Abstract
This study compares two cleaning methods, one involving an ammonium carbonate-EDTA mixture and the other involving the sulfate-reducing bacterium Desulfovibrio vulgaris subsp. vulgaris ATCC 29579, for the removal of black crust (containing gypsum) on marble of the Milan Cathedral (Italy). In contrast to the chemical cleaning method, the biological procedure resulted in more homogeneous removal of the surface deposits and preserved the patina noble under the black crust. Whereas both of the treatments converted gypsum to calcite, allowing consolidation, the chemical treatment also formed undesirable sodium sulfate.
Cleaning is part of the conservation and maintenance program of historical buildings and under current guidelines (1, 3, 11) is described as the removal of harmful deposits from the building fabric to help prevent future decay. Until now, the lack of a shared protocol for in situ evaluation of the cleaning process has often led to the use of cleaning procedures inconsistent with the goals of preservation of monuments. Vergès-Belmin (23) proposed the following criteria for the selection and assessment of a cleaning method: (i) conservation of the patina noble (which imparts an aged character to a surface and has a preservative function); (ii) physical and chemical harm; (iii) homogeneity of removal of the surface deposits; (iv) persistence of the cleaned state; (v) efficiency of cleaning; (vi) color; and (vii) esthetics.
Black crust is a deteriorated surface layer of stone material spontaneously formed from the interaction between a calcareous substrate and the polluted atmosphere in a humid environment and in areas sheltered from rainfall (7, 14, 19). The chemical transformation of the substrate (calcite) into gypsum, together with the deposition of mineral and smog particles, can be assumed to be the mechanism of formation (10). Traditionally, to carefully control the removal of black crust and its effects, only chemical treatment was considered (20). In recent decades, an alternative cleaning technology employing sulfate-reducing bacteria (SRB), in particular Desulfovibrio desulfuricans, has been proposed by Atlas et al. (2) and Gauri and Chowdhury (9). These authors reported that D. desulfuricans converted gypsum to calcite, suggesting that this was both a cleaning treatment and a conservation treatment. Recently, this biotechnology has been optimized to avoid sulfide precipitation by employing an improved delivery system and using the aerotolerant strain D. vulgaris subsp. vulgaris ATCC 29579 (4, 16). Although in their relevant review Webster and May (24) claimed that bioremediation of sulfates is very promising and has received much attention, they also asserted that the risks posed by this technology are still to be addressed and that its advantages and limitations over other cleaning procedures have not yet been fully elucidated.
The aim of this research was to compare the efficacy of and harm caused by a traditional chemical cleaning procedure with the efficacy of and harm caused by a recently improved biological method (4) that employed SRB for in situ removal of black layers on a marble sculpted lunette of the Milan Cathedral (Italy), an important architectural monument.
After collection of a sample (sample 1) representing the surface before any treatment (Fig. 1), the marble relief surface was divided into two areas that were the same size: one area intended for the chemical treatment (right half of the surface) and the other intended for treatment with SRB (left half of the surface). The marble surface to be treated was moistened and covered with tissue paper before any applications began. The tissue paper enabled easy removal of the poultices after the treatments. The chemically and biologically treated areas underwent two 24-h applications for a total duration of 48 h for both treatments, which were performed at the same time to ensure the same treatment conditions. The second application was requested by the conservators, who judged the cleaning after the first 24-h application incomplete. After application, the chemical and biological cleaning systems were covered with a polyvinylchloride film (Silplast srl Italy) to reduce undesirable evaporation of water. In addition, the polyvinylchloride film reduced oxygen diffusion and hence improved the bacterial ability to remove sulfates. After removal of the tissue paper, soft brushing of the wet treated surfaces was performed to remove residual material softened by the effect of the treatments.
FIG. 1.
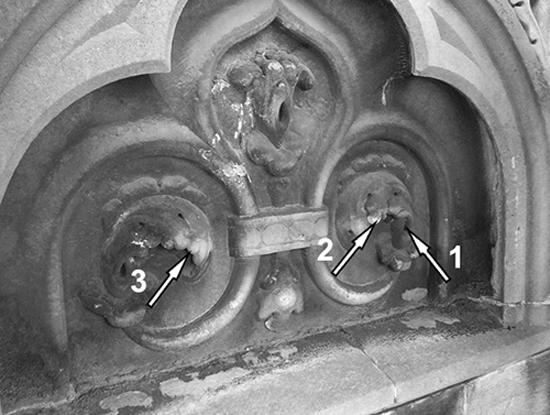
Ornamental high relief frieze of Milan Cathedral chosen as the application site for the cleaning procedures. The arrows indicate the following sampling areas: arrow 1, sample 1; arrow 2, sample 2; and arrow 3, sample 3.
For the chemical cleaning, the poultice was prepared according to a traditional conservation recipe (12) in which the chemical mixture, prepared in situ by mixing 30 g of EDTA, 50 g of ammonium carbonate, and 10 ml of the surfactant Contrad 2000 (a mixture of an anionic surfactant and a nonionic detergent, pH 12.3; Bresciani Srl Italy) in 1,000 ml of distilled water, is suspended in a micronized silica gel (PPG Industries, Pittsburgh, PA).
For the biological treatment, the biomass entrapped in the delivery system was prepared as described by Cappitelli et al. (4). Briefly, the SRB employed was D. vulgaris subsp. vulgaris ATCC 29579 maintained in DSMZ 63 medium (4), and the delivery system was Carbogel (CTS, Vicenza, Italy). Before use in the treatment, the cells were grown in modified DSMZ 63 medium (modified by eliminating any iron source [4]). After centrifugation and prior to mixing with the Carbogel powder, the cell pellet was suspended in deaerated phosphate buffer supplemented with 0.599 g liter−1 sodium lactate at pH 7.0. All the manipulations described above were done under anaerobic conditions in a glove box. No treatment with Carbogel without microorganisms was performed as this experiment was previously carried out in our laboratory (4).
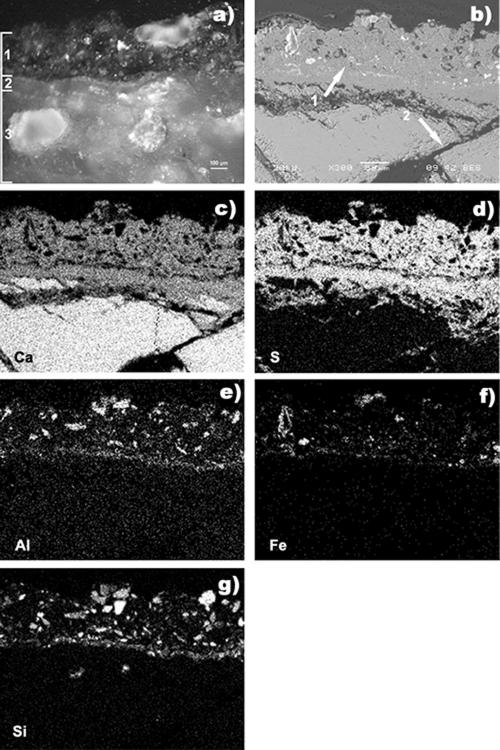
Observation of the untreated marble surface with the naked eye showed the presence of a thick and homogeneous deposit, very dark (brown-black) in color, having a compact texture. Optical microscopy (Leitz Ortholux microscope) of a cross section of a collected sample (Fig. 2a) revealed two very-well-defined layers overlying the substrate, which was extensively decayed, with a pattern of crossed microfractures partially filled with gypsum, as shown by scanning electron microscopy (SEM)-energy dispersive spectroscopy (EDS) analysis (JEOL 5910LV equipped with an IXRF System/EDS 2000) (Fig. 2b to g). The outer layer (average thickness, 150 μm) had the features of a black crust, and SEM-EDS analysis showed that it was composed mainly of gypsum (Ca and S) (Fig. 2b and c) and contained particles rich in iron, silicon, and aluminum. Its structure was not very compact, it contained scattered pores with irregular shapes, and its outer profile was irregular and rough (Fig. 2). The yellow-ochre inner layer (average thickness, 75 μm) had a more compact microstructure, and no atmospheric particles could be detected in it. A Fourier transform infrared (FTIR) analysis (Thermo Nicolet Nexus instrument with a Continuμm microscope equipped with an MCT detector) of the yellow-ochre layer revealed that it was composed of a mixture of calcium oxalate (both weddellite and whewellite; 1,645, 1,324, and 781 cm−1) and gypsum (3,543, 3,407, 1,621, 1,141, 1,119, 670, and 603 cm−1) along with a small amount of calcite (1,423, 876, and 713 cm−1) and traces of organic compounds (2,922 and 2,848 cm−1) (Fig. 3). This layer is the so-called patina noble (18). In contrast to black crusts, patinas are associated with the best-preserved surfaces and should remain intact during cleaning (6, 8, 13).
FIG. 2.
Polished cross section of sample 1 surface before cleaning. (a) Optical microscopy. Layer 1, black crust layer; layer 2, yellow-ochre layer (patina); layer 3, marble substrate. (b) SEM observation (back-scattered image). Arrow 1, black crust; arrow 2, microfractured substrate. (c to g) EDX analysis. (c) Calcium map. (d) Sulfur map. (e) Aluminum map. (f) Iron map. (g) Silicon map.
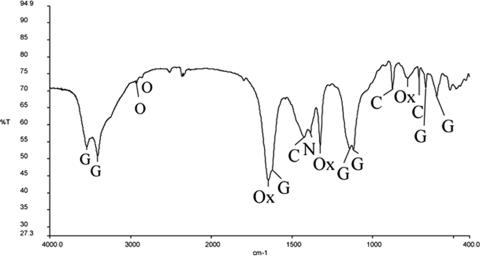
FIG. 3.
Sample 1 FTIR spectrum of patina noble layer micropellet in KBr dispersion. C, calcite; G, gypsum; O, traces of organic compounds; Ox, calcium oxalate.
Two samples were collected after completion of the 48-h treatments and examined with a portable microscope (Hanse Microscope HVS-CM500PC USB); sample 2 was a marble fragment of the chemically cleaned surface, and sample 3 was a fragment of the biologically cleaned surface. Marble samples were collected with a chisel where indicated in Fig. 1.
The chemical treatment cleaned the marble surface incompletely and nonuniformly. In situ observations with a portable microscope revealed the presence of small, diffuse, black-gray residues of crust (spotty pattern) in the areas where the cleaning was more effective next to areas where unaltered crust remained. The small cleaned areas appeared whitened, and no traces of the yellow-ochre layer could be seen. As determined with the stereomicroscope (Leitz Wild M420XRD), sample 2, collected from a cleaned area showing a spotty pattern, had a very nonhomogeneous surface (Fig. 4a). The superficial crust was only partially removed; a sharp separation between cleaned and uncleaned areas was clearly visible. In cleaned areas a diffuse whitening was observed due to the presence of aggregates of white crystals. A yellow layer located underneath the thinner crustal remains, where the thickness was reduced to a few microns, was traceable, but not on the cleaned surface. SEM observations of the loose uncleaned fragments revealed the superficial morphology of residual crust areas with many cavities; it was possible to identify an outer layer of noncoherent material characterized by the diffuse presence of undesirable acicular crystals of sodium sulfate (Fig. 4b), a more compact inner layer with many microfractures, and, deep in the crust, the presence of tabular crystals of gypsum. In the cleaned areas, the harm done by the chemical poultice was well documented by SEM observations of a cross section, which showed marble with a noticeable breakdown in granular cohesion where the crust and the layer beneath it were completely removed (Fig. 4c).
FIG. 4.
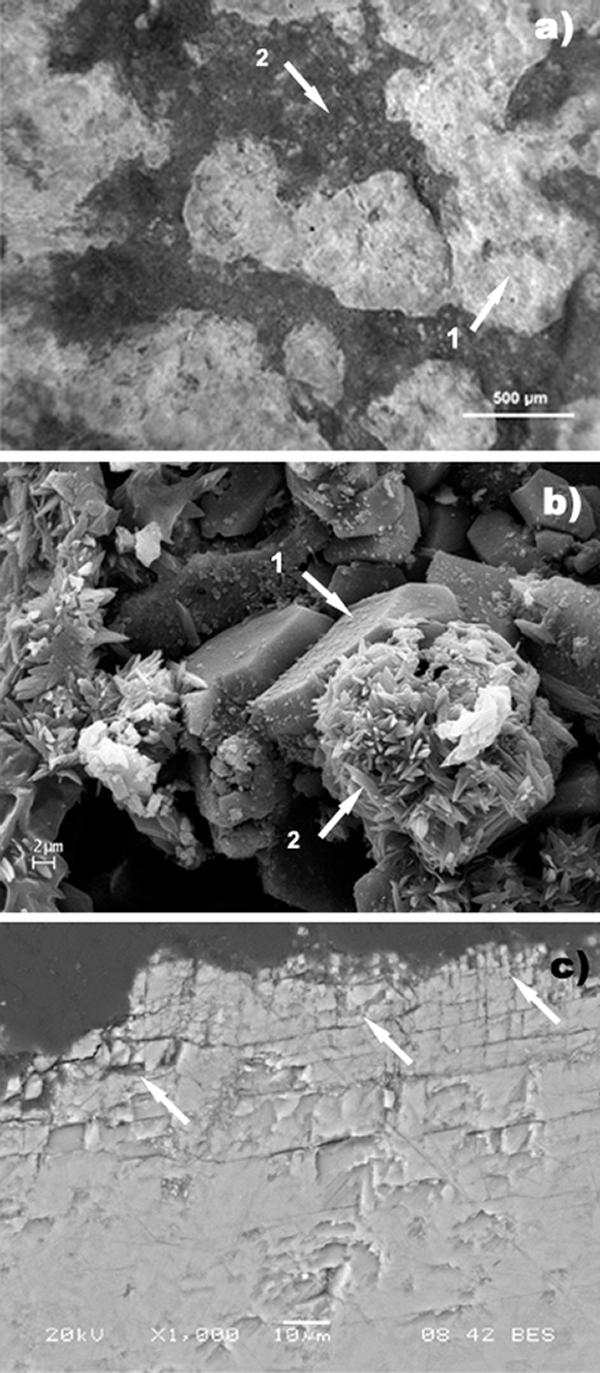
Sample 2 surface after chemical cleaning. (a) Portable microscope observation on site: nonhomogeneous surface from the cleaned area. Arrow 1, clean area; arrow 2, residual crust. (b) SEM observation. Arrow 1, tabular crystals of gypsum; arrow 2, acicular crystals of sodium sulfate. (c) SEM observation of the polished cross section (back-scattered image). The arrows indicate a noticeable network of microfractures close to the surface.
FTIR analyses were done on residual crust, noncoherent material covering the cleaned surface, and cleaned marble. The crust was composed mainly of gypsum together with calcite, and calcium oxalate and quartz (1,080, 797, and 779 cm−1) were minor components. The noncoherent material found on the cleaned marble surface and responsible for the superficial whitening was essentially calcium carbonate (1,794, 1,419, 877, and 713 cm−1). In areas where the chemical cleaning was more complete, only calcite was found. Neither calcium oxalate nor gypsum was present. FTIR spectra and SEM-EDS analyses showed that the chemical poultice seemed to be unable to distinguish between gypsum and calcite present in the black crust or in the yellow layer (lack of selectivity), so when the cleaning was more effective, no yellow layer residues were left.
The biocleaned marble surface had a rather homogeneous aspect and a pale yellow color. Preliminary visual inspection and observations with the portable microscope revealed that most of the crust was removed, even though there were some areas that retained some residues of the atmospheric crust. Under the microscope, the thickness of residues of the atmospheric crust on sample 3 appeared to be reduced (less than 50 μm) compared to the thickness on the untreated sample, sample 1 (ca. 150 μm). There was not a well-defined separation between completely cleaned and incompletely cleaned areas (Fig. 5a), while the yellow layer underneath the crust was maintained everywhere. Actually, the general appearance of the surface was characterized by a warm hue because of the diffuse presence of the yellow layer. Although color measurements are not reported in this paper, the color judged by eye after cleaning differed greatly between cleaned and partially cleaned areas, as well as among cleaned areas, for both the chemical and biological treatments. In completely biocleaned areas, there was a slight whitening over the yellow layer. SEM observations of sample 3 revealed a more homogeneous surface texture with fewer noncoherent crystals than was observed for the chemically cleaned sample, sample 2 (Fig. 5b). The residual yellow-ochre layer, which was observed on the polished cross section, was visible in the whole sample, and the average thickness was around 40 μm (Fig. 5c), indicating that it was left intact by the cleaning agent. Bacteria seemed to be unable to metabolize the gypsum contained in the yellow layer and deep in the stone material. No noticeable breakdown in cohesion was observed; gypsum was still present after the SRB cleaning treatment, both on the surface and between the marble grains. It should be noted that dissolution of gypsum is favored by alkaline conditions, such as those that exist in the chemical poultice, rather than at neutral pH, such as those that exist in the biological poultice, and that the layer corresponding to the patina is more compact and therefore the gypsum contained in it is probably not easily accessible to the bacteria. SEM analysis confirmed that the biocleaning totally removed the gypsum in the black crust but not the gypsum contained in the yellow-ochre layer that covered the substrate. The infrared results for completely cleaned areas showed an extensive presence of gypsum (3,545, 3,407, 1,622, 1,138, and 671 cm−1) together with the presence of calcite (1,457 and 877 cm−1) and calcium oxalate, as whewellite (1,630, 1,317, and 781 cm−1). The FTIR spectrum and SEM observations showed that the whitening over the yellow layer was due to calcite.
FIG. 5.
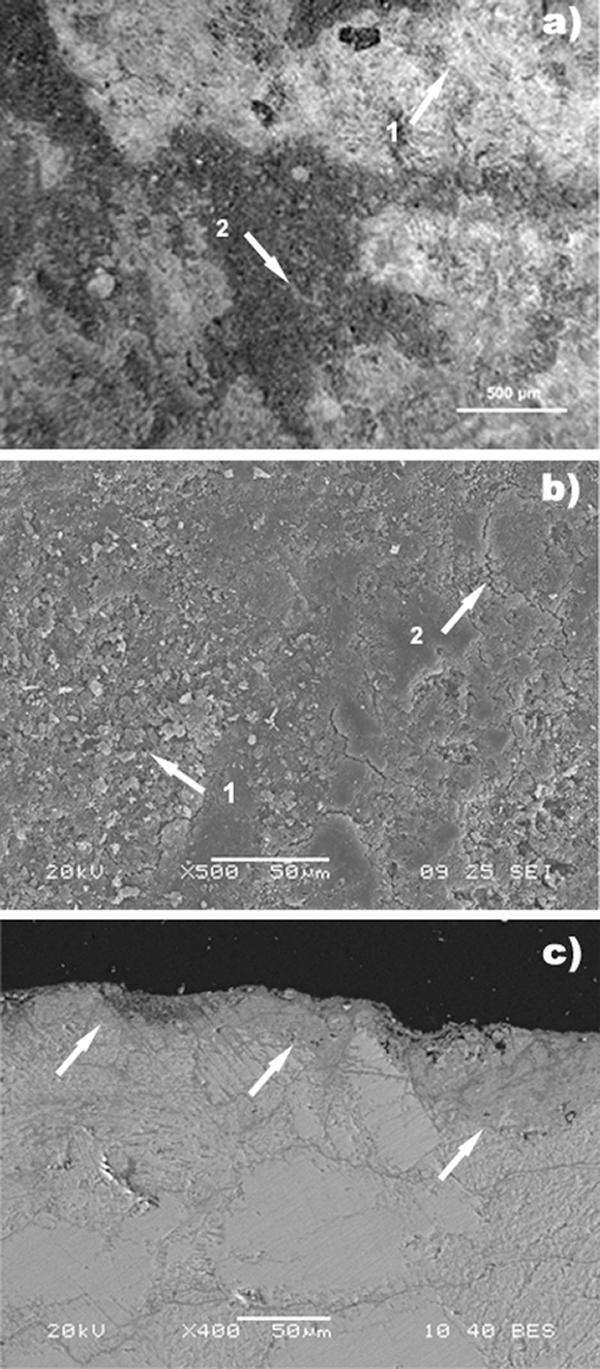
Sample 3 surface after biocleaning. (a) Portable microscope observation on site: surface from the biocleaned area showing rather homogeneous results. Arrow 1, clean area; arrow 2, traces of residual layer of dirt. (b) SEM observation, showing a homogeneous smooth surface. Arrow 1, small microcracks; arrow 2, a few incoherent crystals on the surface. (c) SEM observation of a polished cross section. The arrows indicate the presence of the yellow-ochre layer (patina) in the whole section.
According to Gauri and Chowdhury (9), the presence of calcite after SRB cleaning is probably due to microbial calcification activity; calcium ions released by gypsum dissolution react with carbonate from bacterially produced CO2 and form calcite. This conversion was considered a significant benefit of the microbiological activity, making the biological treatment not only a cleaning procedure but also a consolidation treatment. Many recent investigations have proposed carbonate mineralization as a method to protect works of art consisting of carbonate stone (5, 21). In our experiments, FTIR and SEM-EDS analyses showed that calcite was also formed on surfaces treated with chemical agents. However, it has been suggested that carbonates newly formed by bacteria are (i) more resistant to mechanical stress than calcite formed as a product of other organic processes (17) and (ii) less soluble than inorganically precipitated calcite (15). Tiano et al. (21) suggested the use of Micrococcus and Bacillus to induce carbonate mineralization, and Rodriguez-Navarro et al. (17) suggested the use of Myxococcus for this purpose. However, this type of treatment has some drawbacks, such as the growth of other (detrimental) microorganisms favored by the presence of organic nutrients. Thus, the recent literature recommends the use of organic matrix molecules for mineralization (22). It is worth noting, however, that in the biological procedure used in this research, prior to mixing with the delivery system, SRB were suspended in a phosphate buffer containing only lactate, which is used by SRB as an electron donor. As a consequence, we did not provide organic sources for subsequent microbial colonization.
Our findings showed that for cleaning the ornamental surfaces of Milan Cathedral, chemical treatment based on the use of ammonium carbonate-EDTA was inferior to a biotechnologically based treatment.
Acknowledgments
This work was funded by both the European Union project BIOBRUSH (Bioremediation for Building Restoration of the Urban Stone Heritage in European States; project EVK4-2001-00055) and the MIUR PRIN 2006 “Biotecnologie microbiche per la pulitura di manufatti lapidei di elevato pregio storico artistico: valutazione dell'efficacia e confronto con metodi di pulitura tradizionali” project.
We are very grateful to the Veneranda Fabbrica del Duomo for assistance with and support of the diagnostic work on the Milan Cathedral.
Footnotes
Published ahead of print on 29 June 2007.
REFERENCES
- 1.ASTM International. 2004. Standard guide for selection of cleaning techniques for masonry, concrete, and stucco surfaces. ASTM E1857-97(2004). ASTM International, West Conshohocken, PA.
- 2.Atlas, R. M., A. N. Chowdhury, and L. K. Gauri. 1988. Microbial calcification of gypsum-rock and sulfated marble. Stud. Conserv. 33:149-153. [Google Scholar]
- 3.British Standard Institutions. 2000. Code of practice for cleaning and surface repair of buildings. Part 1. Cleaning of natural stones, brick, terracotta and concrete. British Standards BS 8221-1:2000. British Standard Institutions, London, United Kingdom.
- 4.Cappitelli, F., E. Zanardini, G. Ranalli, E. Mello, D. Daffonchio, and C. Sorlini. 2006. Improved methodology for bioremoval of black crusts on historical stone artworks by use of sulfate-reducing bacteria. Appl. Environ. Microbiol. 72:3733-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castanier, S., G. Le Métayer-Levrel, G. Orial, J. F. Loubière, and J. P. Perthuisot. 2000. Bacterial carbonatogenesis and applications to preservation and restoration of historic property, p. 201-216. In O. Ciferri, P. Tiano, and G. Mastromei (ed.), Of microbes and art: the role of microbial communities in the degradation and protection of cultural heritage. Plenum, New York, NY.
- 6.Del Monte, M., C. Sabbioni, and G. Zappia. 1987. The origin of calcium oxalates on historical buildings, monuments and natural outcrops. Sci. Total Environ. 67:17-39. [Google Scholar]
- 7.Esbert, R. M., F. Díaz-Pache, C. M. Grossi, F. J. Alonso, and J. Ordaz. 2001. Airborne particulate matter around the Cathedral of Burgos (Castilla y León, Spain). Atmos. Environ. 35:441-452. [Google Scholar]
- 8.Fassina, V. 1995. New findings on past treatments carried out on stone and marble monuments’ surfaces. Sci. Total Environ. 167:185-203. [Google Scholar]
- 9.Gauri, K. L., and A. N. Chowdhury. 1988. Experimental studies on conversion of gypsum to calcite by microbes, p. 545-550. In J. Ciabach (ed.), Proceedings of the 6th International Congress on Deterioration and Conservation of Stone, 12 to 14 September 1988. Nicholas Copernicus University Press, Torùn, Poland.
- 10.Grossi, C. M., R. M. Esbert, F. Díaz-Pache, and F. J. Alonso. 2003. Soiling of building stones in urban environments. Build. Environ. 38:147-159. [Google Scholar]
- 11.Istituto Centrale del Restauro and Consiglio Nazionale delle Ricerche. 1985. Interventi conservativi: progettazione esecuzione e valutazione preventiva. Document NorMaL 20/85. ICR-CNR ed., Rome, Italy.
- 12.Lazzarini, L., and M. Laurenzi Tabasso. 1986. Il restauro della pietra. CEDAM, Padua, Italy.
- 13.Maravelaki-Kalaitzaki, P. 2005. Black crusts and patinas on Pentelic marble from the Parthenon and Erechtheum (Acropolis, Athens): characterization and origin. Anal. Chim. Acta 532:187-198. [Google Scholar]
- 14.Moropoulou, A., K. Bisbikou, R. Van Grieken, F. Zezza, and F. Macri. 1998. Origin and growth of weathering crusts on ancient marbles in industrial atmosphere. Atmos. Environ. 32:967-982. [Google Scholar]
- 15.Morse, J. W. 1983. The kinetics of calcium carbonate dissolution and precipitation. Rev. Mineral. 11:227-264. [Google Scholar]
- 16.Ranalli, G., M. Chiavarini, V. Guidetti, F. Marsala, M. Matteini, E. Zanardini, and C. Sorlini. 1997. The use of microorganism for the removal of sulphates on artistic stoneworks. Int. Biodeterior. Biodegrad. 40:255-261. [Google Scholar]
- 17.Rodriguez-Navarro, C., M. Rodriguez-Gallego, K. B. Chekroun, and M. T. Gonzalez-Muñoz. 2003. Conservation of ornamental stone by Myxococcus xanthus-induced carbonate biomineralization. Appl. Environ. Microbiol. 69:2182-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi Manaresi, R. 1996. Oxalate patinas and conservation treatments, p. 113-127. In M. Realini and L. Toniolo (ed.), The oxalate films in the conservation of works of art. Proceedings of the 2nd International Symposium, Milan, 25 to 27 March 1996. EDITEAM, Bologna, Italy.
- 19.Rosvall, J. 1988. Air pollution and conservation. Durabil. Build. Mater. 5:209-237. [Google Scholar]
- 20.Slaton, D., and K. C. Normandin. 2005. Masonry cleaning technologies. J. Architect. Conserv. 11:7-31. [Google Scholar]
- 21.Tiano, P., L. Biagiotti, and G. Mastromei. 1999. Bacterial bio-mediated calcite precipitation for monumental stones conservation: methods of evaluation. J. Microbiol. Methods 36:139-145. [DOI] [PubMed] [Google Scholar]
- 22.Tiano, P., E. Cantisani, I. Sutherland, and J. M. Paget. 2006. Biomediated reinforcement of weathered calcareous stones. J. Cult. Herit. 7:49-55. [Google Scholar]
- 23.Vergès-Belmin, V. 1996. Towards a definition of common evaluation criteria for the cleaning of porous building materials: a review. Sci. Technol. Cult. Herit. 5:69-83. [Google Scholar]
- 24.Webster, A., and E. May. 2006. Bioremediation of weathered-building stone surface. Trends Biotechnol. 24:255-260. [DOI] [PubMed] [Google Scholar]