SUMMARY
Lipids play a multitude of roles in intracellular protein transport and membrane traffic. While a large body of data implicates phosphoinositides in these processes, much less is known about other glycerophospholipids, such as phosphatidic acid, diacylglycerol, and phosphatidylserine. Growing evidence suggests that these lipids may also play an important role, either by mediating protein recruitment to membranes or by directly affecting membrane dynamics. Although membrane lipids are believed to be organized in microdomains, recent advances in cellular imaging methods paired with sophisticated reporters and proteomic analysis have led to the formulation of alternative ideas regarding the characteristics and putative functions of lipid microdomains and their associated proteins. In fact the traditional view that membrane proteins may freely diffuse in a large ‘sea of lipids’ may need to be revised. Lastly, modifications of proteins by lipids or related derivatives have surprisingly complex roles on regulated intracellular transport of a wide range of molecules.
INTRODUCTION
Since the identification of the phosphatidylinositol transfer protein (PITP) Sec14p as an essential factor for protein trafficking from the yeast trans-Golgi [1], the concept that lipids are not passive constituents of membranes, but, rather, are active mediators of membrane trafficking events in cells has rapidly gained popularity [2,3]. There is now overwhelming genetic, biochemical and cell biological evidence for a role of various lipid families in organelle biogenesis and transport. In addition, striking improvements in methodologies designed for their analysis and the ever increasing list of their protein effectors, makes this area of research a passage obligé for the understanding of how cells generate and maintain their complex compartmental organization [4]. How precisely the function of lipids as regulators of protein sorting may relate to the formation of microdomains remains a controversial issue, especially in light of recent cellular imaging and proteomics data. Finally, lipids, fatty acids, and related hydrophobic moieties appear to regulate intracellular protein dynamics by covalent, in many cases reversible attachment to proteins. Here, we summarize three basic mechanisms by which lipids and lipid modifications affect intracellular protein transport: the role of specific lipids, particularly glycerolipids, as protein recruiters and mediators of distinct trafficking steps, the formation of lipid microdomains, and the regulation of sorting by covalent modification of proteins.
GLYCEROLIPIDS AS MEDIATORS OF INTRACELLULAR MEMBRANE TRAFFIC
General considerations on glycerolipids
Phosphatidylcholine (PC) and phosphatidylethanolamine (PE) are clearly the most abundant glycerophospholipids in cells and as such, have been largely regarded as structural components of cellular membranes and thus “passive players” in organelle traffic. Therefore, studies of this process have primarily focused on lipids present at lower levels, which are endowed with major regulatory properties. These include mostly anionic phospholipids, such as phosphatidylinositol (PI) and its phosphorylated derivatives (i.e. phosphoinositides), phosphatidic acid (PA), and phosphatidylserine (PS), in addition to diacylglycerol (DAG), which is uncharged [3,5,6]. Under normal conditions and in a variety of membrane compartments, most if not all of these lipids appear to be concentrated in the cytoplasmic leaflet where they can control the cytosol-membrane interface. The relative amount of each lipid varies from one compartment to another and in several instances specific lipids (e.g. phosphoinositides) were shown to be significantly enriched on particular organelles, thereby acting as spatial landmarks for these compartments [5,6]. These lipids, often with the cooperation of other signals, can in turn recruit effector proteins, such as coat components, signaling scaffolds and cytoskeleton regulators, thereby allowing a plethora of processes to occur at the membrane-cytosol interface. This feature is essential for all aspects of membrane trafficking, including budding, fission, transport, tethering and ultimately, fusion. Superimposed to their roles as signaling molecules, physical features, such as the simple geometry of glycerolipids (e.g. “cone shape” vs “inverted-cone shape”), affect the ability of membranes to bend and fuse, thereby underscoring their importance as key intrinsic components of cellular membranes [3].
Roles of phosphatidic acid in membrane dynamics
PA approximately constitutes 1–5% of total cellular lipids [5,7]. In addition to its fundamental role in the biosynthesis of most other phospholipids and triacylglycerols [7], PA has been directly or indirectly implicated in vesicle trafficking, secretion and endocytosis in a variety of cell types. A major pathway for the synthesis of a pool of PA relevant for membrane traffic involves phospholipases D (PLD), which can hydrolyze a variety of substrates to produce PA [8] (Figure 1). In mammals, the best-characterized members of this family, PLD1 and PLD2, hydrolyze primarily PC and thus release free choline in addition to PA [8].
Figure 1.
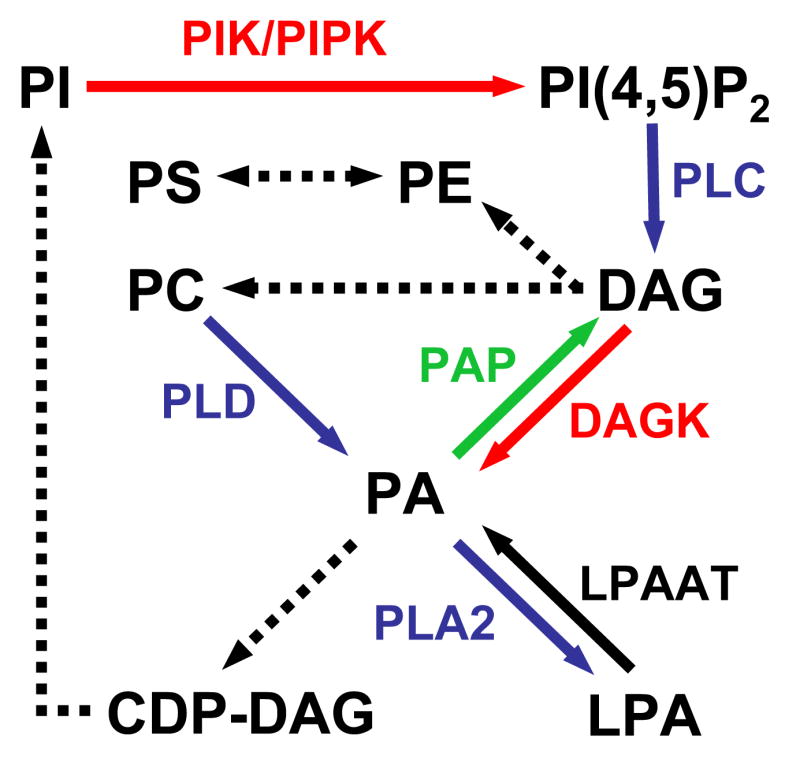
Pathways leading to the synthesis of the main glycerophospholipids. Kinase reactions are shown in red; phosphatase reactions are in green; phospholipases are in blue and acyl transferases are in black. Biosynthetic reactions are indicated by dotted arrows. PIK, phosphatidylinositol kinase; LPAAT, lysophosphatidic acid acyl transferase.
The first evidence for an involvement of PLD in secretion was provided more than a decade ago in permeabilized platelets [9], paving the way for a large number of subsequent studies implicating this pathway and, more specifically PLD1, in the exocytic process in various cell types [8]. While initial functional studies have largely relied on the application of primary alcohols, which divert PLD enzymes from production of PA to phosphatidylalcohol, the recent advance of RNA interference (RNAi) has allowed for a better understanding of the respective PLD isoforms involved and further established a role for these enzymes and their product in membrane fusion.
In a first study, the fusion of GLUT4-containing vesicles with the plasma membrane induced by insulin was shown to be blocked by knocking down PLD1, but rescued by treatment of adipocytes with fusogenic lipids, such as lysoPC [10]. Furthermore, work on the yeast ortholog of PLD, Spo14, has not only provided the first genetic evidence for a role of PLD in membrane fusion, but also identified a SNARE protein, Spo20p (i.e. a SNAP-25 ortholog), as a key effector of PA [11]. Spo14p is dispensable for yeast cells in the vegetative state, but it plays a pivotal role during sporulation following meiosis, where it mediates the coalescence of secretory vesicles into membrane sheets called prospore membrane. In this process, the requirement for PLD reflects, at least in part, the necessity to synthesize on precursor vesicles a pool of PA involved in the recruitment of the t-SNARE protein Spo20, which mediates their fusion [11]. Finally, a recent report has further linked PA to membrane fusion and SNARE proteins in reconstituted liposome fusion assays. A key finding is that PA can either promote or block SNAP23- and VAMP2-mediated liposome fusion, depending on whether it lies on acceptor or donor vesicles, respectively [12]. Interestingly, the converse was observed for PI(4,5)P2, suggesting that the asymmetric distribution of major anionic phospholipids, such as PA and PI(4,5)P2, may be a key determinant of fusion at the plasma membrane [12].
A requirement for PLD and PLD-derived PA in the process of membrane fusion may be broader than previously appreciated. A striking example is that of a mitochondrion-localized PLD isoform (MitoPLD), which is encoded by a distinct ancestral PLD gene and hydrolyzes cardiolipin, rather than PC [13]. This enzyme localizes to the outer membrane of mitochondria, where it appears to act “in trans” (i.e. on a pool of substrate present on a different, but closely localized, mitochondrion) to promote mitochondrion fusion in a mitofusin-dependent fashion [13].
A role for PLD-derived PA was also shown for the endocytic retrieval of various plasma membrane receptors, including the EGF receptor [8]. In these instances, PLD2 appears to be playing a primary role consistent with its enrichment at the plasma membrane in various cells types [8]. Furthermore, an RNAi study has suggested a broader role in the endocytic pathway, i.e. in the recycling of transferrin receptor from recycling endosomes to the cell surface [14]. However, PLD2 has also been localized to compartments distinct from the endosomal system, indicating that it plays multiple roles in vesicular traffic [15]. It is of note that catalysis-independent functions have been also suggested for PLD in the internalization of EGF receptor. More specifically, the Phox homology region of PLD has been shown to directly stimulate the GTPase activity of dynamin (i.e. the main fission factor) [16].
An alternate pathway for the generation of PA involves DAG kinases [17] (Figure 1). These enzymes have been primarily implicated in signaling mechanisms downstream of various cell surface receptors, where they seem to mediate either the termination of DAG signaling, or the synthesis of PA as a bioactive metabolite. Importantly, the pool of PA synthesized by DAG kinases likely differs in many instances from that produced by PLD in terms of fatty acyl composition and may be functionally unrelated [18]. Although there is no clear evidence for the involvement of DAG kinases in membrane traffic, a participation in this process is extremely likely, based on the implication of both DAG (see below) and PA. Furthermore, three independent DAG kinase isoforms were shown to play a role in endocytic traffic based on an RNAi screen of the ”kinome” [19].
PA may be involved in multiple aspects of membrane fusion. While some of its actions likely rely on effector proteins (e.g. SNARE proteins), others may merely reflect its fusogenic properties in lipid bilayers [8]. In the PLD pathway, the hydrolysis of PC into PA converts a cylindrical, non-fusogenic lipid into a cone-shaped, fusogenic lipid that favors negative membrane curvature [3,8]. Furthermore, metabolites originating from the cleavage of PA by other lipases (e.g. the inverted cone-shaped lipid, lysoPA) (Figure 1) may contribute to decreasing the energy required for membrane budding and fusion [8]. Finally, some PA may also fulfill a variety of functions indirectly by promoting PI(4,5)P2 synthesis through a direct activation of Type I PIP kinases [8].
Role of diacylglycerol in vesicle budding and fusion
The first evidence for a role of DAG in membrane trafficking was indirect and came from genetic studies in yeast, where mutations in Sec14p were shown to block transport from the trans-Golgi network (TGN) [1,2]. Sec14p is a PITP that binds to both PI and PC and acts as a negative regulator of PC biosynthesis [2], a pathway that consumes DAG through the CDP-choline metabolic pathway (Figure 1). Thus, in the absence of Sec14p, PC accumulates on Golgi membranes at the expense of DAG. Recently, an RNAi study in mammalian cells has shown that downregulation of Nir2, a protein containing a PITP module, blocks the fission of cell surface-destined transport carriers at the TGN. Because this phenotype correlates with decreased levels of DAG and can be rescued by blocking PC biosynthesis through the CDP-choline pathway, this study reveals a conserved role for PITP family members in the homeostasis of a pool of DAG involved in membrane transport from the TGN [20,21]. Additionally, it further supports a role for DAG in membrane fission at this station, which, as in the case of PA, may result from its cone shape and/or from the action of downstream effectors, such as GTPase-activating proteins (GAPs) for ADP-ribosylation factor (ARF) and protein kinase D (PKD) [21]. In light of the well-known ability of some PITP members to regulate PI metabolism [2] and the recent identification of a PI(4)P-synthesizing enzyme as a physiological substrate for PKD [22], an important question is how DAG and PI(4)P cooperate to mediate transport-carrier formation at the TGN.
DAG has long been known as a regulator of the protein kinase C (PKC) pathway by virtue of its ability to bind to the C1 domain of various PKC family members. Elegant genetic studies have identified Munc13, a C1 domain-containing protein involved in vesicle priming (i.e. the maturation step that precedes full fusion), as another key DAG effector at the plasma membrane [23]. Knock-in mice carrying a point mutation in the C1 domain of Munc13-1, which abolishes binding to DAG, renders this protein insensitive to phorbol ester-stimulated vesicle priming, thereby causing defects in augmentation of neurotransmission [23]. Recent data indicate that the Munc13 pathway may in fact cooperate with PKC-dependent mechanisms in DAG-induced forms of presynaptic plasticity [24]. Consistent with an involvement of DAG in exocytosis, studies in yeast have indicated that vacuolar fusion requires a pool of DAG derived from phospholipase C-mediated cleavage of PI(4,5)P2 [25] (Figure 1).
Recent progress in the analysis of functions of phosphoinositides
Because the properties and biological roles of phosphoinositides have been extensively reviewed elsewhere [4,6], this section will focus more on recent progress in their methods of investigation. Phosphoinositides, which are the best-characterized glycerolipids, are implicated in processes as diverse as signal transduction, membrane trafficking, cytoskeletal rearrangements and the permeability of membranes [4,6]. Through reversible phosphorylation reactions mediated by a variety of kinases on the 3, 4 and 5 positions of the inositol ring, seven phosphoinositide species can be generated. Each of these lipids has a unique distribution in cells, with the quasi-consensual view that PI(4,5)P2 and PI(3,4,5)P3 are preferentially concentrated at the plasma membrane, PI(3)P and PI(3,5)P2 are predominantly localized to the endosomal compartment, and PI(4)P is enriched on the TGN and secretory organelles [6]. These lipids are viewed as spatial landmarks for the respective compartments (i.e. “organelle identity tags”) and their normal balance is required for a large number of processes occurring at the membrane-cytosol interface [6]. Accordingly, a large number of proteins harbor phosphoinositide-binding modules or sequences that mediate their recruitment to membrane compartments enriched for these lipids. Such determinants likely act in conjunction with protein factors to target subpools of phosphoinositides for specific cell physiological functions.
Recently, a series of converging studies have reported the adaptation of existing technologies to allow for the manipulation of phosphoinositide levels in intact cells in a rapid and inducible fashion. These approaches exploit the ability of rapamycin or derivatives thereof (termed “rapalogs”) to induce heterodimerization of two intracellular proteins, mammalian Target of Rapamycin (mTOR) and FK-506 Binding Protein 12 (FKBP12), via their concomitant binding to their FRB and FKBP domains, respectively. While one of these domains harbors a sequence allowing for constitutive targeting to the plasma membrane, the other domain is fused to the catalytic domain of a PI-metabolizing enzyme. In the presence of crosslinking drug, the lipid enzyme is rapidly recruited to the plasma membrane, where it can modify the lipid content of this compartment. This strategy has been used to acutely deplete the plasma membrane of PI(4,5)P2 using inositol 5-phosphatase modules with major consequences on ion channel function [26,27], transferrin and EGF internalization [27] as well as clathrin-coated pits dynamics [28]. A variant of this technology was also used to acutely promote the recruitment of the inositol 3-phosphatase myotubularin to Rab5-positive endosomal compartments. The resulting acute depletion of PI(3)P (and potentially PI(3,5)P2) in this compartment was shown to cause major defects in endosomal trafficking [29], supporting the view that 3-phosphoinositides are essential components in early endosomal membrane dynamics. However, the fundamental question of how subpools of phosphoinositides may regulate specific pathways is still unanswered and remains one of the most important challenges for the future.
Phosphatidylserine: More than a passive player in membrane traffic?
PS is the most abundant anionic phospholipid of cell membranes, where it constitutes approximately 5–10% of total cellular lipids. The main site of synthesis, as for many other lipids, appears to be the ER. Although abundant, PS has been almost exclusively investigated in the context of studies on lipid synthesis, lipid transport (e.g. import into mitochondria), blood coagulation and apoptosis [30]. The latter were motivated by the discovery that during apotosis, PS is translocated from the inner leaflet to the outer leaflet of the plasma membrane, where it constitutes a major cell surface signal for a variety of receptors of phagocytes (e.g. “scavenger” receptor) and sentences dying cells to the engulfment process [30]. Annexin V, which specifically binds PS, has been widely used as a probe for this lipid to detect apoptotic cells [5,30].
Few studies have directly addressed the role of PS in membrane traffic, although some evidence suggest that its homeostasis may be critical for this process. For instance, genetic studies of enzymes (e.g. the P-type ATPases Drs2p, Dnf1p, Dnf2p) believed to “flip” aminophospholipids, such as PS and PE, across membranes have revealed that the asymmetric distibution of these lipids in the bilayers (i.e. with an enrichment in the cytoplasmic leaflet) is critical for budding from the TGN as well as for endocytosis in yeast [31,32]. Second, fluorescent imaging studies evaluating the inner surface charge of the plasma membrane using genetically-encoded cationic probes suggest that PS, likely in combination with PI(4,5)P2, PI(3,4,5)P3 and PA, is a key contributor to the overall negative surface charge of this compartment [33,34]. Thus, membrane domains enriched for these lipids may act as spatial landmarks for a variety of peripheral proteins recognizing these lipids at the plasma membrane through simple electrostatics (e.g. MARCKS) or through more sophisticated interactions involving also hydrophobic residues (e.g. pleckstrin homology domains) [35]. Accordingly, some lipid-binding modules include independent binding pockets for PS and phosphoinositides [5], allowing for coincidental detection of independent signals by proteins that harbor these modules and the generation of high affinity interactions with membranes [6]. However, the high turnover of phosphoinositides, combined with the rapidity and extent to which their levels can be adjusted in cells, suggests that modulation of protein affinity for PS/phosphoinositide-rich membranes may occur primarily by adjusting the levels of phosphoinositides, rather than PS. It is of note that membrane internalization processes may not only be paired with the hydrolysis of phosphoinositides by phospholipases and phosphatases, but also with a de-enrichment of PS from the cytoplasmic leaflet via metabolic conversion into another lipid (i.e. decarboxylation), “flipping” to the luminal/outer leaflet or, in the case of phagocytosis, via a dilution effect mediated by the supply of PS-poor endomembranes to nascent phagocytic structures [33].
CHOLESTEROL AND LIPID MICRODOMAINS
Given that some glycerophospholipids such as PI(4,5)P2 are rare membrane components they have been suggested to become concentrated in microdomains enriched in cholesterol, i.e. to control leading edge motility of cells during polarization [36]. Evidence for the existence of membrane microdomains indeed has been collected from a variety of systems including polarized epithelial cells and primary neurons. However, the chemical and physical nature of membrane microdomains as well as the question of how they are formed have remained a matter of controversial debates [37,38].
Based on the property of membrane lipids to be present in three different phases, the gel phase, the liquid-ordered phase (Lo), and the liquid crystalline phase (Ld), it was proposed that lipid-lipid interactions are required and sufficient for microdomain formation [39]. Membranes enriched in cholesterol, glycosphingolipids, and phospholipids with fully-saturated acyl chains partition into the liquid-ordered phase. The resulting lipid segregation has been suggested to result in the formation of lipid rafts, which then could serve as platforms for protein sorting by differential phase partitioning [37]. Such cholesterol-based lipid rafts (Figure 2A) have been implicated in a wide range of processes including polarized protein sorting within the exo- and endocytic pathways, cell motility [36], cellular entry of toxins [40] and viruses, and signal transduction [41]. However, recent data from Förster resonance energy transfer (FRET) measurements in living cells have cast serious doubt on the existence of stable raft-like entities. Instead it seems that ‘raft’ components such as GPI-linked proteins exist as highly dynamic nanoscale clusters composed of just a handful of protein molecules [42]. Furthermore, confocal fluorescence recovery after photobleaching (FRAP) experiments have shown that putative raft-associated proteins appear to diffuse freely over large distances on the cell surface, suggesting that discrete ‘raft’ domains may not exist as stable entities [43].
Figure 2. Potential forms of cholesterol-based lipid microdomains.
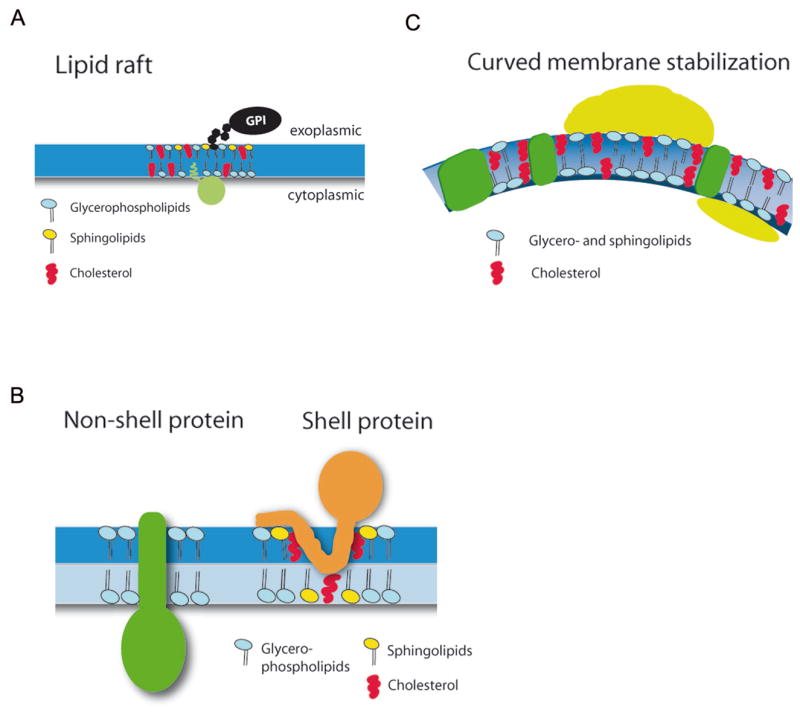
(A) Lipid rafts represent cholesterol- and glycosphingolipid-rich membrane microdomains that have been postulated to act as sorting platforms for the concentration of signaling proteins including many lipid- or GPI-anchored proteins. In light of recent data such rafts are most likely transient, nanoscale structures.
(B) Specific classes of membrane proteins depending on the chemical nature and physical properties of their transmembrane segments have been postulated to laterally organize select lipids including cholesterol (Shell protein). Coalescence of shell proteins into larger complexes could lead to the formation of nanoclusters or protein-lipid-based microdomains similar to those depicted in (A).
(C) The formation of highly curved membrane microdomains might be driven by peripherally associated adaptor and scaffolding proteins, some of which are able to partition predominantly into one leaflet of the bilayer. As the surface area occupied by the cytoplasmic versus the exoplasmic leaflet differs dramatically for highly curved membrane buds and vesicles curved microdomains might require stabilization by cholesterol.
Lipid shells
An important difference between native biological and model membranes is the packing density of membrane proteins. Evidence for a crucial role for protein crowding as a major determinant of slow diffusion over micron scales has recently been provided by elegant photobleaching experiments. Artificial increases in protein density caused by patching of ConA receptors were shown to decrease mobility of GFP-GPI which itself did not undergo co-aggregation [44]. A recent landmark proteomic and lipidomic study in which the molecular constituents of synaptic vesicles had been analyzed indeed suggests that protein transmembrane helices may occupy more than a quarter of the entire membrane volume, a protein density much higher than previously anticipated from the Singer-Nicholson model [45]. This organization implicates that in an average biological membrane integral proteins may be surrounded by a shell composed of just a few rings of lipid. Biological membranes also associate with peripheral adaptor proteins, many of which display medium affinity binding to select membrane lipids such as phosphoinositides [6], and with the cytoskeletal meshwork. Fluorescence correlation spectroscopy (FCS) measurements identify the actin cytoskeleton as a major barrier for confinement of non-’raft’ proteins such as the transferrin receptor [46]. These findings are thus easier to reconcile with the shell model of lipid and protein microdomain formation [43](Figure 2B). According to this view specific classes of membrane proteins may laterally organize select lipids including cholesterol (Fig. 2B, shell protein). Prime examples are represented by the membrane-integral coat proteins caveolin, and reggie/flotillin [47]. By contrast, non-shell proteins would not display any selectivity with regard to their immediate lipid environment (Fig. 2B, non-shell protein). Elegant recent work based on the magnetic purification of endosomes paired with RNA interference has shown that flotillin-1 defines a cholesterol-dependent non-clathrin-mediated endocytic pathway used by GPI-linked proteins and cholera toxin B [48]. The membrane-deforming ability of flotillin-1 may relate to the putative insertion of hairpin loop structures into the plasmalemma, perhaps paired with flotillin oligomerization and association with cholesterol and sphingolipids. Thus, in more general terms coalescence of shell proteins into larger complexes could lead to the formation of nanoclusters or protein-lipid-based microdomains.
Curved membrane microdomains
Protein and lipid segregation has been observed during the budding of coated transport vesicles [49]. A key element of this process is the formation of highly curved membrane microdomains by peripherally associated adaptor and scaffolding proteins, some of which are able to partition predominantly into one leaflet of the bilayer [50]. Lipids with large headgroups prefer positive monolayer curvature, whereas those with a cone-shape will adopt negative curvature [50]. This phenomenon could contribute to curvature acquisition as suggested by molecular dynamics simulations [51]. Thus, lipid segregation may occur based on their geometrical shapes. Moreover, the surface area occupied by the cytoplasmic versus the exoplasmic leaflet differs dramatically for small membrane buds and vesicles, a feature that might require stabilization of the cytoplasmic leaflet by membrane cholesterol (Figure 2C). Small synaptic vesicles indeed display an unusually high content of cholesterol [45]. This feature is paralleled by its direct association with synaptic vesicle membrane proteins including synaptophysin and synaptotagmin 1, which have been found to form cholesterol-dependent complexes within synaptic membranes [52]. Recent progress regarding the advent of two-color high resolution fluorescence nanoscopy [53], FRET techniques [42], and FCS-based protein mobility measurements [46] paired with the use of new polyene-based [54] and other lipid analogs is expected to help unraveling the role of lipids in microdomain formation, dynamics, and disease [55].
LIPID AND RELATED MODIFICATIONS IN PROTEIN SORTING
In addition to the formation of microdomains, lipids or other fatty acyl derivatives can regulate membrane organization and sorting by their covalent attachment to proteins. Modification of otherwise soluble proteins with hydrophobic moieties, such as fatty acyl or isoprenyl groups, regulates their targeting to membranes, their partitioning into lipid microdomains, and perhaps protein-protein or protein-lipid interactions. Lipid modifications of membrane integral proteins may also relieve hydrophobic mismatch between the hydrophobic transmembrane helices and the lipid bilayer [56], resulting in selective concentration of the corresponding proteins in certain membrane areas or microdomains.
Proteins can be modified with fatty acids, lipids, or even cholesterol in several different ways [57] (Table 1). Small GTP-binding proteins of the ADP-ribosylation factor (Arf) subfamily of Ras-related GTPases undergo enzyme-catalyzed N-myristoylation at an amino-terminal glycine residue after its exposure by cleavage of the initiator methionine and this is important for their targeting to membranes. Arf-related proteins (Arl) use a a glycine-linked acetate group, a modification that is required for Arl targeting to membranes, i.e. of Arl3p to the Golgi complex [58,59]. Many important signaling proteins undergo prenylation (farnesylation, geranylgeranylation). For example, Ras as well as Rho proteins are prenylated at a cysteine residue four amino acids upstream from the COOH-terminus via a CaaX box recognition and modification site. Palmitoylation usually reflects the thio-acylation of internal cysteine residues via reversible ester linkage (S-palmitoylation). N-palmitoylation via an amide bond has been reported for the secreted morphogen Hedgehog [60], following its autoproteolytic processing and modification with cholesterol (Table 1). An unusual lipid modification is required for autophagosome formation. Yeast Atg8p or its mammalian counterparts LC3, GABARAP and GATE-16, are ubiquitin-like proteins involved in autophagocytosis, which become attached to the amino group of phosphatidylethanolamine via an E1–E2-analogous modification system [61].
Table 1. Lipid modifications involved in intracellular protein transport.
Characteristics of protein modifications by lipids and related fatty acyl derivatives or cholesterol including example substrates and functions. Gly, glycine; Met, methionine; CaaX, sequence of C-terminal four residues including cysteine (C) that serve as attachment site for a prenyl group; Arf, ADP-ribosylation factor; Arl, Arf-related protein; Gα, α subunit of heterotrimeric G-proteins; PSD-95, postsynaptic density protein of 95 kDa; AMPA, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid; PE, phosphatidylethanolamine, PS, phosphatidylserine.
| Modification | Characteristics | Enzymatic Regulators | Example Substrates | Exemplary function in sorting | Refs. |
|---|---|---|---|---|---|
| N-myristoylation | Attachment of myristate to N- terminal Gly residue following removal of initiator Met; irreversible | N-myristoyl- transferase | Src-family kinases (Fyn, Lck); Gα; Arf1-6; yeast vacuolar protein Vac8p | Weak & reversible membrane attachment, i.e. regulating Arf targeting to membranes | 57 and refs. therein |
| N-acetylation | Attachment of acetate to N-terminal Met, penultimate Thr, or other residues; co- or posttranslational; presumably irreversible | N-terminal acetyltransferase (Nat A, B, C in yeast) | β-endorphin & other hormones; Arl3p; Arl8a/b; | Golgi or lysosome targeting of Arls | 58,59 |
| N-palmitoylation | Attachment of palmitate to Cys at protein N-terminus followed by rearrangement to stable amide linkage; irreversible | Palmitoyltransferase (PAT) | Hedgehog; possibly Spitz (spi) | Spread of Hedgehog signaling within tissues; regulation of Hedgehog targeting & localization | 60 |
| S-palmitoylation | Thioacylation of Cys residue via thioester linkage; reversible | Palmitoyltransferases (PATs) usually characterized by DHHC motif; acylprotein thioesterases carry out depalmitoylation | Ras; human δ-opioid receptor; AMPA-type glutamate receptors (AMPARs); psotsynaptic scaffold PSD-95; vacuolar protein Vac8p; yeast SNARE Tlg1; SNAP-25, SNAP-23 | Antero- or retrograde trafficking (Ras, AMPARs); targeting to postsynaptic clusters (PSD-95); protection from ubiquitination (Tlg1) | 62–70 |
| Prenylation | Attachment of farnesyl or geranylgeranyl moiety to CaaX sequence at C-terminal end, usually followed by aaX cleavage & carboxymethylation; irreversible | Farnesyltransfe rase (FTase); geranylgeranyl- transferase (GGTase) | Ras(farnesylation); Rabs (geranyl-geranylation) | Antero- and retrograde trafficking of Ras between the Golgi and the PM; membrane recruitment of Rabs to organelles | 57 and refs. therein |
| Cholesteroylation | Attachment of cholesterol to proteins, i.e. Hedgehog following autocatalytic cleavage | Cholesterol transferase | Hedgehog | Spread of Hedgehog signaling within tissues; exact relationship to sorting is unknown | 57 and refs. therein |
| Lipidation | Attachment of amino group of PE (perhaps also PS) to C-terminal Gly of ubiquitin-like proteins | E1- and E2-like enzymes, i.e. Atg7p/hAtg7 and Atg3p/hAtg3 | Ubiquitin-like protein Atg8p in yeast & its mammalian homologs LC3, GABARAP, GATE-16 | Regulates autophagy in yeast and presumably mammals | 61 |
Recent studies on Ras have unravelled an acylation cycle that regulates subcellular trafficking of H-Ras and N-Ras by dual lipid modification. Constitutive farnesylation targets H- or N-Ras to the endoplasmic reticulum (ER) where post-prenylation processing of -aaX and carboxy-methylation occur. Reversible palmitoylation of Ras as demonstrated by elegant quantitative fluorescence microscopy and photobleaching techniques [62] by a so far unidentified Golgi-localized acyltransferase (PAT) facilitates trafficking of dual lipid-modified Ras to the plasma membrane. At the cell surface Ras has been observed in spatially distinct cholesterol-dependent microdomains and this may be related to the insertion of palmitate into the inner leaflet [63]. Depalmitoylation via a putative acylprotein thioesterase (APT) allows non-vesicular exchange with endomembranes [64]. These findings predict a prominent role for the Ras acylation cycle in cell signaling. Similar mechanisms appear to regulate the subcellular localization of phospholipase D1 [65].
Over the past several years a number of proteins have been identified whose sorting is dependent on S-palmitoylation including the presynaptic vesicle proteins synaptotagmin 1, SNAP-25 (as well as its close relative SNAP-23), and cysteine-string protein [66], postsynaptic AMPA-type glutamate receptors [67] and the associated postsynaptic density protein PSD-95. The latter is consistent with a role for palmitate-based acylation cycles in synaptic plasticity [68]. A prominent role for palmitoylation in membrane sorting is underscored by the recent global analysis of protein palmitoylation in yeast. Using acyl-biotinyl exchange and mass spectrometry-based proteomic methodology palmitoyl proteins were identified. The 47 protein factors included a variety of factors with proven functions in membrane traffic and cell signaling such as SNARE and Rho protein family members as well as amino acid permeases [69]. The precise mechanisms by which palmitoylation regulates protein function and intracellular targeting have remained elusive in many cases, but may involve masking of specific association sites for proteinaceous binding partners. Indeed, elegant studies by Pelham and colleagues have indicated a regulatory interplay between protein palmitoylation and ubiquitination. Palmitoylation of the yeast Golgi/endosomal SNARE protein Tlg1 by the DHHC-cysteine-rich domain palmitoyl transferase (PAT) Swf1 was shown to protect Tlg1 from ubiquitination, whereas inhibition of palmitate addition onto Tlg1 caused missorting to the vacuole followed by its degradation [70]. Surprisingly, cysteine mutants of Tlg1 did not mislocalize in ubiquitin ligase deficient yeast strains, suggesting that in this case palmitoylation is merely used to regulate ubiquitination cycles rather than membrane targeting per se. Whether palmitoylation is a general regulator of ubiquitin attachment remains to be investigated.
In summary, a mechanistic understanding of protein modification by lipids and other hydrophobic moieties and the elucidation of the enzymatic control mechanisms involved appears to be one of the most important challenges for the near future. This will require us to devise better methods for detection and acute manipulation of select subgroups or pools of lipids at distinct intracellular sites and to then assess their contribution to cellular function in real time.
Acknowledgments
We would like to thank Belle Chang, Sergey Voronov, Michael Krauss and Pietro De Camilli for critical reading of the manuscript. G.D.P. is funded by grants from the NIH (1R01 NS056049 and 1R21 HD047733), the Whitehall Foundation (2005-12-28-APL) and the March of Dimes (Basil O’Connor, 5-FY05-1216). V.H. acknowledges support by grants from the German research funding agency DFG (HA2686/2-1, FOR806, GRK1123, and SFB449, TP A11), the German Ministry of Science (BMBF, BioDISC/RENTRAFF), the European Molecular Biology Organization (EMBO YIP Programme), and the Fonds der Chemischen Industrie (FCI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- 2.Bankaitis VA, Morris AJ. Lipids and the exocytotic machinery of eukaryotic cells. Curr Opin Cell Biol. 2003;15:389–395. doi: 10.1016/s0955-0674(03)00076-0. [DOI] [PubMed] [Google Scholar]
- 3.van Meer G, Sprong H. Membrane lipids and vesicular traffic. Curr Opin Cell Biol. 2004;16:373–378. doi: 10.1016/j.ceb.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Krauss M, Haucke V. Phosphoinositide-metabolizing enzymes at the interface between membrane traffic and cell signalling. EMBO Rep. 2007;8:241–246. doi: 10.1038/sj.embor.7400919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stace CL, Ktistakis NT. Phosphatidic acid- and phosphatidylserine-binding proteins. Biochim Biophys Acta. 2006;1761:913–926. doi: 10.1016/j.bbalip.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 7.Athenstaedt K, Daum G. Phosphatidic acid, a key intermediate in lipid metabolism. Eur J Biochem. 1999;266:1–16. doi: 10.1046/j.1432-1327.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins GM, Frohman MA. Phospholipase D: a lipid centric review. Cell Mol Life Sci. 2005;62:2305–2316. doi: 10.1007/s00018-005-5195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haslam RJ, Coorssen JR. Evidence that activation of phospholipase D can mediate secretion from permeabilized platelets. Adv Exp Med Biol. 1993;344:149–164. doi: 10.1007/978-1-4615-2994-1_11. [DOI] [PubMed] [Google Scholar]
- 10.Huang P, Altshuller YM, Hou JC, Pessin JE, Frohman MA. Insulin-stimulated plasma membrane fusion of Glut4 glucose transporter-containing vesicles is regulated by phospholipase D1. Mol Biol Cell. 2005;16:2614–2623. doi: 10.1091/mbc.E04-12-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakanishi H, Morishita M, Schwartz CL, Coluccio A, Engebrecht J, Neiman AM. Phospholipase D and the SNARE Sso1p are necessary for vesicle fusion during sporulation in yeast. J Cell Sci. 2006;119:1406–1415. doi: 10.1242/jcs.02841. ●An elegant genetic study in yeast demonstrating a role for Spo14p, a phospholipase D, in the fusion of prospore membrane precursors during the sporulation process via regulating the recruitment and the function of the t-SNARE protein Spo20p. The accumulation of membrane precursors observed in SPO14 mutants is also observed as a result of mutations in SSO1, a gene encoding another t-SNARE involved in prospore membrane formation. [DOI] [PubMed] [Google Scholar]
- 12.Vicogne J, Vollenweider D, Smith JR, Huang P, Frohman MA, Pessin JE. Asymmetric phospholipid distribution drives in vitro reconstituted SNARE-dependent membrane fusion. Proc Natl Acad Sci U S A. 2006;103:14761–14766. doi: 10.1073/pnas.0606881103. ●The authors identify the asymmetric distribution of acidic phospholipids, such as phosphatidic acid and PI(4,5)P2, as key determinants for SNARE protein–mediated membrane fusion in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi SY, Huang P, Jenkins GM, Chan DC, Schiller J, Frohman MA. A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat Cell Biol. 2006;8:1255–1262. doi: 10.1038/ncb1487. ●●This original study further connects the PLD family and their enzymatic reaction product PA to the process of membrane fusion. In this instance, the site of action of the PLD isoform described is the mitochondrion and the fusion machinery involves mitofusin, rather than SNARE proteins. In essence, this study highlights the diversity of molecular mechanisms underlying PLD- and PA-mediated membrane fusion and points to a cellular context, apoptosis, where this process may be tightly regulated. [DOI] [PubMed] [Google Scholar]
- 14.Padron D, Tall RD, Roth MG. Phospholipase D2 is required for efficient endocytic recycling of transferrin receptors. Mol Biol Cell. 2006;17:598–606. doi: 10.1091/mbc.E05-05-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freyberg Z, Bourgoin S, Shields D. Phospholipase D2 is localized to the rims of the Golgi apparatus in mammalian cells. Mol Biol Cell. 2002;13:3930–3942. doi: 10.1091/mbc.02-04-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CS, Kim IS, Park JB, Lee MN, Lee HY, Suh PG, Ryu SH. The phox homology domain of phospholipase D activates dynamin GTPase activity and accelerates EGFR endocytosis. Nat Cell Biol. 2006;8:477–484. doi: 10.1038/ncb1401. [DOI] [PubMed] [Google Scholar]
- 17.Topham MK. Signaling roles of diacylglycerol kinases. J Cell Biochem. 2006;97:474–484. doi: 10.1002/jcb.20704. [DOI] [PubMed] [Google Scholar]
- 18.Hodgkin MN, Pettitt TR, Martin A, Michell RH, Pemberton AJ, Wakelam MJ. Diacylglycerols and phosphatidates: which molecular species are intracellular messengers? Trends Biochem Sci. 1998;23:200–204. doi: 10.1016/s0968-0004(98)01200-6. [DOI] [PubMed] [Google Scholar]
- 19.Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, Krausz E, Zerial M. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. ●●This large scale RNAi screen implicates a significant fraction of human kinases in various aspects of endocytosis in HELA cells. Three DAG kinase isoforms out of the ten present in the human genome were identified as regulators of endocytic membrane traffic. [DOI] [PubMed] [Google Scholar]
- 20.Litvak V, Dahan N, Ramachandran S, Sabanay H, Lev S. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat Cell Biol. 2005;7:225–234. doi: 10.1038/ncb1221. ●These authors identify Nir2 as a functional homolog of yeast Sec14p in mammalian cells, based on its ability to regulate DAG levels at the TGN and to mediate transport-carrier formation at this compartment. Nir2 shares a PITP-like domain in common with Sec14p. [DOI] [PubMed] [Google Scholar]
- 21.Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–455. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- 22.Hausser A, Storz P, Martens S, Link G, Toker A, Pfizenmaier K. Protein kinase D regulates vesicular transport by phosphorylating and activating phosphatidylinositol-4 kinase IIIbeta at the Golgi complex. Nat Cell Biol. 2005;7:880–886. doi: 10.1038/ncb1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, Hesse D, Sudhof TC, Takahashi M, Rosenmund C, et al. Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–133. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- 24.Wierda KD, Toonen RF, de Wit H, Brussaard AB, Verhage M. Interdependence of PKC-Dependent and PKC-Independent Pathways for Presynaptic Plasticity. Neuron. 2007;54:275–290. doi: 10.1016/j.neuron.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Jun Y, Fratti RA, Wickner W. Diacylglycerol and its formation by phospholipase C regulate Rab- and SNARE-dependent yeast vacuole fusion. J Biol Chem. 2004;279:53186–53195. doi: 10.1074/jbc.M411363200. [DOI] [PubMed] [Google Scholar]
- 26.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314:1454–1457. doi: 10.1126/science.1131163. ●●Together with [26] this study describes an elegant tool to acutely deplete PI(4,5)P2 from the plasma membrane resulting in profound changes with regard to ion channel function and endocytosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varnai P, Thyagarajan B, Rohacs T, Balla T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J Cell Biol. 2006;175:377–382. doi: 10.1083/jcb.200607116. ●●Together with [26] this study describes an elegant tool to acutely deplete PI(4,5)P2 from the plasma membrane resulting in profound changes with regard to ion channel function and endocytosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D, De Camilli PV. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci U S A. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. ●Using the technology described in [26] combined with total internal reflection fluorescence microscopy, this live-imaging study demonstrates that the presence of PI(4,5)P2 at the plasma membrane is an absolute requirement for the integrity of clathrin-coated pits, consistent with other studies showing that endocytic clathrin adaptors bind this lipid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fili N, Calleja V, Woscholski R, Parker PJ, Larijani B. Compartmental signal modulation: Endosomal phosphatidylinositol 3-phosphate controls endosome morphology and selective cargo sorting. Proc Natl Acad Sci U S A. 2006;103:15473–15478. doi: 10.1073/pnas.0607040103. Using similar technology as described in [26,27] the authors show that acute depletion of endosomal PI(3)P and perhaps PI(3,5)P2 leads to major defects in endosomal trafficking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henson PM, Bratton DL, Fadok VA. The phosphatidylserine receptor: a crucial molecular switch? Nat Rev Mol Cell Biol. 2001;2:627–633. doi: 10.1038/35085094. [DOI] [PubMed] [Google Scholar]
- 31.Hua Z, Fatheddin P, Graham TR. An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol Biol Cell. 2002;13:3162–3177. doi: 10.1091/mbc.E02-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pomorski T, Lombardi R, Riezman H, Devaux PF, van Meer G, Holthuis JC. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol Biol Cell. 2003;14:1240–1254. doi: 10.1091/mbc.E02-08-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeung T, Terebiznik M, Yu L, Silvius J, Abidi WM, Philips M, Levine T, Kapus A, Grinstein S. Receptor activation alters inner surface potential during phagocytosis. Science. 2006;313:347–351. doi: 10.1126/science.1129551. ●●These authors design a genetically-encoded fluorescent probe in order to assess the surface charge of the plasma membrane in intact cells. This probe, which was modeled after the COOH-terminus of the small GTPase K-Ras, binds to anionic phospholipids and appears to interact with cell membranes strictly on the basis of electrostatics. Using this tool, changes in inner surface potentials are revealed during the process of phagocytosis. [DOI] [PubMed] [Google Scholar]
- 34.Heo WD, Inoue T, Park WS, Kim ML, Park BO, Wandless TJ, Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 36.Golub T, Caroni P. PI(4,5)P2-dependent microdomain assemblies capture microtubules to promote and control leading edge motility. J Cell Biol. 2005;169:151–165. doi: 10.1083/jcb.200407058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 38.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 39.de Almeida RF, Loura LM, Fedorov A, Prieto M. Lipid rafts have different sizes depending on membrane composition: a time-resolved fluorescence resonance energy transfer study. J Mol Biol. 2005;346:1109–1120. doi: 10.1016/j.jmb.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 40.Deinhardt K, Berninghausen O, Willison HJ, Hopkins CR, Schiavo G. Tetanus toxin is internalized by a sequential clathrin-dependent mechanism initiated within lipid microdomains and independent of epsin1. J Cell Biol. 2006;174:459–471. doi: 10.1083/jcb.200508170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 42.Sharma P, Varma R, Sarasij RC, Ira, Gousset K, Krishnamoorthy G, Rao M, Mayor S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116:577–589. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]
- 43.Kenworthy AK, Nichols BJ, Remmert CL, Hendrix GM, Kumar M, Zimmerberg J, Lippincott-Schwartz J. Dynamics of putative raft-associated proteins at the cell surface. J Cell Biol. 2004;165:735–746. doi: 10.1083/jcb.200312170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frick M, Schmidt K, Nichols BJ. Modulation of lateral diffusion in the plasma membrane by protein density. Curr Biol. 2007;17:462–467. doi: 10.1016/j.cub.2007.01.069. ●Using photobleaching the authors provide evidence that protein density may be a major parameter for determining rates of lateral diffusion. [DOI] [PubMed] [Google Scholar]
- 45.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. ●●A landmark study providing the first detailed molecular description of a trafficking organelle, the synaptic vesicle. The study reveals a surprisingly high coverage of the vesicle surface by proteins. [DOI] [PubMed] [Google Scholar]
- 46.Lenne PF, Wawrezinieck L, Conchonaud F, Wurtz O, Boned A, Guo XJ, Rigneault H, He HT, Marguet D. Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. Embo J. 2006;25:3245–3256. doi: 10.1038/sj.emboj.7601214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bauer M, Pelkmans L. A new paradigm for membrane-organizing and -shaping scaffolds. FEBS Lett. 2006;580:5559–5564. doi: 10.1016/j.febslet.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 48.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8:46–54. doi: 10.1038/ncb1342. ●●Starting from the magnetic ferro-fluid-based purification of endosomes the authors define a clathrin-independent endocytic pathway that requires the hairpin-loop transmembrane protein flotillin-1. Flotillin-1 thus represents the first protein component of clathrin-independent non-caveolar fluid-phase uptake in mammalian cells. [DOI] [PubMed] [Google Scholar]
- 49.Brugger B, Sandhoff R, Wegehingel S, Gorgas K, Malsam J, Helms JB, Lehmann WD, Nickel W, Wieland FT. Evidence for segregation of sphingomyelin and cholesterol during formation of COPI-coated vesicles. J Cell Biol. 2000;151:507–518. doi: 10.1083/jcb.151.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
- 51.Markvoort AJ, van Santen RA, Hilbers PA. Vesicle shapes from molecular dynamics simulations. J Phys Chem B. 2006;110:22780–22785. doi: 10.1021/jp064888a. [DOI] [PubMed] [Google Scholar]
- 52.Jia JY, Lamer S, Schumann M, Schmidt MR, Krause E, Haucke V. Quantitative proteomics analysis of detergent-resistant membranes from chemical synapses: evidence for cholesterol as spatial organizer of synaptic vesicle cycling. Mol Cell Proteomics. 2006;5:2060–2071. doi: 10.1074/mcp.M600161-MCP200. [DOI] [PubMed] [Google Scholar]
- 53.Donnert G, Keller J, Wurm C, Rizzoli S, Westphal V, Schoenle A, Jahn R, Jakobs S, Eggeling C, Hell SW. Two-Color Far-field Fluorescence Nanoscopy. Biophys J. 2007 doi: 10.1529/biophysj.107.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuerschner L, Ejsing CS, Ekroos K, Shevchenko A, Anderson KI, Thiele C. Polyene-lipids: a new tool to image lipids. Nat Methods. 2005;2:39–45. doi: 10.1038/nmeth728. [DOI] [PubMed] [Google Scholar]
- 55.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 56.Kandasamy SK, Larson RG. Molecular dynamics study of the lung surfactant peptide SP-B1-25 with DPPC monolayers: insights into interactions and peptide position and orientation. Biophys J. 2005;88:1577–1592. doi: 10.1529/biophysj.104.038430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magee T, Seabra MC. Fatty acylation and prenylation of proteins: what’s hot in fat. Curr Opin Cell Biol. 2005;17:190–196. doi: 10.1016/j.ceb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Behnia R, Panic B, Whyte JR, Munro S. Targeting of the Arf-like GTPase Arl3p to the Golgi requires N-terminal acetylation and the membrane protein Sys1p. Nat Cell Biol. 2004;6:405–413. doi: 10.1038/ncb1120. [DOI] [PubMed] [Google Scholar]
- 59.Setty SR, Shin ME, Yoshino A, Marks MS, Burd CG. Golgi recruitment of GRIP domain proteins by Arf-like GTPase 1 is regulated by Arf-like GTPase 3. Curr Biol. 2003;13:401–404. doi: 10.1016/s0960-9822(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 60.Pepinsky RB, Zeng C, Wen D, Rayhorn P, Baker DP, Williams KP, Bixler SA, Ambrose CM, Garber EA, Miatkowski K, et al. Identification of a palmitic acid-modified form of human Sonic hedgehog. J Biol Chem. 1998;273:14037–14045. doi: 10.1074/jbc.273.22.14037. [DOI] [PubMed] [Google Scholar]
- 61.Nair U, Klionsky DJ. Molecular mechanisms and regulation of specific and nonspecific autophagy pathways in yeast. J Biol Chem. 2005;280:41785–41788. doi: 10.1074/jbc.R500016200. [DOI] [PubMed] [Google Scholar]
- 62.Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PI. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–1752. doi: 10.1126/science.1105654. An elegant study unravelling a palmitoylation/depalmitoylation cycle on Ras as an essential regulatory mechanism to control its intracellular localization and growth factor-induced activation. [DOI] [PubMed] [Google Scholar]
- 63.Prior IA, Muncke C, Parton RG, Hancock JF. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J Cell Biol. 2003;160:165–170. doi: 10.1083/jcb.200209091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goodwin JS, Drake KR, Rogers C, Wright L, Lippincott-Schwartz J, Philips MR, Kenworthy AK. Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J Cell Biol. 2005;170:261–272. doi: 10.1083/jcb.200502063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han JM, Kim Y, Lee JS, Lee CS, Lee BD, Ohba M, Kuroki T, Suh PG, Ryu SH. Localization of phospholipase D1 to caveolin-enriched membrane via palmitoylation: implications for epidermal growth factor signaling. Mol Biol Cell. 2002;13:3976–3988. doi: 10.1091/mbc.E02-02-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greaves J, Chamberlain LH. Dual role of the cysteine-string domain in membrane binding and palmitoylation-dependent sorting of the molecular chaperone cysteine-string protein. Mol Biol Cell. 2006;17:4748–4759. doi: 10.1091/mbc.E06-03-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hayashi T, Rumbaugh G, Huganir RL. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron. 2005;47:709–723. doi: 10.1016/j.neuron.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 68.Greaves J, Chamberlain LH. Palmitoylation-dependent protein sorting. J Cell Biol. 2007;176:249–254. doi: 10.1083/jcb.200610151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roth AF, Wan J, Bailey AO, Sun B, Kuchar JA, Green WN, Phinney BS, Yates JR, 3rd, Davis NG. Global analysis of protein palmitoylation in yeast. Cell. 2006;125:1003–1013. doi: 10.1016/j.cell.2006.03.042. ●●A global mass spectrometry-based analysis of protein palmitoylation in yeast reported here identifies a variety of factors with proven functions in membrane traffic and cell signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valdez-Taubas J, Pelham H. Swf1-dependent palmitoylation of the SNARE Tlg1 prevents its ubiquitination and degradation. Embo J. 2005;24:2524–2532. doi: 10.1038/sj.emboj.7600724. ●●Using yeast genetics the authors demonstrate that Swf1-mediated palmitoylation regulates ubiquitination of the yeast Golgi/endosomal SNARE Tlg1. This elegant study therefore suggests a new function of protein palmitoylation. [DOI] [PMC free article] [PubMed] [Google Scholar]


