Abstract
The antibiotic resistance (AR) patterns of 462 Escherichia coli isolates from wastewater, surface waters, and oysters were determined. Rates of AR and multiple-AR among isolates from surface water sites adjacent to wastewater treatment plant (WWTP) discharge sites were significantly higher (P < 0.05) than those among other isolates, whereas the rate of AR among isolates from oysters exposed to WWTP discharges was low (<10%).
The aim of this study was to investigate the rates of antibiotic resistance among Escherichia coli isolates from a variety of sources, including wastewater treatment plants (WWTPs), surface waters (including those directly influenced by WWTP discharges), and oysters affected by WWTP discharges. This information will contribute to our understanding of antibiotic resistance in the aquatic environment and the potential environmental and public health risks associated with exposure to aquatic bacteria.
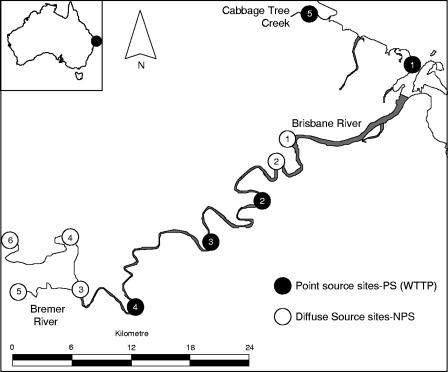
This study was conducted on the central east coast of Australia on the Brisbane and Bremer Rivers and Cabbage Tree Creek, which are subtropical city-dominated systems with both estuarine and freshwater regions. Samples from each stage of the treatment process at five regional WWTPs (42 samples) were collected (Table 1), as well as samples from surface waters in the Brisbane River, including waters at sites adjacent to the investigated WWTPs (five sites referred to as point sources [PS]) and waters at sites distant from PS (six sites referred to as nonpoint sources [NPS]) (Fig. 1). Approximately 30 native oysters (Saccostrea commercialis) were collected at low tide from a small estuarine creek (Cabbage Tree Creek), approximately 300 m downstream from a WWTP discharge site (Fig. 1).
TABLE 1.
Operational parameters of investigated WWTPs
| WWTP | Population served | Avg daily dry-weather flow (ml) | Effluent disinfection method | Typical coliform range (CFU 100 ml−1) |
|---|---|---|---|---|
| 1 | 700,000 | 140 | None | 1e4-1e5 |
| 2 | 11,000 | 2.8 | Chlorination | 10-170 |
| 3 | 228,000 | 60 | Chlorination | 2-88 |
| 4 | 65,000 | 15.4 | Chlorination | <2 |
| 5 | 80,000 | 20 | UV radiation | 1-150 |
FIG. 1.
Map of study area and site locations.
The isolation of E. coli from WWTP and surface water samples was performed using membrane filtration (20). Oysters were washed and scrubbed in 70% ethanol (ChromAR [purity, 99.9%]; Mallinckrodt Chemicals) before shucking. The isolation of E. coli from oysters was carried out using a standard food technique (21).
A total of 462 isolates were tested for sensitivity to six antibiotics by using the CLSI disk susceptibility testing method (5). The antibiotics chosen were ampicillin (10 μg; Oxoid), cephalothin (30 μg; Oxoid), nalidixic acid (30 μg; Oxoid), sulfafurazole (300 μg; Oxoid), gentamicin (10 μg; Oxoid), and tetracycline (30 μg; Oxoid). A one-way analysis of variance followed by a posthoc Tukey honestly significant difference means test was used to determine significant differences (P < 0.05) among sources of E. coli isolates for each antibiotic and among multiple-antibiotic-resistance (MAR) indices based on zones of inhibition. For MAR patterns, data were converted into a binary code (resistant or nonresistant) and differences (P < 0.05) among sources for each of the resistance patterns were evaluated by paired t tests for all combinations.
The observed level of antibiotic resistance among all investigated isolates, 59%, was markedly lower than those demonstrated previously in similar studies, typically around 90% (11, 16). It is hard to draw direct comparisons with the results of these studies due to differences in the antibiotics investigated, the levels of fecal pollution and the therapeutic drug practices in the study areas, and the wastewater treatment practices employed.
The highest incidence of bacterial resistance recorded was that for tetracycline (51%), followed by those for cephalothin (41%) and sulfafurazole (32%). The rate of resistance among bacteria in the aquatic environment to tetracycline has previously been reported to range from 1 to 24% (3, 7, 16, 22), indicating that quite high levels were identified in the present study. Results for cephalothin and sulfafurazole were more typical of previously reported findings, with ranges from 8 to 98% and 21 to 100%, respectively (7, 16, 22).
E. coli concentrations at PS (average, 1,730 CFU 100 ml−1) were significantly higher (P < 0.05) than those at NPS (average, 22 CFU 100 ml−1). Additionally, antibiotic resistance among E. coli isolates from PS was higher and more frequent than that among isolates from NPS (Table 2). Similarly, Boon and Cattanach (3) and Parveen et al. (16) demonstrated previously that antibiotic resistance was more highly associated with PS than with NPS. E. coli has long been used as an indicator of fecal contamination; however, differentiation between human and agricultural sources has been limited. The use of MAR indices has demonstrated potential for differentiating E. coli sources (16). MAR indices for each isolate were calculated by dividing the number of antibiotics to which the isolate was resistant by the total number of antibiotics tested (Table 3). The MAR indices identified in this study (0.26 for PS isolates and 0.15 for NPS isolates) were directly comparable with those previously reported in the literature for PS isolates (∼0.25) and NPS isolates (∼0.13) (16), emphasizing the potential of MAR indices in identifying the source of fecal contamination. The higher MAR levels of PS isolates are most probably due to the larger range of drugs utilized in human therapeutic treatment than in agriculture and the potential exchange of resistance elements (e.g., plasmids and integrons) during the treatment process. Research has demonstrated high rates of exchange of transferable elements within WWTPs, even in the absence of antibiotics as selective agents (9, 12).
TABLE 2.
Percentages of antibiotic-resistant E. coli isolates from WWTPs, surface waters, and oystersa
| Source of isolates (no. of isolates) | % of isolates from indicated source resistant to:
|
||||||
|---|---|---|---|---|---|---|---|
| ≥1 antibiotic | Ampicillin | Cephalothin | Nalidixic acid | Sulfafurazole | Gentamicin | Tetracycline | |
| WWTPs (100) | 31 A | 15 A | 14 A | 5 A | 17 A | NR A | 10 A |
| PS (74) | 87 B | 19 A | 41 B | 5 A | 32 B | 8 B | 51 B |
| NPS (88) | 62 C | 7 B | 30 C | NR B | 20 A | NR A | 34 C |
| Oysters (50) | 4 D | NR C | 2 D | NR B | NR C | 4 C | NR D |
Letters indicate significant differences (P < 0.05) between sources. NR, no resistance.
TABLE 3.
Antibiotic resistance patterns of E. coli isolates from investigated sourcesa
| Resistance pattern | % of isolates from indicated source with indicated resistance pattern
|
|||
|---|---|---|---|---|
| WWTPs (n = 100) | PS (n = 74) | NPS (n = 88) | Oysters (n = 50) | |
| A | 4 A | 14 A | 3 A | NR B |
| A-C | 5 A | NR B | NR B | NR B |
| A-N | 1 A | NR A | NR A | NR A |
| A-S | 1 A | NR A | NR A | NR A |
| A-T | NR A | NR A | 2.3 A | NR A |
| A-C-S | 3 A | NR B | NR B | NR B |
| A-C-T | NR A | 5.4 B | NR A | NR A |
| A-S-T | 2 A | NR A | NR A | NR A |
| A-C-S-T | 1 A | NR A | NR A | NR A |
| C | 5 A | 11 A | 11 A | NR B |
| C-N | NR A | 2.7 A | NR A | NR A |
| C-S | 1 A | 5.4 A | 6.8 A | NR A |
| C-G | NR A | NR A | NR A | 2 A |
| C-T | NR A | 11 B | 9 B | NR A |
| C-S-T | NR A | 2.7 A | 2.3 A | NR A |
| C-N-S-G-T | NR A | 2.7 A | NR A | NR A |
| N | NR A | NR A | NR A | NR A |
| N-T | 1 A | NR A | NR A | NR A |
| N-S-T | 3 A | NR B | NR B | NR B |
| S | 5 A | 3 A | 10 A | NR B |
| S-T | 1 A | 8 B | NR A | NR A |
| S-G-T | NR A | 5.4 B | NR A | NR A |
| G | NR A | NR A | NR A | 2 A |
| T | 2 A | 11 B | 18 B | NR A |
| Avg MAR index | 0.11 A | 0.26 B | 0.15 A | 0.01 C |
Letters indicate significant differences (P < 0.05) between sources. A, ampicillin; C, cephalothin; N, nalidixic acid; S, sulfafurazole; T, tetracycline; G, gentamicin; NR, no resistance.
Somewhat surprisingly, the incidence of antibiotic resistance among WWTP E. coli isolates was significantly lower than that among the PS isolates. It is difficult to assess the implications of this observation without knowing the concentrations of E. coli bacteria in the investigated WWTP samples, which were not possible to determine during this study. Given the substantial reduction of E. coli levels during wastewater treatment, particularly with disinfection, this observation may be misleading; although the proportion of resistant E. coli isolates in PS samples was higher, one would expect a much higher number of resistant E. coli isolates in WWTP samples, even considering the lower rates of resistance. Wastewater treatment has been shown to have many effects on the prevalence of resistant E. coli bacteria. Reinthaler et al. (18) have demonstrated that the rate of antibiotic resistance in WWTP effluent is greater than that in wastewater before treatment and have proposed that treatment selects for more-resistant organisms. This selection can occur through numerous pathways. Firstly, the presence of low concentrations of antibiotics within WWTPs has been documented previously (1, 2, 4, 13, 14), and these antibiotics may exert selective pressure favoring resistant organisms. Effluent disinfection, while lowering the total bacterial count, has also been shown to increase the prevalence of antibiotic-resistant bacteria and MAR (15). These observations, when combined, may possibly account for the higher incidence of antibiotic resistance among PS isolates than among WWTP isolates due to the isolation of PS bacteria from areas receiving final effluent in contrast to the isolation of WWTP bacteria from samples at each stage of sewage treatment, including raw effluent that has not yet undergone treatment.
The concentration of E. coli bacteria detected in oysters (most probable number [MPN], 17 g−1) is considered low compared to values reported in the literature. The numbers of E. coli cells isolated from shellfish associated with PS discharges have been shown to range from an MPN of 200 g−1 to an MPN of 46,000 g−1 and reflect concentrations of E. coli bacteria in the surrounding waters (8, 10, 17, 19), although comparative data are scarce. Levels of antibiotic resistance among oyster E. coli isolates were low, with only 10% of isolates indicating resistance to at least one antibiotic. Limited comparative data are available; however, in a study by Cooke (6), rates of antibiotic resistance among oyster E. coli isolates from WWTP outfalls were found to range from 56 to 100%, in complete contrast to the results of the present study. Additionally, the incidence of MAR among oyster isolates ranged from 11 to 81% (6), whereas no incidence of MAR among oyster isolates was seen in this study.
Antibiotic resistance, particularly MAR, is a major public health threat, and the presence of resistant organisms in environmental waters is an emerging concern around the world. The potential for this resistance to be transferred to native populations or other pathogenic species is largely unknown and warrants further investigation. This study has further emphasized the potential application of MAR indices for identifying the origin of fecal pollution. This application would provide a beneficial tool for administrators in managing waste discharge to the aquatic environment.
Acknowledgments
This project was supported through an Australian Research Council Linkage grant (LP0453-708) and in part by the Wastewater Program of the Cooperative Research Centre for Water Quality and Treatment (project number 666003).
The use of trade, firm, or brand names in this paper is for identification purposes only and does not constitute endorsement by the National Research Centre for Environmental Toxicology or the Cooperative Research Centre for Water Quality and Treatment.
Footnotes
Published ahead of print on 6 July 2007.
REFERENCES
- 1.Alder, A. C., C. S. McArdell, E. M. Golet, S. Ibric, E. Molnar, N. S. Nipales, and W. Giger. 2001. Occurrence and fate of fluoroquinolone, macrolide and sulfonamide antibiotics during wastewater treatment and in ambient waters in Switzerland, p. 39-54. In C. G. Daughton and T. L. Jones-Lepp (ed.), Pharmaceuticals and personal care products in the environment: scientific and regulatory issues. American Chemical Society, Washington, DC.
- 2.Batt, A. L., I. B. Bruce, and D. S. Aga. 2006. Evaluating the vulnerability of surface waters to antibiotic contamination from varying wastewater treatment plant discharges. Environ. Pollut. 142:295-302. [DOI] [PubMed] [Google Scholar]
- 3.Boon, P. I., and M. Cattanach. 1999. Antibiotic resistance of native and faecal bacteria isolated from rivers, reservoirs and sewage treatment facilities in Victoria, south-eastern Australia. Lett. Appl. Microbiol. 28:164-168. [DOI] [PubMed] [Google Scholar]
- 4.Brown, K. D., J. Kulis, B. Thomson, T. H. Chapman, and D. B. Mawhinney. 2006. Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Sci. Total Environ. 366:772-783. [DOI] [PubMed] [Google Scholar]
- 5.CLSI. 2003. Performance standards for antimicrobial disk susceptibility tests; approved standard M2-A8, 8th ed. CLSI, Wayne, PA.
- 6.Cooke, M. D. 1976. Antibiotic resistance among coliform and fecal coliform bacteria isolated from sewage, seawater, and marine shellfish. Antimicrob. Agents Chemother. 9:879-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edge, T. A., and H. Stephen. 2005. Occurrence of antibiotic resistance in Escherichia coli from surface waters and fecal pollution sources near Hamilton, Ontario. Can. J. Microbiol. 51:501. [DOI] [PubMed] [Google Scholar]
- 8.Eyles, M. J., and G. R. Davey. 1988. Vibrio cholerae and enteric bacteria in oyster-producing areas of two urban estuaries in Australia. Int. J. Food Microbiol. 6:207-218. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Astorga, A., A. Muela, R. Cisterna, J. Iriberri, and I. Barcina. 1992. Biotic and abiotic factors affecting plasmid transfer in Escherichia coli strains. Appl. Environ. Microbiol. 58:392-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussong, D., R. R. Colwell, and R. M. Weiner. 1980. Seasonal concentration of coliform bacteria by Crassostrea virginica, the eastern oyster, in Chesapeake Bay. J. Food Prot. 44:201-203. [DOI] [PubMed] [Google Scholar]
- 11.Kaspar, C. W., J. L. Burgess, I. T. Knight, and R. R. Colwell. 1990. Antibiotic resistance indexing of Escherichia coli to identify sources of fecal contamination in water. Can. J. Microbiol. 36:891-894. [DOI] [PubMed] [Google Scholar]
- 12.Mach, P. A., and D. J. Grimes. 1982. R-plasmid transfer in a wastewater treatment plant. Appl. Environ. Microbiol. 44:1395-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McArdell, C. S., E. Molnar, M. J. F. Suter, and W. Giger. 2003. Occurrence and fate of macrolide antibiotics in wastewater treatment plants and in the Glatt Valley watershed, Switzerland. Environ. Sci. Technol. 37:5479-5486. [DOI] [PubMed] [Google Scholar]
- 14.Miao, X. S., F. Bishay, M. Chen, and C. D. Metcalfe. 2004. Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. Environ. Sci. Technol. 38:3533-3541. [DOI] [PubMed] [Google Scholar]
- 15.Murray, G. E., R. S. Tobin, B. Junkins, and D. J. Kushner. 1984. Effect of chlorination on antibiotic resistance profiles of sewage-related bacteria. Appl. Environ. Microbiol. 48:73-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parveen, S., R. Murphree, L. Edmiston, C. Kaspar, K. Portier, and M. Tamplin. 1997. Association of multiple-antibiotic-resistance profiles with point and nonpoint sources of Escherichia coli in Apalachicola Bay. Appl. Environ. Microbiol. 63:2607-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pommepuy, M., F. Dumas, M. P. Caprais, P. Camus, C. Le Mennec, S. Parnaudeau, L. Haugarreau, B. Sarrette, P. Vilagines, P. Pothier, E. Kholi, and F. Le Guyader. 2004. Sewage impact on shellfish microbial contamination. Water Sci. Technol. 50:117-124. [PubMed] [Google Scholar]
- 18.Reinthaler, F. F., J. Posch, G. Feierl, G. Wust, D. Haas, G. Ruckenbauer, F. Mascher, and E. Marth. 2003. Antibiotic resistance of E. coli in sewage and sludge. Water Res. 37:1685-1690. [DOI] [PubMed] [Google Scholar]
- 19.Silva, A. I. M., R. H. S. F. Vieira, F. G. R. Menezes, A. A. Fonteles-Filho, R. C. O. Torres, and E. S. Sant'Anna. 2004. Bacteria of faecal origin in mangrove oysters (Crassostrea rhizophorae) in the Coco River estuary, Ceara State, Brazil. Braz. J. Microbiol. 35:126-130. [Google Scholar]
- 20.Standards Australia. 1995. Thermotolerant coliforms and Escherichia coli: membrane filtration method. AS4273.7. Standards Australia, Sydney, Australia.
- 21.Standards Australia. 2004. Examination of foods for E. coli: estimation of most probable number (MPN). AS5013.15. Standards Australia, Sydney, Australia.
- 22.Watkinson, A. J., G. B. Micalizzi, J. B. Bates, and S. D. Costanzo. 2007. Novel method for rapid assessment of antibiotic resistance in Escherichia coli isolates from environmental waters by use of a modified chromogenic agar. Appl. Environ. Microbiol. 73:2224-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]