Abstract
Coprophilous and litter-decomposing species (26 strains) of the genus Coprinus were screened for peroxidase activities by using selective agar plate tests and complex media based on soybean meal. Two species, Coprinus radians and C. verticillatus, were found to produce peroxidases, which oxidized aryl alcohols to the corresponding aldehydes at pH 7 (a reaction that is typical for heme-thiolate haloperoxidases). The peroxidase of Coprinus radians was purified to homogeneity and characterized. Three fractions of the enzyme, CrP I, CrP II, and CrP III, with molecular masses of 43 to 45 kDa as well as isoelectric points between 3.8 and 4.2, were identified after purification by anion-exchange and size exclusion chromatography. The optimum pH of the major fraction (CrP II) for the oxidation of aryl alcohols was around 7, and an H2O2 concentration of 0.7 mM was most suitable regarding enzyme activity and stability. The apparent Km values for ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)], 2,6-dimethoxyphenol, benzyl alcohol, veratryl alcohol, and H2O2 were 49, 342, 635, 88, and 1,201 μM, respectively. The N terminus of CrP II showed 29% and 19% sequence identity to Agrocybe aegerita peroxidase (AaP) and chloroperoxidase, respectively. The UV-visible spectrum of CrP II was highly similar to that of resting-state cytochrome P450 enzymes, with the Soret band at 422 nm and additional maxima at 359, 542, and 571 nm. The reduced carbon monoxide complex showed an absorption maximum at 446 nm, which is characteristic of heme-thiolate proteins. CrP brominated phenol to 2- and 4-bromophenols and selectively hydroxylated naphthalene to 1-naphthol. Hence, after AaP, CrP is the second extracellular haloperoxidase-peroxygenase described so far. The ability to extracellularly hydroxylate aromatic compounds seems to be the key catalytic property of CrP and may be of general significance for the biotransformation of poorly available aromatic substances, such as lignin, humus, and organopollutants in soil litter and dung environments. Furthermore, aromatic peroxygenation is a promising target of biotechnological studies.
The introduction of oxygen functionalities into aromatic compounds is a key step in the synthesis of specific metabolites (hormones, antibiotics, phytoalexines, etc.) in numerous organisms as well as of particular importance for the initiation of specific degradation and detoxification pathways. Thus, environmental pollutants such as benzene, toluene, and polycyclic aromatic hydrocarbons have been found to be subject to enzymatic epoxidation/hydroxylation, leading to the formation of assimilable metabolites (in bacteria and fungi) or water-soluble products which can be excreted (in animals) (12, 13). Furthermore, the incorporation of oxygen increases the reactivities of natural and xenobiotic molecules that are prerequisites for spontaneous coupling reactions resulting in the humification of organic materials. Oxygenation is usually catalyzed by complex intracellular enzymes transferring either one (monooxygenases) or two (dioxygenases) oxygen atoms from dioxygen (O2) to the substrate (39). Examples are toluene monooxygenase (28), nonspecific cytochrome P450 monooxygenases (13), and naphthalene dioxygenase (9), which all need NAD(P)H as a cosubstrate (electron donor) and specific transport proteins (reductases and ferredoxins) supplying the electrons derived from NAD(P)H to the catalytic oxygenase component. Heme-thiolate haloperoxidases make an exception to this rule; they are extracellular fungal biocatalysts that need only peroxide (e.g., H2O2) for function and catalyze, in addition to classic peroxidase reactions (e.g., phenol oxidation) and halogenations (18), the oxygenation of certain substrates (39, 42).
The first haloperoxidase, chloroperoxidase (CPO) from the sooty mold Caldariomyces fumago, was already described more than 50 years ago and was thought to be involved in the chlorination of fungal metabolites via the intermediary formation of hypochloric acid (29, 34). Later, it turned out that CPO is a heme-thiolate protein, i.e., the fifth ligand of the iron in the heme ring is a cysteine, as in cytochrome P450 monooxygenases (3). As the latter, CPO shows specific oxygenating activities, such as sulfoxidation (5), epoxidation of dienes (45), and benzylic hydroxylation (27). The second enzyme of this type, Agrocybe aegerita peroxidase (AaP), was described in 2004 for the agaric basidiomycete Agrocybe aegerita (18, 41), but in contrast to CPO, AaP also hydroxylates aromatic substrates. Thus, we recently demonstrated the hydroxylation of toluene at different positions and the regioselective hydroxylation of naphthalene by AaP (40). Interestingly, spectral studies on AaP and CPO suggested a closer relationship of AaP to cytochrome P450s than to CPO and led to the conclusion that AaP may be regarded as a functional hybrid of CPO and cytochrome P450 enzymes acting as an extracellular peroxygenase (18). Since the catalytic properties of aromatic peroxygenases are of interest from the environmental and biotechnological points of view (for activation and biotransformation of poorly available aromatics, such as lignin, humic substances, and polycyclic aromatic hydrocarbons), we wanted to know whether such enzymes are also found in other fungi. To this end, we screened mushrooms of the genus Coprinus (ink caps), in the course of which two further heme-thiolate haloperoxidase-peroxygenase producers were identified. Here we describe the purification and partial characterization of one of these enzymes, from Coprinus radians.
MATERIALS AND METHODS
Organisms.
Fungal strains belonging to 26 species of the genus Coprinus (Table 1) were obtained from the German collection of microorganisms and cell cultures (designation DSMZ; Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany), the Baarn filamentous fungal collection (designation CBS; Centraalbureau voor Schimmelcultures, Baarn, The Netherlands), the fungal culture collection of the Department of Applied Chemistry and Microbiology, University of Helsinki, Helsinki, Finland (designation K), and the culture collection of the International Graduate School of Zittau, Zittau, Germany (designation C or Zi).
TABLE 1.
Peroxidase and laccase activities (towards veratryl alcohol and ABTS, respectively), benzyl alcohol oxidation, and final pH in liquid surface cultures of 26 Coprinus spp. after 21 days of culturea
| Coprinus species | Strain | ABTS oxidation in agar platesb | Peroxidase activity (U liter−1)c | Laccase activity (U liter−1)d | Benzyl alcohol oxidatione | pH of the medium after 3 wk |
|---|---|---|---|---|---|---|
| Coprinus alcalinus | C 149-1 | + | ND | Y | 8.37 | |
| Coprinus aureogranulatus | CBS 973.95 | + | ND | N | 7.70 | |
| Coprinus atramentarius | K 119 | + | ND | N | 6.29 | |
| Coprinus clastophylus | DSMZ 8306 | + | ND | Y | 7.41 | |
| Coprinus comatus | CCB 053 | + | ND | N | 6.64 | |
| Coprinus disseminatus | CBS 506.78 | + | ND | N | 7.61 | |
| Coprinus domesticus | CBS 378.90 | + | ND | Y | 8.04 | |
| Coprinus erythrocephalus | C 022-1 | + | ND | Y | 6.9 | |
| CBS 534.87 | + | ND | Y | 7.86 | ||
| Coprinus laanii | CBS 476.70 | − | ND | N | 7.35 | |
| Coprinus marculentus | CBS 433.86 | − | ND | Y | 7.89 | |
| Coprinus micaceus | DSMZ 1704 | + | ND | N | 7.84 | |
| Coprinus miser | CBS 680.70 | + | ND | N | 7.27 | |
| Coprinus narcoticus | CBS 681.70 | + | ND | Y | 8.02 | |
| Coprinus phaeosporus | CBS 894.70 | + | ND | N | 8.14 | |
| Coprinus picaceus | CBS 790.84 | + | ND | Y | 8.00 | |
| Coprinus psydromorbird | CBS 209.89 | + | ND | N | 6.21 | |
| Coprinus radians | DSMZ 888 | + | 178 ± 24 | 71 ± 12 | Y | 8.30 |
| Coprinus silvaticus | CBS 588.84 | + | ND | Y | 7.74 | |
| Coprinus sp. | Zi 12 | − | ND | N | 7.70 | |
| Z 3 | + | ND | Y | 7.86 | ||
| Coprinus sterquilinus | DSMZ 3341 | + | ND | Y | 6.55 | |
| Coprinus truncorum | CBS 640.66 | + | ND | N | 8.16 | |
| Coprinus tuberosus | CBS 590.80 | + | ND | N | 8.24 | |
| Coprinus verticillatus | DSMZ 3397 | + | 14 ± 8 | 388 ± 39 | Y | 8.04 |
| Coprinus xanthothrix | DSMZ 4916 | + | ND | Y | 7.95 |
The medium contained 30 g liter−1 soybean flour, and the initial pH was 6.7. Values given represent means ± standard deviations for three replicates.
+, dark violet coloring around and below the fungal mycelium; −, no coloring reaction (growth period, 7 to 14 days).
Peroxidase activity was determined by the oxidation of veratryl alcohol to veratraldehyde at pH 7 (41).
Laccase activity was measured by the oxidation of ABTS at pH 4.5 after 21 days (only in the case of C. radians and C. verticillatus). ND, not determined.
Benzyl alcohol (1.8 mM) was added just after inoculation and was quantified by HPLC after 3 weeks. Y, benzyl alcohol conversion of >10% and detection of benzaldehyde and/or benzoic acid; N, benzyl alcohol conversion of <10%, with detection of neither benzaldehyde nor benzoic acid.
Culture conditions.
Fungal stock cultures were grown on 2% malt extract agar (MEA) at 24°C in culture slants and stored at 4°C in the dark. All fungal strains were precultured on MEA plates for 14 days. The basic liquid medium consisted simply of soybean meal (30 g liter−1) (Hensel Voll-Soja; Schoneberge GmbH, Magstadt, Germany) suspended in distilled water. In the case of Coprinus radians, selected as the most interesting peroxidase producer, the soybean medium was supplemented with various concentrations of glucose (10 to 40 g liter−1) to improve growth and enzyme production.
In all screening tests, 100-ml Erlenmeyer flasks containing 40 ml liquid medium were inoculated with three agar plugs (diameter, 1 cm) and supplemented with 1.8 mM benzyl alcohol. Cultivation occurred at 24°C for 3 weeks as surface cultures. In addition to being screened in liquid culture, fungal strains were tested using a special agar medium containing soybean meal (30 g liter−1) and ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid; 250 mg liter−1], an indicator substrate for extracellular phenol oxidases and peroxidases (37).
Larger amounts of peroxidase from C. radians were produced in 500-ml flasks containing 200 ml liquid soybean medium supplemented with glucose (3%), which was inoculated with the contents of an overgrown MEA plate homogenized in 80 ml sterile water (5% [vol/vol]). Fungal cultures were agitated on a rotary shaker (100 rpm) at 24°C, and samples were taken every 2 to 3 days to measure enzyme activities and pH.
Enzyme assays.
Specific peroxidase activity was measured at 310 nm following the oxidation of veratryl alcohol to veratraldehyde at 310 nm (ɛ310, 9.3 mM−1 cm−1) in sodium phosphate-citrate buffer at pH 7 (41). The reaction was started by the addition of 0.7 mM H2O2. Aryl alcohol oxidase was detected under the same conditions, but in the absence of H2O2 (26). Laccase activity was determined with ABTS following its oxidation at 420 nm (ɛ420, 36.0 mM−1 cm−1) (8). Peroxygenase activity was determined by following the hydroxylation of naphthalene into 1-naphthol at 303 nm (ɛ303, 2.01 cm−1 mM−1) (23). The assay mixture consisted of 500 μl potassium phosphate buffer (100 mM; pH 7.0), 200 μl naphthalene (5 mM) dissolved in acetonitrile, 10 to 200 μl enzyme solution, and distilled water to give a final volume of 1 ml. The reaction was started by the addition of 20 μl hydrogen peroxide (50 mM).
Enzyme purification and characterization.
Culture liquid (total volume, 4 liters) of C. radians was centrifuged, and the supernatant was filtered through glass fiber filters (GF 6; Whatman GmbH, Dassel, Germany). The culture filtrate was concentrated 60-fold by two steps of ultrafiltration, using a tangential-flow cassette (Omega Minisette, with a cutoff of 10 kDa; Pall Corporation, Hauppauge, NY) and a 150-ml stirred-cell system (10-kDa-cutoff modified polyethersulfone membrane; Pall Life Sciences, Dreieich, Germany).
Crude enzyme preparations were further purified by two steps of anion-exchange chromatography, using Q Sepharose FF and Mono Q as separation media, followed by size exclusion chromatography (SEC; Superdex 200). All chromatographic steps were performed with an ÄKTA fast-performance liquid chromatography (FPLC) system (GE Healthcare Europe GmbH, Freiburg, Germany). Anion-exchange chromatography was carried out with sodium acetate (10 mM; pH 5.5 to 6.5) as the solvent, eluting the proteins with an increasing sodium chloride gradient of 0 to 0.3/0.6 M (flow rate, 1 ml min−1). SEC was performed under isocratic conditions (50 mM sodium acetate, 100 mM NaCl, pH 6.0) at a flow rate of 1 ml min−1. AaP used for comparison was produced and purified as described previously (40, 41).
Molecular masses of Coprinus radians peroxidase (CrP) isoforms were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Novex XCell; Invitrogen GmbH, Karlsruhe, Germany) using a 12% NuPage gel. A low-molecular-mass calibration kit (MBI Fermentas, St. Leon Roth, Germany) served as a protein standard. The same electrophoresis system was used for analytical isoelectric focusing (IEF), applying precast IEF gels (pH 3 to 7; Invitrogen) with pH 3 to 10 IEF markers (Serva, Heidelberg, Germany). Electrophoretically separated protein bands were visualized with a colloidal blue staining kit (Invitrogen).
For N-terminal amino acid analysis, purified CrP was treated with an enzymatic protein deglycosylation kit (Sigma-Aldrich, Steinheim, Germany) and stained with Coomassie blue (see Fig. 3B), and protein bands were transferred by electroblotting to a hydrophobic polyvinylidene difluoride membrane (GE Healthcare) by using an XCell II blotting module (Invitrogen).
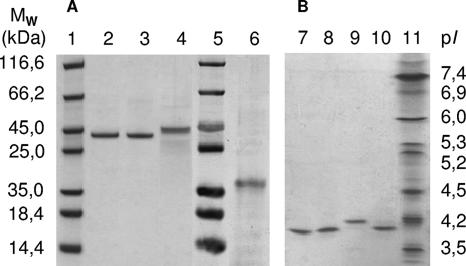
FIG. 3.
Electrophoretic characterization of purified CrP isoforms. (A) SDS-PAGE of CrP II (lane 2), CrP III (lane 3), and CrP I (lane 4) after Mono Q separation. Lanes 1 and 5, protein standards; lane 6, CrP II after deglycosylation. (B) IEF of purified CrP I, CrP II, and CrP III after SEC separation. Lanes 7 and 8, CrP I; lane 9, CrP II; lane 10, CrP III; lane 11, protein standards.
For de novo peptide sequencing of CrP II and AaP, proteins purified by FPLC were separated by SDS electrophoresis and stained with Coomassie blue. Single bands were excised and digested overnight with trypsin (33). The extracted peptides were separated by reversed-phase nano-LC (LC1100 series; Agilent Technologies, Palo Alto, CA) with a Zorbax 300SB-C18 column (3.5 μm by 150 mm by 0.075 mm; eluent, 0.1% formic acid, 0 to 60% acetonitrile) and analyzed by tandem mass spectrometry (LC/MSD TRAP XCT mass spectrometer; Agilent Technologies, Palo Alto, CA). Database searches were carried out with MS/MS Ion Search (MASCOT [http://www.matrixscience.com]) against the NCBI nonredundant database. The software Spectrum Mill (Agilent Technologies, Palo Alto, CA) was used for de novo sequencing of tryptic peptides.
In a second approach, the gel bands were cut in half and transferred to a 96-well microtiter plate (Greiner Bio-One, Solingen, Germany). The protein cysteine bonds were reduced in the gel bands by soaking them in 20 μl 10 mM dithiothreitol (in 25 mM NH4HCO3) for 1 h at 56°C, and the free cysteines were alkylated by further soaking in 20 μl 55 mM iodoacetamide at room temperature in the dark, with occasional shaking. The bands were subsequently processed on an automatic Ettan TA digester (Amersham, Freiburg, Germany) (see reference 14 for details).
The tryptic peptides were reconstituted in 5 μl aqueous 0.1% formic acid, and aliquots (2 to 5 μl) were injected onto a CapLC XE nano-LC system (Waters, Milford, MA). De novo peptide sequencing was performed as described previously (14). The ProteinLynx Global Server v.2.2.5 browser (Waters) was used for data preparation and de novo peptide sequence identification. The determined sequences were searched against the Swissprot database, downloaded on 12 December 2006. The search parameters were as follows: parent peptide tolerance, 20 ppm, with a minimum of two peptides found; fragment tolerance, 0.05 Da; estimated calibration error, 0.025 Da; one possible missed cleavage; carbamidomethylation of cysteines; and possible oxidation of methionines. The amino acid sequences of peptides were subjected to searches (minimum of two identified peptides, with an accuracy of ≤20 ppm) and homology searches (MS-BLAST server installed in-house [further information can be found at http://dove.embl-heidelberg.de/Blast2/], with a PAM30MS matrix and an expect factor of 10) using a nonredundant consolidated database (sp_nrdb) comprising several nonredundant protein sequence databases (SwissProt, SwissProtNew, SptremblNew, and Sptrembl), with no significant hits.
Kinetic constants (Km and kcat) of CrP were determined for veratryl alcohol (pH 7), benzyl alcohol (pH 7), 2,6-dimethoxyphenol (DMP; pH 4.5), ABTS (pH 4.5), and naphthalene (pH 7) by following the formation of veratraldehyde, benzaldehyde (ɛ280, 1.4 cm−1 mM−1), dimeric DMP quinone (ɛ569, 49.6 cm−1 mM−1), ABTS cation radical (ɛ420, 36.0 cm−1 mM−1), and 1-naphthol (ɛ303, 2.01 cm−1 mM−1), respectively. Km and kcat values for H2O2 were determined in the presence of 5 mM benzyl alcohol at pH 7. Lineweaver-Burk plots were made from the initial rates obtained at various substrate concentrations while the concentration of the second substrate was held constant.
UV-Vis spectroscopy.
UV-visible (UV-Vis) spectra of resting-state CrP (4.65 μM) as well as of its reduced CO complex were recorded in 100 mM sodium phosphate buffer (pH 7.0) in the range of 200 to 700 nm, using a Cary 50 spectrophotometer (Varian, Darmstadt, Germany). To obtain the reduced CO-enzyme complex, samples were reduced with a few grains of sodium dithionite and flushed with CO for 2 min.
HPLC.
High-performance liquid chromatography (HPLC) was used to determine the concentrations of aromatic compounds in fungal cultures (benzyl alcohol and its oxidation products) or in cell-free reaction mixtures (phenol, halogenated phenols, naphthalene, and its oxygenation/oxidation products). Samples (1 to 1.5 ml) were routinely filtered using AcrodiscSyringe filters (0.45-μm pore size) (SuporMembrane; Pall Corporation, Hauppauge, NY) and transferred to 2-ml HPLC vials. An Agilent HPLC system (1100 series; Agilent, Waldbronn, Germany) equipped with a diode array detector and LiChrospher reversed-phase (C18) columns (4.6 mm by 125 mm by 5 μm; Merck) were used for all analyses. A mixture of acetonitrile and 15 mM phosphoric acid (30:70 [vol/vol]) served as the solvent, at a flow rate of 1 ml min−1, under isocratic conditions. Eluted substances were detected in the wavelength range from 190 to 550 nm and identified/quantified by means of authentic standards (benzyl alcohol, benzaldehyde, benzoic acid, phenol, 2- and 4-bromophenol, and 2-chlorophenol; Sigma-Aldrich).
Enzymatic in vitro tests.
Halogenation by CrP was tested by the bromination and chlorination of phenol. The reaction mixtures (total volume, 1 ml in 2-ml vials) contained 20 mM potassium phosphate buffer (pH 3.0), 10 mM KBr or KCl, 100 μM phenol, and 110 nM CrP. Reactions were started by the addition of 0.7 mM H2O2 at 25°C and stopped after 10 min by the addition of 20 μl HCl (36%). Halogenated phenols were analyzed using the HPLC system and conditions mentioned above.
Aromatic hydroxylation was proven by the oxidation of naphthalene. The latter (100 μM) was treated with purified CrP in 2-ml reaction vials containing 50 mM citrate-phosphate buffer (pH 7) and 110 nM CrP. The reaction was initiated by the addition of 0.7 mM H2O2 and stopped after 10 min by the addition of 40 μl trichloroacetic acid (50%). After 10 min under reaction conditions at pH 7, CrP still exhibited about 80% of its initial activity (merely 60% was recovered after bromination at pH 3). Samples were analyzed by HPLC, using the system described above but applying a gradient of 20 to 80% acetonitrile (0 and 5 min, 20%; 20 min, 80%) in 15 mM phosphoric acid, with a constant flow rate of 1 ml min−1 over 20 min. Authentic naphthalene, 1-naphthol, 2-naphthol, and 1,4-naphthoquinone (all from Merck) served as standards.
RESULTS
Screening.
Within a two-stage screening, 26 species of the genus Coprinus were tested for oxidative enzyme activities in nitrogen-rich media (soybean-ABTS agar plates and a liquid soybean medium supplemented with benzyl alcohol). The first criterion for strain selection was a strong coloring of the ABTS plates from light yellowish to dark violet, particularly around and below the fungal mycelium. The second criterion was the fungal capability to oxidize benzyl alcohol to benzaldehyde and/or benzoic acid in the surface cultures (Table 1). Fungal strains which fulfilled both criteria were checked further for peroxidase activity towards veratryl alcohol. As a result, the strains Coprinus radians DSM 888 and Coprinus verticillatus DSM 3397 were selected as particularly promising species. Both produced a peroxidase that oxidized veratryl alcohol at pH 7, indicating the presence of a haloperoxidase; maximum levels were 176 ± 24 U liter−1 and 14 ± 8 U liter−1 for C. radians and C. verticillatus, respectively. C. radians was chosen for all further studies due to its rapid growth and relatively high peroxidase titer.
In addition to the two peroxidase-producing species, 23 of 26 Coprinus species (including C. radians and C. verticillatus) were observed to oxidize ABTS on agar plates (Table 1). Interestingly, ABTS oxidation in the presence of soybean meal did not result in the characteristic blue-green of ABTS+· radicals (37) but in a dark violet coloring. Maybe the color change was caused by subsequent coupling of ABTS cation radicals to phenolic soybean ingredients (e.g., flavonoids) or by their coupling with each other. HPLC elution profiles showed that 14 of 26 Coprinus species (again including C. radians and C. verticillatus) oxidized benzyl alcohol to benzaldehyde and benzoic acid. The pH in most cultures increased noticeably within the cultivation period of 3 weeks (from 6.7 to values between 6.9 and 8.3; nine species reached a pH above 8, including C. radians [pH 8.3] and C. verticillatus [pH 8.04]) (Table 1).
Peroxidase production by Coprinus radians.
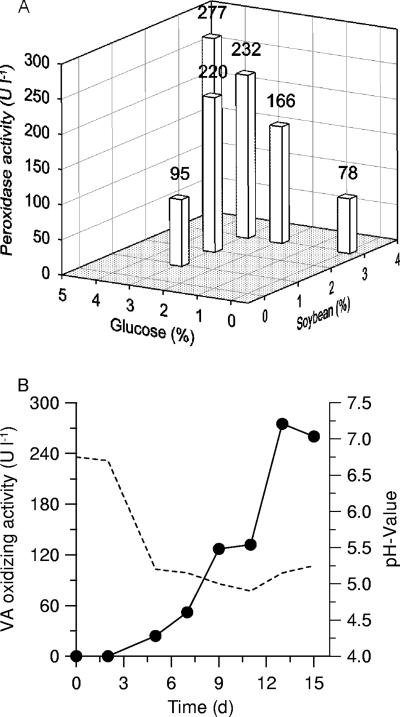
Soybean meal and glucose proved to be the key factors influencing/promoting the production of CrP in agitated liquid culture. Changes in their content led to drastic changes in enzyme yield. Thus, CrP activity was not detectable in the absence of soybean meal, and omitting glucose led to peroxidase levels of <80 U liter−1. The increase of glucose concentration while the amount of soybean meal was held constant at a relatively low level (10 g liter−1) resulted in an almost linear increase in CrP activity (Fig. 1A). The maximum CrP level (277 U liter−1) was obtained in the presence of 3% (wt/vol) soybean meal and 4% (wt/vol) glucose and was reached on day 13 (Fig. 1B), and then the activity slowly decreased until the end of the experiment.
FIG. 1.
(A) Effects of different amounts of soybean meal and glucose on peroxidase production by Coprinus radians DSM 888 in surface culture (data points represent maximum levels obtained within a cultivation period of 3 weeks). (B) Time course of peroxidase production by C. radians DSMZ 888 in the presence of 3% soybean meal and 4% glucose in agitated culture. Data points represent mean values for three parallel cultures (standard deviations, <10%). The dotted line marks the time course of pH. VA, veratryl alcohol.
Using optimal substrate concentrations and agitated cultures, sufficient amounts of CrP were produced (average final peroxidase activity, 157 U liter−1), but only in the pH range from 4.8 to 5.2 that was maintained throughout the whole cultivation. In contrast to surface cultures (where the pH increased during peroxidase production), fungal growth and CrP production were inhibited in agitated cultures when the pH did not fall or even increased (data not shown). The form of the fungal mycelium was amorphous (flake-like rather than pellet-shaped) under the culture conditions described, and activities of manganese peroxidase, other peroxidases, aryl alcohol oxidase, and laccase were not observed.
Purification of CrP.
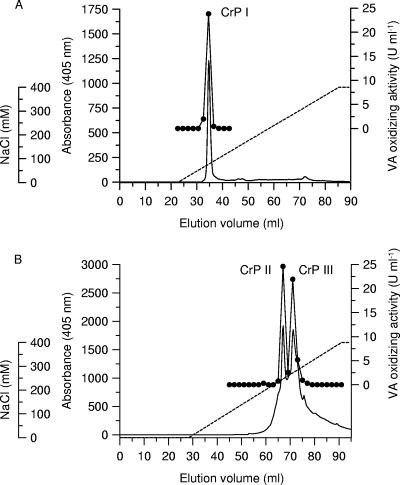
Agitated cultures of the fungus (total volume, 4 liters) were harvested after 13 days and centrifuged to separate the mycelium from the culture liquid. The latter was concentrated by ultrafiltration and separated by three chromatographic steps, using an FPLC system fitted with a Q Sepharose FF, Mono Q, or SEC column. Throughout the purification process, three fractions of the enzyme were detected and named CrP I, CrP II, and CrP III. Figure 2 shows the chromatograms of fractions CrP I (Fig. 2A), CrP II, and CrP III (Fig. 2B) obtained after elution from a Mono Q column. All fractions corresponding to different isoforms of the enzyme showed peroxidase activity towards veratryl alcohol at pH 7. The final specific activities of the three CrP fractions were similar (30 to 38 U mg−1), but since the total activity of CrP II (60 U) was more than twice as high as those of CrP I and III, it was chosen for further studies (Table 2).
FIG. 2.
FPLC elution profiles of CrP I (A) and CrP II and III (B) from Coprinus radians DSMZ 888. Separation was performed on a Mono Q column. Absorption at 405 nm (solid line), CrP activity detected by the oxidation of veratryl alcohol (VA) to veratraldehyde at pH 7 (•), and the NaCl gradient (dotted line) are shown.
TABLE 2.
Purification of veratryl alcohol-oxidizing peroxidases from Coprinus radians DSMZ 888a
| Purification step | Total activity | Total amt of protein (mg) | Sp act (U mg−1) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Culture liquid | 627 | 752.4 | 0.83 | 100 | 1.0 |
| First ultrafiltration step (10 kDa) | 613 | 691.5 | 0.89 | 97.8 | 1.1 |
| Second ultrafiltration step (10 kDa) | 547 | 316.2 | 1.73 | 87.2 | 2.1 |
| Q Sepharose FF fraction I | 143 | 25.0 | 5.7 | 22.8 | 6.9 |
| Q Sepharose FF fraction II | 341 | 18.2 | 18.7 | 54.4 | 22.5 |
| Mono Q CrP I | 40 | 1.6 | 25.1 | 6.4 | 30.1 |
| Mono Q CrP II | 150 | 7.2 | 20.6 | 23.9 | 24.8 |
| Mono Q CrP III | 100 | 3.3 | 30.5 | 15.9 | 36.6 |
| SEC CrP I | 27 | 0.9 | 31.5 | 4.3 | 37.8 |
| SEC CrP II | 60 | 2.0 | 30.6 | 9.6 | 36.7 |
| SEC CrP III | 21 | 0.55 | 38.5 | 3.3 | 46.2 |
Enzyme activities are based on the oxidation of veratryl alcohol to veratraldehyde at pH 7 (according to the method described in reference 41).
Characterization of CrP II.
SDS-PAGE revealed molecular masses of 43 kDa for CrP II and III and 45 kDa for CrP I (Fig. 3). IEF led to the estimation of the following four pIs, indicating the presence of four isoforms of the enzyme (Fig. 3): 3.8 and 3.9 (two close bands) for CrP I, 4.2 for CrP II, and 4.0 for CrP III. The purified major isoform CrP II was deglycosylated to remove bound carbohydrates and blotted (Western blotting) to prepare the protein for determination of the N-terminal amino acid sequence (Fig. 3). The deglycosylated protein had a molecular mass of 27 kDa, indicating a high carbohydrate content, of 37%, of the mature protein.
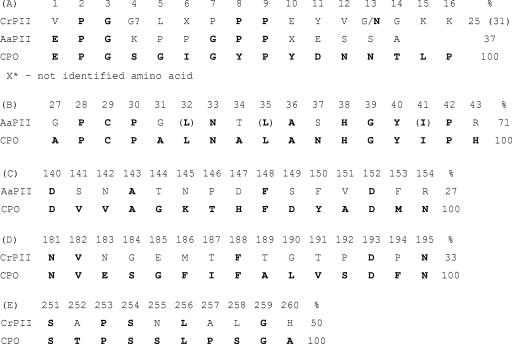
N-terminal sequencing was performed by Sequence Laboratories GmbH (Göttingen, Germany). The obtained CrP N-terminal sequence showed 29% identity to the N-terminal sequence of AaP and 25% identity to that of C. fumago CPO (Fig. 4A). These identities are lower than that of AaP and CPO (43%) (41). An additional NCBI database search with MS/MS data for tryptic peptides of purified CrP and AaP failed, and therefore de novo peptide sequencing was performed (Fig. 4B to E). The data obtained for an AaP peptide consisting of 16 amino acids show 71% identity to the CPO sequence around its active site (positions 27 to 43) and include the Cys29 that is responsible for the binding of heme (fifth ligand in CPO) (Fig. 4B). A second AaP fragment shows 27% identity with the CPO sequence between positions 140 and 154 (Fig. 4C). Unfortunately, a respective peptide fragment for CrP was not obtained, but the two CrP fragments sequenced (Fig. 4D and E) show 33% and 50% identities to the CPO sequence, between positions 181 and 195 and positions 251 and 260, respectively. All in all, the data imply a certain structural relationship of the three enzymes.
FIG. 4.
N-terminal sequences (A) and peptide fragment alignments (B to E) of CrP II (Coprinus radians), AaP (Agrocybe aegerita), and CPO (Caldariomyces fumago). The numbering of amino acid residues was done on the basis of the known total sequence of CPO (30). (B) A peptide fragment of AaP consisting of 16 amino acids shows 71% identity to the sequence around the active site of CPO (positions 27 to 43) and includes the Cys29 that is responsible for the binding of heme (fifth ligand [heme-thiolate]). (C) A second AaP fragment shows 27% identity. (D and E) Peptide sequences obtained for CrP II fragments showing 33% and 50% identities to the CPO sequence towards the C terminus.
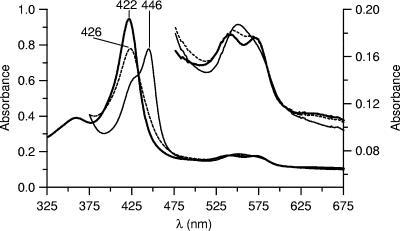
The UV-Vis spectrum of the enzyme's resting state (purified CrP II) shows a characteristic absorbance at 422 nm (Soret band) as well as α- and β-bands at 542 and 571 nm (Fig. 5). Furthermore, there is a clear band in the near-UV spectrum (δ-band) at 359 nm. The addition of sodium dithionite to the resting enzyme caused a peak shift from 422 to 426 nm. The spectrum of the CO complex of CrP obtained after it was flushed with carbon monoxide has its Soret absorption maximum at 446 nm, which is in the typical range for heme-thiolate proteins (Fig. 5).
FIG. 5.
UV-Vis absorption spectra of resting-state CrP II (4.6 μM) (thick line) and its reduced CO complex (thin line). The dotted line belongs to the spectrum of the dithionite-reduced enzyme.
Oxidation of different substrates and kinetic parameters.
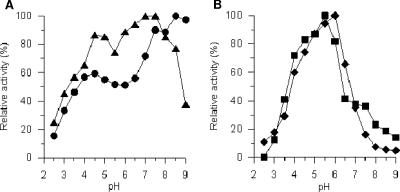
Purified CrP II oxidized veratryl alcohol and benzyl alcohol, as well as typical peroxidase substrates, such as ABTS and DMP, into the corresponding aldehydes (and further into benzoic acids) (Fig. 6A and B). The pH dependence of these reactions is shown in Fig. 6. The pH profile for ABTS has a sharp maximum at pH 5, and that of the phenolic substrate DMP shows a similar curve, with the maximum at pH 6 (Fig. 6B). Oxidation of veratryl alcohol and benzyl alcohol occurred in a broad pH range, between 2.5 and 9, and the maxima appear at pH 7.5 and 8, respectively (Fig. 6A). Interestingly, both pH profiles have a second distinct maximum at pH 4.5. Different polarization of the carbonyl group at different pHs possibly affects substrate binding as well as the electron transfer between aryl alcohol and the enzyme's active site.
FIG. 6.
Effects of pH on the CrP-catalyzed oxidation of veratryl alcohol (5 mM) (▴) and benzyl alcohol (5 mM) (•) (A) as well as DMP (2 mM) (⧫) and ABTS (0.6 mM) (▪) (B). Data points are means for three parallel measurements (standard deviations, <5%).
Using an aryl alcohol concentration of 5 mM, the highest CrP activities were observed between 0.5 and 0.7 mM H2O2 (at pH 7), but even in the presence of 4 mM H2O2, CrP still reached 60% of the maximum activity. The addition of higher concentrations of H2O2 (10 to 100 mM) resulted in the immediate formation of gas bubbles, indicating a catalase activity of CrP that produces O2.
Table 3 summarizes the apparent Michaelis-Menten (Km) and catalytic constants (kcat) of all substrates tested. Under the conditions used, ABTS was the best substrate and aryl alcohols were better substrates than the phenolic compound DMP, whose catalytic efficiency (kcat/Km) was about 50 times lower than those of the aryl alcohols. Turnover numbers of CrP ranged between 120 min−1 (DMP) and 10,560 min−1 (benzyl alcohol). The affinity of CrP for H2O2 seems to be comparatively low (Km, 1.25 mM), but due to the high turnover number (28,260 min−1), the catalytic efficiency is in the same range as those of aryl alcohols.
TABLE 3.
Kinetic parameters of purified CrP II from Coprinus radiansa
| Substrate | Km (μM) | kcat (s−1) | kcat/Km (s−1 M−1) |
|---|---|---|---|
| Veratryl alcohol | 88 | 34 | 3.86 × 105 |
| Benzyl alcohol | 635 | 176 | 2.77 × 105 |
| ABTS | 49 | 123 | 2.51 × 106 |
| DMP | 342 | 2 | 5.85 × 103 |
| Naphthalene | 584 | 15 | 2.57 × 104 |
| H2O2 | 1,201 | 471 | 3.92 × 105 |
Reactions were performed with various substrate concentrations at pH 4.5 (ABTS and DMP) or pH 7 (aryl alcohols and naphthalene). Constants for H2O2 were determined in the presence of 5 mM benzyl alcohol.
Halogenation of phenol and hydroxylation of naphthalene.
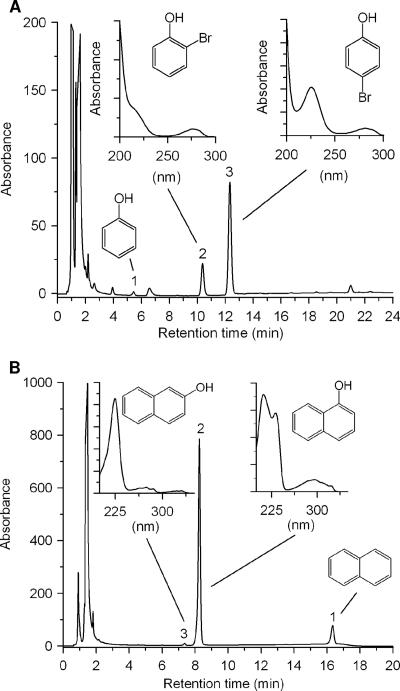
The HPLC elution profile of the bromination experiment shows the conversion of phenol into 2-bromo- and 4-bromophenol at a ratio of 1:4 (yields, 10% and 40%, respectively) (Fig. 7A). The chlorination test performed under identical conditions (pH 3, 0.7 mM H2O2, 10 mM KBr, 0.11 μM CrP II) yielded only traces of 2-chlorophenol (yield, <1% of the converted phenol), along with a number of unidentifiable oxidation and coupling products (data not shown).
FIG. 7.
Bromination of phenol (A) and hydroxylation of naphthalene (B) by CrP II. HPLC elution profiles were recorded at 275 and 220 nm, respectively. (A) Peak 1, residual phenol; peak 2, 2-bromophenol; peak 3, 4-bromophenol. Insets show the UV spectra of 2-bromo- and 4-bromophenol. (B) Peak 1, residual naphthalene; peak 2, 1-naphthol; peak 3, 2-naphthol. The insets show the UV spectra of 1-naphthol (right) and 2-naphthol (left).
To prove the oxygenating activity of CrP, naphthalene (100 μM) was treated at pH 7 with CrP II (0.11 μM) in the presence of 0.7 mM H2O2. About 50% of the naphthalene was converted to 1-naphthol, while only traces of 2-naphthol were detected (yield, <1%), which demonstrates the ability of CrP to catalyze aromatic hydroxylation and the regioselectivity of this reaction (ratio of 1-naphthol to 2-naphthol, 60:1) (Fig. 7B). The catalytic parameters of naphthalene hydroxylation are given in Table 3. The catalytic efficiency is about an order of magnitude lower than those of aryl alcohols but five times higher than that of the phenolic substrate DMP.
DISCUSSION
During growth in soybean medium, Coprinus radians produced a multifunctional peroxidase that oxidized aryl alcohols and aldehydes as well as typical peroxidase substrates, such as DMP and ABTS. Moreover, CrP was found to brominate phenol, resulting in the formation of 2-bromo- and 4-bromophenol, and remarkably, it was capable of selectively hydroxylating naphthalene. The latter reaction is of particular interest since the incorporation of oxygen into aromatic substrates is necessary to activate inert organic substances and is thought to be a specific feature of intracellular mono- and dioxygenases (2, 19, 39). The results of our study give reason to assume that enzymes acting as extracellular aromatic peroxygenases are widespread among agaric basidiomycetes.
CrP is the third heme-thiolate haloperoxidase that has been found so far and is the second one that catalyzes aromatic hydroxylation. The absorption spectrum of its reduced CO complex shows a characteristic shift of the Soret band from 422 nm to 446 nm, which is typical for all heme-thiolate proteins, including cytochrome P450 enzymes and the two other peroxidases of this type, AaP and CPO (18, 31). The molecular mass of CrP (43 to 45 kDa) and its isoelectric points (3.8 to 4.2) are in the same ranges as those of AaP (45 to 46 kDa; pI, 4.6 to 5.6) (41) and CPO (40 to 46 kDa; pI, 4.0) (20, 32). The three enzymes are heavily glycosylated proteins, with CrP having the highest carbohydrate content (37%) (this paper), while AaP and CPO consist of 20% and 25 to 30% sugars, respectively (18, 32). In the case of CPO, different glycosylation patterns were reported to result in different CPO isoforms (16), a fact that may also apply to CrP (three fractions and four isoforms) and AaP (up to six isoforms) (41).
Although the catalytic properties of CrP resemble those of AaP and CPO, there are considerable differences, particularly with respect to CPO, concerning substrate spectra, H2O2 requirements, kinetic parameters, and spectral properties (18, 38). Thus, the three enzymes were found to oxidize DMP, ABTS, and aryl alcohols at neutral pH and to brominate phenol at acidic pHs, but CrP (and AaP) cannot efficiently chlorinate phenol (only traces of 2-chlorophenol were found) and CPO cannot hydroxylate aromatics such as naphthalene (27, 40, 41). CrP hydroxylated naphthalene even more selectively than AaP did, with a product ratio of 1-naphthol to 2-naphthol of 60:1 (for comparison, the respective ratio obtained with AaP was 40:1) (40). A further distinction between CrP and AaP concerns the peroxide requirement; whereas CrP showed the highest activity for veratryl alcohol (5 mM) at 0.5 to 0.7 mM H2O2, AaP did so at 2 mM H2O2 under identical conditions (41). Although CPO does not hydroxylate aromatic rings, it is able to transfer oxygen from H2O2 to more activated carbon atoms, for example, those found in cyclic and aliphatic dienes (4, 11, 25).
The similarity of CrP and AaP becomes further evident by comparing their spectral data. The resting state of both enzymes shows Soret bands, at 422 nm and 420 nm, respectively, whereas resting-state CPO has its maximum at 401 nm. Also, the other maxima (α, β, and δ) of resting CrP and AaP are almost identical (Table 4). Interestingly, such spectral properties are more characteristic of cytochrome P450 enzymes than of peroxidases, including CPO (Table 4) (6, 18, 24).
TABLE 4.
Spectral characteristics of CrP in comparison to respective data for CPO, AaP, and two cytochrome P450 enzymesa
| Organism | Enzyme, function(s) | Soret maximum (nm)
|
Additional maximum of the native enzyme (nm)
|
||||
|---|---|---|---|---|---|---|---|
| Resting | Reduced | CO adduct | α | β | δ | ||
| Coprinus radians | CrP II, haloperoxidase, aromatic peroxygenase | 422 | 426 | 446 | 571 | 542 | 359 |
| Caldariomyces fumago | CPO, chloroperoxidase, nonaromatic peroxygenase | 401 | 409 | 443 | —b | 545 | — |
| Agrocybe aegerita | AaP, haloperoxidase, aromatic peroxygenase | 420 | 413 | 447 | 572 | 540 | 359 |
| Fusarium oxysporum | P450nor, nitric oxide reductase | 414 | 406 | 447 | 565 | 533 | — |
| Oryctolagus cuniculus | P450LM2, aromatic monooxygenase | 418 | 413 | 451 | 569 | 535 | 361 |
The C. radians strain used in this study was isolated from horse dung (DSMZ 888; ATCC 20014). The species also colonizes wet and moldy hardwood, often indoors in basements and in contact with soil or brickwork (1). A. aegerita, the black poplar mushroom, prefers hardwood (e.g., poplar stumps) and grows on subsurface wood as well as on mulch-like materials (36). These habitats are characterized by relatively large amounts of nitrogen and a high pH (pH 6 to 9), which gives reason to the assumption that the production of peroxygenases may be a characteristic feature of alkaliphilic and coprophilous mushrooms. This fact is further supported by the selection of a second Coprinus species, C. verticillatus (DSM 3397) isolated from camel dung, which also produced a peroxidase that converted veratryl alcohol at pH 7, within our screening (currently, this fungus is being studied in more detail in our lab). In this context, it is interesting that neither A. aegerita nor C. radians and C. verticillatus produced classic ligninolytic peroxidases (lignin, manganese, or versatile peroxidases). The latter are active only at low pHs (2.5 to 5.5), and possibly in more alkaline environments, mushrooms have developed alternative strategies to transform aromatic substances, for example, using peroxygenases.
Under laboratory conditions, CrP production was strongly dependent on soybean meal, and in its absence, no peroxidase activity was detectable. The same phenomenon was observed for AaP (41), and soybeans were also reported to stimulate the production of other peroxidases in Coprinus spp. (17, 21, 22). In the case of C. radians, supplementation of the soybean medium with glucose (2 to 3%) additionally stimulated peroxidase production, which was not observed for A. aegerita (41).
Aromatic hydroxylation and bromination are certainly the most striking properties of CrP and AaP. Hence, these enzymes can be regarded as functional hybrids of cytochrome P450 monooxygenases and haloperoxidases. Since bromination can also be catalyzed by other peroxidases, including CPO and lignin and manganese peroxidases (10, 15, 35), and may have little significance in terrestrial ecosystems (where bromide is rare), the specific catalytic feature of CrP and AaP may be their peroxygenase activity. Whether these hydroxylating activities are somehow related to lignin/humus degradation or rather to detoxification reactions is still elusive. In case studies with Coprinus spp., extensive hydroxylation of the herbicide isoproturon was recently observed for Coprinus lagopus (31a).
In future, it should be taken into consideration to designate enzymes which hydroxylate aromatic substrates by means of H2O2 as aromatic peroxygenases, irrespective of their other side activities, such as halogenation or phenol or alcohol oxidation (the abbreviations CrP and AaP could be used in the sense of Coprinus radians and Agrocybe aegerita peroxygenases). Moreover, it seems worthwhile to think about an individual EC number for aromatic peroxygenases. They act on a peroxide as an electron acceptor (peroxidases; EC 1.11.1.x) and convert a substrate (naphthalene) that is usually not susceptible to direct oxidation by other peroxidases (43). Further studies will have to substantiate this fact and clarify which aromatic substrates can be hydroxylated by CrP and how selectively these reactions occur in comparison to those mediated by CPO and AaP.
Acknowledgments
Financial support by the European Union (integrated project Biorenew), the Bundesministerium für Bildung, Wissenschaft und Forschung (BMBF; project 0313433D), and the German Academic Exchange Service (DAAD; projects A/04/20213 and D/05/11714) is gratefully acknowledged.
We thank C. Liers, M. Kluge (Inge), M. Kinne, M. Brandt, and U. Schneider for technical and scientific assistance as well as K. Steffen (University of Helsinki) for providing fungal strains.
Footnotes
Published ahead of print on 29 June 2007.
REFERENCES
- 1.Arora, D. 1986. Mushrooms demystified: a comprehensive guide to the fleshy fungi. Ten Speed Press, Berkeley, CA.
- 2.Bernhardt, R. 2006. Cytochromes P450 as versatile biocatalysts. J. Biotechnol. 124:128-145. [DOI] [PubMed] [Google Scholar]
- 3.Blanke, S. R., and L. P. Hager. 1988. Identification of the fifth axial heme ligand of chloroperoxidase. J. Biol. Chem. 263:18739-18743. [PubMed] [Google Scholar]
- 4.Colonna, S., N. Gaggero, C. Richelmi, and P. Pasta. 1999. Recent biotechnological developments in the use of peroxidases. Trends Biotechnol. 17:163-168. [DOI] [PubMed] [Google Scholar]
- 5.Colonna, S. G., N. Manfredi, A. Casella, L. Gulotti, M. Carrea, and G. Pasta. 1990. Enantioselective oxidations of sulfides catalyzed by chloroperoxidase. Biochemistry 29:10465-10468. [DOI] [PubMed] [Google Scholar]
- 6.Correia, M. A. 2005. Human and rat liver cytochromes P450: functional markers, diagnostic inhibitor probes, and parameters frequently used in P450 studies, p. 619-657. In P. R. Ortiz de Montellano (ed.), Cytochrome P450: structure, mechanism and biochemistry, 3rd ed. Plenum Press, New York, NY.
- 7.Daiber, A., H. Shoun, and V. Ullrich. 2005. Nitric oxide reductase (P450nor) from Fusarium oxysporum. J. Inorg. Biochem. 99:185-193. [DOI] [PubMed] [Google Scholar]
- 8.Eggert, C., U. Temp, J. F. D. Dean, and K.-E. L. Eriksson. 1995. Laccase-mediated formation of the phenoxazinone derivative, cinnabarinic acid. FEBS Lett. 376:202-206. [DOI] [PubMed] [Google Scholar]
- 9.Ensley, B. D., and D. T. Gibson. 1983. Naphthalene dioxygenase: purification and properties of a terminal oxygenase component. J. Bacteriol. 155:505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farhangrazi, Z. S., R. Sinclair, I. Yamazaki, and L. S. Powers. 1992. Haloperoxidase activity of Phanerochaete chrysosporium lignin peroxidases H2 and H8. Biochemistry 31:10763-10768. [DOI] [PubMed] [Google Scholar]
- 11.Geigert, J., D. J. Dalietos, D. S. Hirano, and S. L. Neidleman. 1986. Epoxidation of alkenes by chloroperoxidase catalysis. Biochem. Biophys. Res. Commun. 136:778-782. [DOI] [PubMed] [Google Scholar]
- 12.Gibson, D. T., and R. E. Parales. 2000. Aromatic hydrocarbon dioxygenases in environmental biotechnology. Curr. Opin. Biotechnol. 11:236-243. [DOI] [PubMed] [Google Scholar]
- 13.Gillam, E. M. J., and D. J. B. Hunter. 2007. Chemical defense and exploitation. Biotransformation of xenobiotics by cytochrome P450 enzymes, p. 477-560. In A. Sigel, H. Sigel, and R. K. O. Sigel (ed.), The ubiquitous roles of cytochrome P450 proteins, vol. 3. John Wiley & Sons Ltd., Chichester, United Kingdom. [Google Scholar]
- 14.Giri, A. P., H. Wunsche, S. Mitra, J. A. Zavala, A. Muck, A. Svatos, and I. T. Baldwin. 2006. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant's proteome. Plant Physiol. 142:1621-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hager, L. P., D. R. Morris, F. S. Brown, and H. Eberwein. 1966. Chloroperoxidase. II. Utilization of halogen anions. J. Biol. Chem. 241:1769-1777. [PubMed] [Google Scholar]
- 16.Hashimoto, A., and P. A. Pickard. 1984. Chloroperoxidases from Caldariomyces (Leptoxyphium) cultures: glycoproteins with variable carbohydrate content and isoenzymic forms. J. Gen. Microbiol. 130:2051-2058. [Google Scholar]
- 17.Heinzkill, M., L. Bech, T. Halkier, P. Schneider, and T. Anke. 1998. Characterization of laccases and peroxidases from wood-rotting fungi (family Coprinaceae). Appl. Environ. Microbiol. 64:1601-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofrichter, M., and R. Ullrich. 2006. Heme-thiolate haloperoxidases: versatile biocatalysts with biotechnological and environmental significance. Appl. Microbiol. Biotechnol. 71:276-288. [DOI] [PubMed] [Google Scholar]
- 19.Holland, H. L. 1998. Hydroxylation and dihydroxylation, p. 475-533. In D. R. Kelly (ed.), Biotransformations I, vol. 8a. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- 20.Hollenberg, P. F., and L. P. Hager. 1973. The P-450 nature of the carbon monoxide complex of ferrous chloroperoxidase. J. Biol. Chem. 248:2630-2633. [PubMed] [Google Scholar]
- 21.Ikehata, K., I. D. Buchanan, M. A. Pickard, and D. W. Smith. 2005. Purification, characterization and evaluation of extracellular peroxidase from two Coprinus species for aqueous phenol treatment. Bioresour. Technol. 96:1758-1770. [DOI] [PubMed] [Google Scholar]
- 22.Kjalke, M., M. B. Andersen, P. Schneider, B. Christensen, M. Schulein, and K. G. Welinder. 1992. Comparison of structure and activities of peroxidases from Coprinus cinereus, Coprinus macrorhizus and Arthromyces ramosus. Biochim. Biophys. Acta 1120:248-256. [DOI] [PubMed] [Google Scholar]
- 23.Kluge, M., R. Ullrich, K. Scheibner, and M. Hofrichter. 2007. Spectrophotometric assay for detection of aromatic hydroxylation catalyzed by fungal haloperoxidase-peroxygenase. Appl. Microbiol. Biotechnol. 75:1473-1478. [DOI] [PubMed] [Google Scholar]
- 24.Lewis, D. F. V. 2001. Guide to cytochromes P450: structure and function. Taylor & Francis, London, United Kingdom.
- 25.Manoj, K. M., X. Yi, G. P. Rai, and L. P. Hager. 1999. A kinetic epoxidation assay for chloroperoxidase. Biochem. Biophys. Res. Commun. 266:301-303. [DOI] [PubMed] [Google Scholar]
- 26.Marzullo, L., R. Cannio, P. Giardina, M. T. Santini, and G. Sannia. 1995. Veratryl alcohol oxidase from Pleurotus ostreatus participates in lignin biodegradation and prevents polymerization of laccase-oxidized substrates. J. Biol. Chem. 270:3823-3827. [DOI] [PubMed] [Google Scholar]
- 27.Miller, V. P., R. A. Tschirret-Guth, and P. R. Ortiz de Montellano. 1995. Chloroperoxidase-catalyzed benzylic hydroxylation. Arch. Biochem. Biophys. 319:333-340. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell, K. H., C. E. Rogge, T. Gierahn, and B. G. Fox. 2003. Insight into the mechanism of aromatic hydroxylation by toluene 4-monooxygenase by use of specifically deuterated toluene and p-xylene. Proc. Natl. Acad. Sci. USA 100:3784-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris, D. R., and L. P. Hager. 1966. Chloroperoxidase. I. Isolation and properties of the chrystalline glycoprotein. J. Biol. Chem. 241:1763-1768. [PubMed] [Google Scholar]
- 30.Nuell, M. J., G. H. Fang, M. J. Axley, P. Kenigsberg, and L. P. Hager. 1988. Isolation and nucleotide sequence of the chloroperoxidase gene from Caldariomyces fumago. J. Bacteriol. 170:1007-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omura, T. 2006. Mitochondrial P450s. Chem. Biol. Interact. 163:86-93. [DOI] [PubMed] [Google Scholar]
- 31a.Rønhede, S., B. Jensen, S. Rosendahl, B. B. Kragelund, R. K. Juhler, and J. Aamand. 2005. Hydroxylation of the herbicide isoproturon by fungi isolated from agricultural soil. Appl. Environ. Microbiol. 71:7927-7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sae, A. S. W., and B. A. Cunningham. 1979. Isolation and properties of chloroperoxidase isoenzymes. Phytochemistry 18:1785-1787. [Google Scholar]
- 33.Santos, P. M., D. Benndorf, and I. Sa-Correia. 2004. Insights into Pseudomonas putida KT2440 response to phenol-induced stress by quantitative proteomics. Proteomics 4:2640-2652. [DOI] [PubMed] [Google Scholar]
- 34.Shaw, P. D., and L. P. Hager. 1959. Biological chlorination. III. Beta-ketoadipate chlorinase: a soluble enzyme system. J. Biol. Chem. 234:2565-2569. [PubMed] [Google Scholar]
- 35.Sheng, D., and M. H. Gold. 1997. Haloperoxidase activity of manganese peroxidase from Phanerochaete chrysosporium. Arch. Biochem. Biophys. 345:126-134. [DOI] [PubMed] [Google Scholar]
- 36.Stamets, P., and J. S. Chilton. 1983. The mushroom cultivator: a practical guide to growing mushrooms at home. Agarikon Press, Olympia, WA.
- 37.Steffen, K. T., M. Hofrichter, and A. Hatakka. 2000. Mineralisation of 14C-labelled synthetic lignin and ligninolytic enzyme activities of litter-decomposing basidiomycetous fungi. Appl. Microbiol. Biotechnol. 54:819-825. [DOI] [PubMed] [Google Scholar]
- 38.Sun, W., T. A. Kadima, M. A. Pickard, and H. B. Dunford. 1994. Catalase activity of chloroperoxidase and its interaction with peroxidase activity. Biochem. Cell Biol. 72:321-331. [DOI] [PubMed] [Google Scholar]
- 39.Ullrich, R., and M. Hofrichter. 2007. Enzymatic hydroxylation of aromatic compounds. Cell. Mol. Life Sci. 64:271-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ullrich, R., and M. Hofrichter. 2005. The haloperoxidase of the agaric fungus Agrocybe aegerita hydroxylates toluene and naphthalene. FEBS Lett. 579:6247-6250. [DOI] [PubMed] [Google Scholar]
- 41.Ullrich, R., J. Nüske, K. Scheibner, J. Spantzel, and M. Hofrichter. 2004. Novel haloperoxidase from the agaric basidiomycete Agrocybe aegerita oxidizes aryl alcohols and aldehydes. Appl. Environ. Microbiol. 70:4575-4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Rantwijk, F., and R. A. Sheldon. 2000. Selective oxygen transfer catalysed by heme peroxidases: synthetic and mechanistic aspects. Curr. Opin. Biotechnol. 6:554-564. [DOI] [PubMed] [Google Scholar]
- 43.Vazquez-Duhalt, R., D. W. S. Westlake, and P. M. Fedorak. 1994. Lignin peroxidase oxidation of aromatic compounds in systems containing organic solvents. Appl. Environ. Microbiol. 60:459-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White, R. E., and M. J. Coon. 1982. Heme ligand replacement reactions of cytochrome P-450. Characterization of the bonding atom of the axial ligand trans to thiolate as oxygen. J. Biol. Chem. 257:3073-3083. [PubMed] [Google Scholar]
- 45.Zhu, G., and P. Wang. 2005. Novel interface-binding chloroperoxidase for interfacial epoxidation of styrene. J. Biotechnol. 117:195-202. [DOI] [PubMed] [Google Scholar]