Protein Utilizing Principle Of Protein-Dye Binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryson JM, Coy PE, Gottlob K, Hay N, Robey RB. Increased hexokinase activity, of either ectopic or endogenous origin, protects renal epithelial cells against acute oxidant-induced cell death. J Biol Chem. 2002;277:11392–11400. doi: 10.1074/jbc.M110927200. [DOI] [PubMed] [Google Scholar]
- Burns FJ, Uddin AN, Wu F, Nadas A, Rossman TG. Arsenic-induced enhancement of ultraviolet radiation carcinogenesis in mouse skin: A dose-response study. Environ Health Perspect. 2004;112:599–603. doi: 10.1289/ehp.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante E, Morris HP, Pedersen PL. Energy-Metabolism Of Tumor-Cells - Requirement For A Form Of Hexokinase With A Propensity For Mitochondrial Binding. J Biol Chem. 1981;256:8699–8704. [PubMed] [Google Scholar]
- Bustamante E, Pedersen PL. High Aerobic Glycolysis Of Rat Hepatoma-Cells In Culture - Role Of Mitochondrial Hexokinase - (L-Lactic Acid-D-Glucose-D-Galactose-Liver-Neoplasia) Proc Natl Acad Sci U S A. 1977;74:3735–3739. doi: 10.1073/pnas.74.9.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante E, Pedersen PL. Mitochondrial Hexokinase Of Rat Hepatoma-Cells In Culture - Solubilization And Kinetic-Properties. Biochemistry. 1980;19:4972–4977. doi: 10.1021/bi00563a006. [DOI] [PubMed] [Google Scholar]
- Centeno JA, Mullick FG, Martinez L, Page NP, Gibb H, Longfellow D, Thompson C, Ladich ER. Pathology related to chronic arsenic exposure. Environ Health Perspect. 2002;110:883–886. doi: 10.1289/ehp.02110s5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HF, Yang CY. Decreasing trend in renal disease mortality after cessation from arsenic exposure in a previous arseniasis-endemic area in southwestern Taiwan. J Toxicol Env Health Part A. 2005;68:319–327. doi: 10.1080/15287390590900804. [DOI] [PubMed] [Google Scholar]
- Cui X, Li S, Shraim A, Kobayashi Y, Hayakawa T, Kanno S, Yamamoto M, Hirano S. Subchronic exposure to arsenic through drinking water alters expression of cancer-related genes in rat liver. Toxicol Pathol. 2004;32:64–72. doi: 10.1080/01926230490261348. [DOI] [PubMed] [Google Scholar]
- Frumkin H, Thun MJ. Arsenic. CA-Cancer J Clin. 2001;51:254–262. doi: 10.3322/canjclin.51.4.254. [DOI] [PubMed] [Google Scholar]
- Germolec DR, Spalding J, Yu HS, Chen GS, Simeonova PP, Humble MC, Bruccoleri A, Boorman GA, Foley JF, Yoshida T, Luster MI. Arsenic enhancement of skin neoplasia by chronic stimulation of growth factors. Am J Pathol. 1998;153:1775–1785. doi: 10.1016/S0002-9440(10)65692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering PL, Aposhian HV, Mass MJ, Cebrian M, Beck BD, Waalkes MP. The enigma of arsenic carcinogenesis: Role of metabolism. Toxicol Sci. 1999;49:5–14. doi: 10.1093/toxsci/49.1.5. [DOI] [PubMed] [Google Scholar]
- Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneda M, Koya D, Isono M, Kikkawa R. Overview of glucose signaling in mesangial cells in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1374–1382. doi: 10.1097/01.asn.0000064500.89551.76. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Biggs ML, Smith AH. Lung and kidney cancer mortality associated with arsenic in drinking water in Cordoba, Argentina. Int J Epidemiol. 1998;27:561–569. doi: 10.1093/ije/27.4.561. [DOI] [PubMed] [Google Scholar]
- Katzen HM, Soderman DD, Wiley CE. Multiple Forms Of Hexokinase - Activities Associated With Subcellular Particulate And Soluble Fractions Of Normal And Streptozotocin Diabetic Rat Tissues. J Biol Chem. 1970;245:4081–&. [PubMed] [Google Scholar]
- Kitchin KT. Recent advances in arsenic carcinogenesis: Modes of action, animal model systems, and methylated arsenic metabolites. Toxicol Appl Pharmacol. 2001;172:249–261. doi: 10.1006/taap.2001.9157. [DOI] [PubMed] [Google Scholar]
- Lee MG, Pedersen PL. Glucose metabolism in cancer - Importance of transcription factor-DNA interactions within a short segment of the proximal region of the type II hexokinase promoter. J Biol Chem. 2003;278:41047–41058. doi: 10.1074/jbc.M307031200. [DOI] [PubMed] [Google Scholar]
- Lu T, Liu J, LeCluyse EL, Zhou YS, Cheng ML, Waalkes MP. Application of cDNA microarray to the study of arsenic-induced liver diseases in the population of Guizhou, China. Toxicol Sci. 2001;59:185–192. doi: 10.1093/toxsci/59.1.185. [DOI] [PubMed] [Google Scholar]
- Majewski N, Nogueira V, Robey RB, Hay N. Akt inhibits apoptosis downstream of BID cleavage via a glucose-dependent mechanism involving mitochondrial hexokinases. Mol Cell Biol. 2004;24:730–740. doi: 10.1128/MCB.24.2.730-740.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1358–1373. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- Mass MJ, Tennant A, Roop BC, Cullen WR, Styblo M, Thomas DJ, Kligerman AD. Methylated trivalent arsenic species are genotoxic. Chem Res Toxicol. 2001;14:355–361. doi: 10.1021/tx000251l. [DOI] [PubMed] [Google Scholar]
- Okoji RS, Yu RC, Maronpot RR, Froines JR. Sodium arsenite administration via drinking water increases genome-wide and Ha-ras DNA hypomethylation in methyl-deficient C57BL/6J mice. Carcinogenesis. 2002;23:777–785. doi: 10.1093/carcin/23.5.777. [DOI] [PubMed] [Google Scholar]
- Parry DM, Pedersen PL. Intracellular-Localization Of Rat-Kidney Hexokinase - Evidence For An Association With Low-Density Mitochondria. J Biol Chem. 1984;259:8917–8923. [PubMed] [Google Scholar]
- Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- Pedersen PL, Mathupala S, Rempel A, Geschwind JF, Ko YH. Mitochondrial bound type II hexokinase: a key player in the growth and survival of many cancers and an ideal prospect for therapeutic intervention. Biochim Biophys Acta-Bioenerg. 2002;1555:14–20. doi: 10.1016/s0005-2728(02)00248-7. [DOI] [PubMed] [Google Scholar]
- Ratnaike RN. Acute and chronic arsenic toxicity. Postgrad Med J. 2003;79:391–396. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MA, Gregg JP, Qin Q, Phillips MA, Rice RH. Global alteration of gene expression in human keratinocytes by inorganic arsenic. Carcinogenesis. 2003;24:747–756. doi: 10.1093/carcin/bgg010. [DOI] [PubMed] [Google Scholar]
- Robey RB, Ma JF, Santos AVP, Noboa OA, Coy PE, Bryson JM. Regulation of mesangial cell hexokinase activity and expression by heparin-binding epidermal growth factor-like growth factor - Epidermal growth factors and phorbol esters increase glucose metabolism via a common mechanism involving classic mitogen-activated protein kinase pathway activation and induction of hexokinase II expression. J Biol Chem. 2002;277:14370–14378. doi: 10.1074/jbc.M111722200. [DOI] [PubMed] [Google Scholar]
- Robey RB, Raval BJ, Ma JF, Santos AVP. Thrombin is a novel regulator of hexokinase activity in mesangial cells. Kidney Int. 2000;57:2308–2318. doi: 10.1046/j.1523-1755.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: An animal model for arsenic carcinogenesis. Toxicol Appl Pharmacol. 2001;176:64–71. doi: 10.1006/taap.2001.9277. [DOI] [PubMed] [Google Scholar]
- Schlondorff D. The Glomerular Mesangial Cell - an Expanding Role for a Specialized Pericyte. Faseb J. 1987;1:272–281. doi: 10.1096/fasebj.1.4.3308611. [DOI] [PubMed] [Google Scholar]
- Schoen A, Beck B, Sharma R, Dube E. Arsenic toxicity at low doses: epidemiological and mode of action considerations. Toxicol Appl Pharmacol. 2004;198:253–267. doi: 10.1016/j.taap.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Simeonova PP, Luster MI. Arsenic and atherosclerosis. Toxicol Appl Pharmacol. 2004;198:444–449. doi: 10.1016/j.taap.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Simeonova PP, Wang SY, Toriuma W, Kommineni V, Matheson J, Unimye N, Kayama F, Harki D, Ding M, Vallyathan V, Luster MI. Arsenic mediates cell proliferation and gene expression in the bladder epithelium: Association with activating protein-1 transactivation. Cancer Res. 2000;60:3445–3453. [PubMed] [Google Scholar]
- Steer KA, Sochor M, Gonzalez AM, McLean P. Regulation Of Pathways Of Glucose-Metabolism In Kidney - Specific Linking Of Pentose-Phosphate Pathway Activity With Kidney Growth In Experimental Diabetes And Unilateral Nephrectomy. FEBS Lett. 1982;150:494–498. doi: 10.1016/0014-5793(82)80797-7. [DOI] [PubMed] [Google Scholar]
- Styblo M, Drobna Z, Jaspers I, Lin S, Thomas DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic: A research update. Environ Health Perspect. 2002;110:767–771. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GF. Arsenic contamination and arsenicosis in China. Toxicol Appl Pharmacol. 2004;198:268–271. doi: 10.1016/j.taap.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Tseng CH. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol Appl Pharmacol. 2004;197:67–83. doi: 10.1016/j.taap.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Tseng CH, Chong CK, Heng LT, Tseng CP, Tai TY. The incidence of type 2 diabetes mellitus in Taiwan. Diabetes Res Clin Pract. 2000a;50:S61–S64. doi: 10.1016/s0168-8227(00)00180-7. [DOI] [PubMed] [Google Scholar]
- Tseng CH, Tai TY, Chong CK, Tseng CP, Lai MS, Lin BJ, Chiou HY, Hsueh YM, Hsu KH, Chen CJ. Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: A cohort study in arseniasis-hyperendemic villages in Taiwan. Environ Health Perspect. 2000b;108:847–851. doi: 10.1289/ehp.00108847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauson EM, Langan AS, Vorce RL. Sodium arsenite inhibits and reverses expression of adipogenic and fat cell-specific genes during in vitro adipogenesis. Toxicol Sci. 2002;65:211–219. doi: 10.1093/toxsci/65.2.211. [DOI] [PubMed] [Google Scholar]
- Whiteside CI, Dlugosz JA. Mesangial cell protein kinase C isozyme activation in the diabetic milieu. Am J Physiol-Renal Physiol. 2002;282:F975–F980. doi: 10.1152/ajprenal.00014.2002. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Yamauchi H, Sun GF. Chronic health effects in people exposed to arsenic via the drinking water: dose-response relationships in review. Toxicol Appl Pharmacol. 2004;198:243–252. doi: 10.1016/j.taap.2003.10.022. [DOI] [PubMed] [Google Scholar]


