Abstract
Volatiles of Stenotrophomonas, Serratia, and Bacillus species inhibited mycelial growth of many fungi and Arabidopsis thaliana (40 to 98%), and volatiles of Pseudomonas species and Burkholderia cepacia retarded the growth to lesser extents. Aspergillus niger and Fusarium species were resistant, and B. cepacia and Staphylococcus epidermidis promoted the growth of Rhizoctonia solani and A. thaliana. Bacterial volatiles provide a new source of compounds with antibiotic and growth-promoting features.
The rhizosphere of plants is the habitat of a community comprising many different organisms. Soil bacteria often possess traits that enable them to act as antagonists by suppressing soilborne plant diseases, for example, by excreting antifungal metabolites that directly or indirectly support plant growth (7, 8, 9, 19). Many of these specialized compounds, such as antibiotics, are either liquid or solid at room temperature, and little is known about volatiles (with molecular masses less than 300 Da, low polarity, and a high vapor pressure) that can act as antibiotics and cause growth inhibition or have more deleterious effects on organisms. The microbial world synthesizes and emits many volatile compounds (1, 3, 4, 6, 16, 17). We previously showed that rhizobacterial isolates of Serratia plymuthica, Serratia odorifera, Stenotrophomonas maltophilia, Stenotrophomonas rhizophila, Pseudomonas fluorescens, and Pseudomonas trivialis emit complex blends of volatiles that inhibit the growth of Rhizoctonia solani (12). These rhizobacterial isolates and one human pathogen isolate were used to investigate their biological effects on 14 fungi, as well as on the model plant Arabidopsis thaliana.
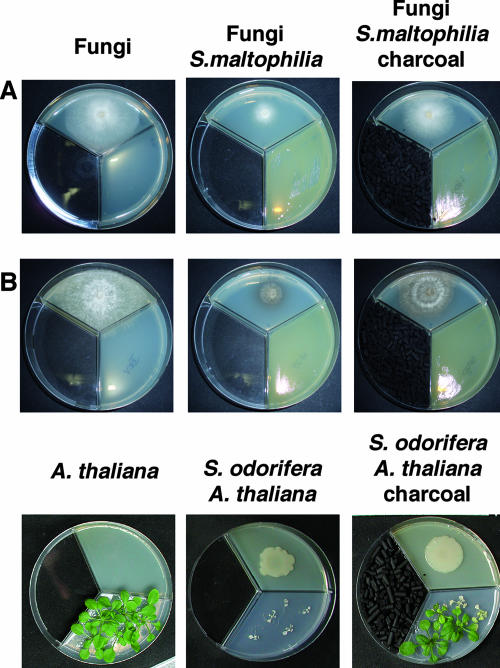
Fungi were cocultivated with rhizobacteria in divided petri dishes; the fungal medium contained 20 g liter−1 peptone, 10 g liter−1 glucose, and 20 g liter−1 agar-agar (pH 6.8) at 20°C, and the bacterial medium contained 5 g liter−1 peptone from casein, 2.5 g liter−1 peptone from meat, 2.5 g liter−1 peptone from gelatin, 1.5 g liter−1 yeast extract, l 5 g liter−1 NaCl, and 15 g liter−1 agar-agar (pH 7.2) at 20°C. Two representative examples are shown in Fig. 1. Growth of the mycelium was recorded from the first to the seventh or ninth day after inoculation using a digital camera (C-3030 Zoom Camedia; Olympus) and the Image Gauge software of the image analyzer LAS-1000 (Fujifilm, Tokyo, Japan). Fungal mycelial growth and plant growth were visible in the absence of bacteria (Fig. 1, left panels), but mycelial development of Paecilomyces carneus and R. solani was strongly inhibited by adjacent growth of S. maltophilia R3089 and A. thaliana development was strongly inhibited by adjacent growth of S. odorifera 4Rx13 (Fig. 1, middle panels). To prove that bacterial volatiles were the principal component impeding mycelial and plant growth, charcoal was added to the test system to trap the volatiles. These coincubations showed that growth inhibition was partly abrogated in the charcoal test system (Fig. 1, right panels). In the presence of charcoal the fungal mycelial size reached 50% or more of the mycelial size of the control, and 80% of A. thaliana plants exhibited normal growth.
FIG. 1.
Cocultivation of rhizobacteria with fungi or A. thaliana in the presence or absence of charcoal. Nutrient broth (left panels) or overnight rhizobacterial cultures (20 or 50 μl) (middle and right panels) were plated in one compartment of a tripartite petri dish. After 2 days of incubation, a fungal mycelium plug (P. carneus [A] or R. solani [B]) or 10 A. thaliana seedlings with or without charcoal (right and middle panels, respectively) were placed in the other compartments, and incubation was continued for 4 days for the fungi or for 14 days for A. thaliana.
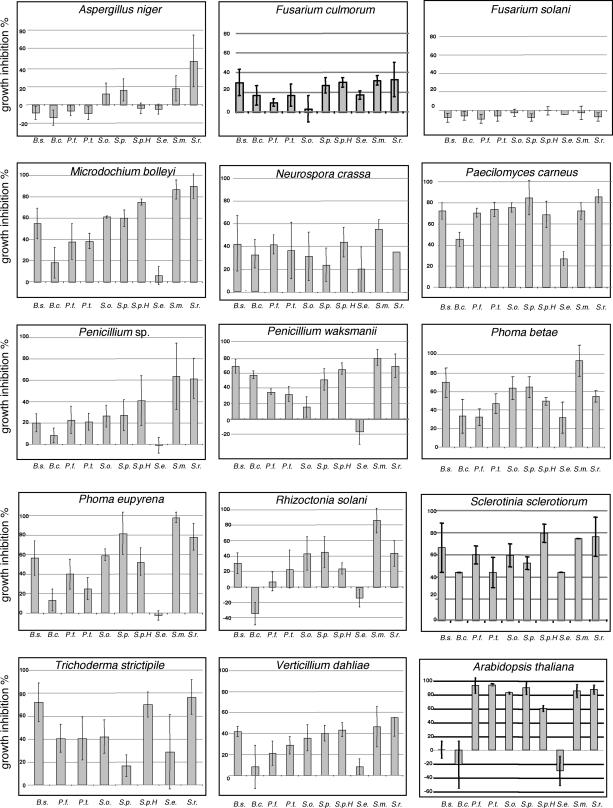
The strength of fungal growth inhibition depends on the rhizobacterial isolate. Growth retardation was often visible after the second day of incubation, and in most cases this inhibition accelerated after the fourth day. To allow comparisons, growth alterations (expressed in percentages) were calculated for all fungus-bacterium combinations on the fourth day (Fig. 2). Aspergillus niger and Fusarium culmorum were inhibited by approximately 20% or less or mycelial growth was promoted (Fusarium solani and A. niger, 3 to 10%) by all or many bacteria. All tested fungi showed individual inhibition patterns. Microdochium bolleyi, P. carneus, Phoma betae, and Sclerotinia sclerotiorum were strongly inhibited, by 40% or more. The inhibition of Verticillium dahliae, R. solani, Penicillium sp., and Neurospora crassa was moderate in comparison to the inhibition of the other fungi tested. At present, the biologically active volatiles causing the inhibition are not known because many volatiles have not been detected or identified (12). Antifungal effects of organic volatiles were previously shown to inhibit germination or mycelial growth of S. sclerotiorum (6, 11), and unidentified compounds from Bacillus subtilis cause structural deformation of pathogenic fungi (2). Inorganic volatiles also control growth or inhibit hyphal formation and extension (10, 13). Bacterial volatiles can also promote fungal growth (11, 18).
FIG. 2.
Determination of growth inhibition. Mycelial growth inhibition or promotion was calculated after 4 days of cocultivation. The fresh weight of A. thaliana was determined after 14 days of cocultivation. Experiments were repeated three times, and each experiment comprised three to seven replicates with each bacterial isolate. The error bars indicate standard errors. The bacterial isolates used were B. subtilis B2g (B.s.), B. cepacia 1S18 (B.c.), P. fluorescens L13-6-12 (P.f.), P. trivialis 3Re2-7 (P.t.), S. odorifera 4Rx13 (S.o.), S. plymuthica 3Re4-18 (S.p.), S. plymuthica HRO C48 (S.p.H.), S. epidermidis 2P13-18 (S.e.), S. maltophilia R3089 (S.m.), and S. rhizophila P69 (S.r.). The soilborne fungi used were F. culmorum PR 19-12-11, F. solani, M. bolleyi PR 5-11-6, P. carneus PR 16-10-1, Penicillium sp. strain 2-1-20, Penicillium waksmanii PR 17-11-8, P. betae, Phoma eupyrena PC 17-12-10, R. solani AG3, S. sclerotiorum, Trichoderma strictipile PC26-12-6, and V. dahliae V25. In addition, A. niger and N. crassa wild-type 1202A were used.
Bacteria and plants were incubated together for 14 days before the fresh weight of the plants was determined. The following conditions were used for plant growth: surface-sterilized vernalized seeds, half-strength Murashige-Skoog medium, a cycle consisting of 16 h of light and 8 h of darkness, a light intensity of 84 μmol/m2/s, and 20°C. Drastic growth inhibition (>80%) leading to small, pale yellow, and dead A. thaliana plants was obtained with P. fluorescens, P. trivialis, both S. plymuthica isolates, S. odorifera, S. rhizophila, and S. maltophilia (Fig. 1 and 2). In comparison to controls (nutrient broth), the fresh weight of A. thaliana increased by 20 to 30% under the influence of B. cepacia and S. epidermidis, and cocultivation with B. subtilis had no significant effect on plant development. The latter result seems to contradict observations made by Ryu et al. (14, 15) and Farag et al. (5), who showed that B. subtilis GB03 and Bacillus amyloliquefaciens IN937a promote growth and trigger induced systemic resistance in A. thaliana. The use of different bacillus species or isolates and variations in the growth conditions and test systems may account for the different results. Growth promotion of A. thaliana and R. solani, however, was observed when these organisms were cocultivated with B. cepacia or S. epidermidis.
Concluding remarks.
The experiments described here clearly demonstrate that bacterial volatiles add another component to the action profile of relevant growth-promoting or -inhibiting strategies of rhizobacteria. Volatiles can be an advantageous tool for rhizobacteria because they are small molecules that can easily diffuse through the porous structure of the soil and over great distances in the atmosphere. Many situations can be anticipated where this communication among plants, fungi, and rhizobacteria may be advantageous to at least one of the parties involved and has consequences for the organisms living in a community. The growth-inhibiting effects of rhizobacterial volatiles on phytopathogenic fungi, which cause many economically relevant crop diseases, have potential for agronomical applications, for example, by using the volatiles or rhizobacteria as biological control agents. Most of the investigated fungi also cause diseases in humans, such as opportunistic infections, mycoses, and allergic reactions (e.g., Aspergillus, Fusarium, Paecilomyces, or Penicillium species). Therefore, medicinal applications for rhizobacterial volatiles can also be anticipated.
Acknowledgments
We thank G. Berg (University of Graz, Austria) and Hella Goschke (University of Rostock, Germany) for the bacterial and fungal isolates, T. Rönneberg (University of Munich, Germany) for N. crassa, and A. Podbielski (University of Rostock, Germany) for A. niger.
This research was financially supported by a grant from the University of Rostock to B.P.
Footnotes
Published ahead of print on 29 June 2007.
REFERENCES
- 1.Alstrom, S. 2001. Characteristics of bacteria from oil seed rape in relation to their biocontrol activity against Verticillium dahliae. J. Phytopathol. 149:57-64. [Google Scholar]
- 2.Chaurasia, B., A. Pandey, L. M. S. Palni, P. Trivedi, B. Kumar, and N. Colvin. 2005. Diffusible and volatile compounds produced by an antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiol. Res. 160:75-81. [DOI] [PubMed] [Google Scholar]
- 3.Dickschat, J. S., S. C. Wenzel, H. B. Bode, R. Müller, and S. Schulz. 2004. Biosynthesis of volatiles by the Myxobacterium Myxococcus xanthus. Chem. Biol. Chem. 5:778-787. [DOI] [PubMed] [Google Scholar]
- 4.Dickschat, J. S., R. Martens, T. Brinkhoff, M. Simon, and S. Schulz. 2005. Volatiles released by a Streptomyces species isolated from the North Sea. Chem. Biodivers. 2:837-865. [DOI] [PubMed] [Google Scholar]
- 5.Farag, M. A., C. M. Ryu, L. W. Summer, and P. W. Pare. 2006. GC-MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry 67:2262-2268. [DOI] [PubMed] [Google Scholar]
- 6.Fernando, W. G., R. Ramarathnam, A. S. Krishnamoorthy, and S. C. Savchuk. 2005. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 37:955-964. [Google Scholar]
- 7.Gupta, A. M., K. V. B. Gopal, and R. Tilak. 2000. Mechanism of plant growth promotion by rhizobacteria. Indian J. Exp. Biol. 38:856-862. [PubMed] [Google Scholar]
- 8.Haas, D., and G. Defago. 2005. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3:307-319. [DOI] [PubMed] [Google Scholar]
- 9.Handelsman, J., and E. V. Stabb. 1996. Biocontrol of soilborne plant pathogens. Plant Cell 8:1855-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howell, C. R., R. C. Beier, and R. D. Stipanovic. 1988. Reproduction of ammonia by Enterobacter cloacae and its possible role in the biological control of Pythium preemergence damping-off by the bacterium. Phytopathology 78:1075-1078. [Google Scholar]
- 11.Huang, H., J. W. Huang, G. Saidon, and R. Erickson. 1997. Effect of allyl alcohol and fermented agricultural wastes on carpogenic germination of sclerotia of Sclerotinia sclerotiorum and colonization of Trichoderma spp. Can. J. Plant Pathol. 9:43-46. [Google Scholar]
- 12.Kai, M., U. Effmert, G. Berg, and B. Piechulla. 2007. Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch. Microbiol. 187:351-360. [DOI] [PubMed] [Google Scholar]
- 13.Robinson, P. M., N. D. McKee, L. A. A. Thompson, D. B. Harper, and J. T. G. Hamilton. 1989. Autoinhibition of germination and growth in Geotrichum candidum. Mycol. Res. 93:214-222. [Google Scholar]
- 14.Ryu, C. M., M. A. Farag, C. H. Hu, M. S. Reddy, H. X. Wie, P. W. Pare, and J. W. Kloepper. 2003. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 100:4927-4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu, C. M., M. A. Farag, C. H. Hu, M. S. Reddy, J. W. Kloepper, and P. W. Pare. 2004. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 134:1017-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schöller, C. E. G., H. Gürtler, R. Petersen, S. Molin, and K. Wilkins. 2002. Volatile metabolites from Actinomycetes. J. Agric. Food Chem. 50:2615-2621. [DOI] [PubMed] [Google Scholar]
- 17.Stotzky, G., and S. Schenk. 1976. Volatile organic compounds and microorganisms. Crit. Rev. Microbiol. 4:333-382. [DOI] [PubMed] [Google Scholar]
- 18.Wheatley, R. E. 2002. The consequences of volatile organic compound mediated bacterial and fungal interactions. Antonie Leeuwenhoek 81:357-364. [DOI] [PubMed] [Google Scholar]
- 19.Whipps, J. M. 2001. Microbial interaction and biocontrol in the rhizosphere. J. Exp. Bot. 52:487-511. [DOI] [PubMed] [Google Scholar]