Abstract
The phosphotransferase system regulation domain (PRD)-containing activator, ManR, is required for glucose-controlled transcription of the mannose permease two (mpt) operon in Listeria innocua. His-871 in ManR PRD-II is needed for mpt repression in glucose-free media. His-506 in PRD-I is needed for mpt induction by glucose.
Listeria monocytogenes is a gram-positive, food-borne bacterium that is responsible for the severe disease listeriosis. Its close relative, L. innocua, is the most common nonpathogenic species isolated from foods. L. monocytogenes and L. innocua share many phenotypic properties and differ in the content of only a few hundred genes (10).
The principal high-affinity glucose transporter (EIItMan) in L. innocua and L. monocytogenes is encoded by the mpt (mannose permease two) operon (5, 7, 26). EIItMan belongs to the mannose structural family of phosphoenolpyruvate-dependent phosphotransferase system (PTS) permeases (1, 16). EIItMan contains three subunits—IIABMan, IICMan, and IIDMan—that are encoded by the mptA, mptC, and mptD genes (7, 10). Expression of the mpt operon is induced by glucose and mannose (7, 26). EIItMan serves as a docking site for class IIa bacteriocins in Listeria cells (7, 17, 18).
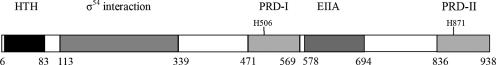
Transcription of mpt is controlled by the sigma factor RpoN (σ54), the σ54-associated transcription activator ManR, and the Lin0142 protein (7, 19, 26). ManR contains an N-terminal helix-turn-helix DNA binding domain, a σ54 interaction domain, a IIAFru domain, and two PTS regulation domains (PRDs) (Fig. 1). ManR probably assists σ54 in melting mpt promoter DNA during transcription initiation (3). The regulatory functions of the two PRDs have not been previously analyzed. Lin0142 is related to the CRP-FNR (cyclic AMP receptor protein-fumarate and nitrate reductase regulator) group of transcription factors (26). The role played by Lin0142 in mpt transcription is unknown.
FIG. 1.
Domain organization of the L. innocua Lin11 ManR protein. Protein domains were examined using the InterProScan server (http://www.ebi.ac.uk/InterProScan). Conserved histidines and the boundaries of each domain are numbered. HTH, helix-turn-helix DNA binding domain; EIIA, fructose PTS permease EIIA-like domain. Note that unlike the B. subtilis LevR protein (8), ManR does not appear to contain an EIIB-like domain.
In both gram-positive and gram-negative bacteria, carbon catabolite repression of catabolic operons is achieved in part by phosphorylation of conserved histidine residues within the PRDs of transcription antiterminator and activator proteins (1, 6, 8, 14, 20-23). PRD phosphorylation drives conformational changes that modulate nucleic acid binding activity (24). PRD-containing proteins often are negatively regulated by phosphorylation of one PRD by the cognate PTS permease in the absence of the transported sugar and are positively regulated by histidine protein (HPr)-mediated phosphorylation of the other PRD in the presence of the sugar and in the absence of glucose (8, 23). However, studies of closely related proteins, such as the Bacillus subtilis LicT and Escherichia coli BglG antiterminators, have shown that the response (e.g., activation) of a protein to PRD phosphorylation is variable and cannot be easily predicted (2, 11, 24, 25).
In the present study, we extended research on PRD-containing regulators to Listeria by elucidating the functions of conserved histidines in the PRDs of the L. innocua ManR protein. We also analyzed the general function of ManR in control of mpt transcription. Because L. monocytogenes virulence gene expression is regulated by glucose (8, 9, 12, 15), the present study has ramifications for L. monocytogenes pathogenesis.
Contributions of ManR to mpt transcription.
A manR in-frame deletion strain, JXD1, was constructed using L. innocua Lin11 (26). The chromosomal copy of the manR gene, which encodes the 938-amino-acid ManR protein, was replaced by homologous recombination with a truncated manR gene, which encoded only the N-terminal 22 amino acids and C-terminal 41 amino acids of ManR. The in-frame deletion gene was created by splice-by-overlap extension (SOE) PCR (13) in plasmid pKSV7 (4). The PCR primers used for construction of JXD1 are listed in Table 1.
TABLE 1.
Primers used for construction of manR deletion and substitution mutant strainsa
| Primer | Primer no. | Sequence |
|---|---|---|
| Outside | ||
| manR-A1-BamHI F | 1 | CGGGATCCCGGCCAGCTGTCATTAATTC |
| manR-A2-BamHI F | 2 | CGGGATCCCCCTTCAACTAAGTCGATTCGTGAG |
| manR-D1-SalI R | 3 | ACGCGTCGACCATGTGTGCCAATAATAATTGC |
| Internal deletion | ||
| manR-B1 R | 4 | GTTTGTTCTAATGCACTGAAAATCTCACGAATCGACTTAGTTGAAG |
| manR-B2 R | 5 | GTTTGTTCTAATGCACTGAAAATGCGAGAAACGATACATTAAGTTAGC |
| manR-C1 F | 6 | ATTTTCAGTGCATTAGAACAAAC |
| Internal substitution | ||
| manR-PA1 F | 7 | AGTCTGGCCTTGACTTCCTTTATTA |
| manR-PA2 R | 8 | AAAGGAAGTCAAGGCCAGACTAAAGGC |
| manR-PA3 F | 9 | ATCGCTGTAGGATGCGCCC |
| manR-PA4 R | 10 | AGCGCATCCTACAGCGATGAGTAAATT |
The PCR primer combinations used in the synthesis of mutant manR genes by SOE PCR were as follows, in the order 5′ outside (upstream)-internal (deletion or substitution site)/internal (complementary deletion or substitution site)-3′ outside (downstream): for JXD1, primer 1-primer 4/primer 6-primer 3; for JXD2, primer 2-primer 5/primer 6-primer 3; for manR-H506A, primer 2-primer 8/primer 7-primer 3; for manR-H871A, primer 1-primer 10/primer 9-primer 3; and for manR-H506A/H871A, primer 2-primer 10/primer 9-primer 3. manR-H506A genomic DNA was used as the template for synthesis of the manR-H506A/H871A gene. Wild-type Lin11 DNA was used for synthesis of all other substitution mutant genes and the two deletion mutant genes. The BamHI and SalI restriction sites incorporated into primers for cloning purposes are underlined. The overhangs complementary to the manR-C1 F primer used for SOE PCR are underlined in the manR-B1 R and manR-B2 R primers. Triplets encoding alanine residues are in bold type and underlined in the primers used for construction of substitution mutants. Primers were designed using the L. innocua CLIP 11262 genome sequence available at the ListiList server (http://genolist.pasteur.fr/ListiList). F, forward primer; R, reverse primer.
The effects of gene disruption on mpt transcription were studied by real-time reverse transcription-PCR measurement of mpt mRNA levels using primers specific for the mptA (lin0143) gene (26). mRNA levels for the lin0142, manR (lin0778), and rpoN genes also were measured to screen for regulatory interactions between mpt control genes. Cells were grown in Luria-Bertani (LB) media with or without glucose to an optical density at 600 nm (OD600) of 0.3 to 0.4 for RNA isolation. Note that the induction of the mpt operon is greater in LB media with glucose at this OD600 than at the slightly higher OD600 (0.4 to 0.6) used previously in a study of mpt control by the Lin0142 protein (26). The mRNA quantitation method used and the primers used for the four genes and a 16S rRNA endogenous control gene have been described previously (26). The REST statistics program was used to compare mRNA levels.
Inactivation of the manR gene reduced the level of mpt mRNA in the JXD1 manR deletion strain 100-fold relative to the basal level measured in the Lin11 control in the absence of glucose (Table 2). In addition, the 17-fold induction of mpt observed in the wild-type strain grown in the presence of glucose was abolished in the deletion mutant. manR inactivation did not significantly affect the transcript levels for the lin0142 and rpoN genes. In addition, the manR deletion strain (and all other mutant strains listed in Table 2) did not exhibit a reduced growth rate in LB media with or without glucose. No changes in the growth rate were observed, possibly because there is more than one glucose transporter in Listeria (5, 7).
TABLE 2.
mpt mRNA levels in manR deletion and substitution mutant strainsa
| Strain | Medium | Relative mRNA level
|
|||
|---|---|---|---|---|---|
| lin0142 | mptAb | manR | rpoN | ||
| Lin11 | LB | 1.0 (0.6-1.7) | 1.0 (0.8-1.3) | 1.0 (0.8-1.2) | 1.0 (0.6-1.6) |
| LB + Glc | 1.3 (0.9-1.9) | 16.9 (13.5-21.2)* | 1.1 (0.8-1.5) | 1.0 (0.7-1.6) | |
| JXD1 | LB | 0.8 (0.4-1.5) | 0.01 (0.005-0.02)* | NDc | 0.9 (0.6-1.6) |
| LB + Glc | 0.9 (0.4-2.2) | 0.01 (0.005-0.02)* | ND | 0.8 (0.4-1.5) | |
| manR-H506A | LB | 0.8 (0.4-1.5) | 1.6 (0.9-2.9) | 0.9 (0.5-1.7) | 0.8 (0.5-1.3) |
| LB + Glc | 0.9 (0.6-1.6) | 0.7 (0.4-1.1) | 1.0 (0.6-1.7) | 0.7 (0.4-1.0) | |
| manR-H871A | LB | 1.4 (0.9-2.2) | 51.5 (33.6-78.9)* | 1.6 (1.2-2.0) | 0.6 (0.5-0.8) |
| LB + Glc | 0.8 (0.5-1.3) | 18.7 (13.9-25.2)* | 1.1 (0.7-1.6) | 0.6 (0.4-0.9) | |
| manR-H506A/H871A | LB | 1.2 (0.7-1.9) | 8.6 (3.7-19.9)* | 1.5 (0.7-3.0) | 0.7 (0.4-1.2) |
| LB + Glc | 0.7 (0.4-1.2) | 1.5 (0.8-2.8) | 1.6 (0.8-2.9) | 0.8 (0.5-1.3) | |
A real-time reverse transcription-PCR analysis of the relative levels of the lin0142, mptA, manR, and rpoN mRNAs in wild-type (Lin11) and mutant strains grown in LB medium with or without glucose was performed. The values are the averages obtained using three independent RNA preparations. Transcript levels were measured in triplicate for each RNA preparation. The cycle threshold values for PCR products were compared to the values measured in strain Lin11 grown in LB medium without glucose to determine differences. The values in parentheses are ranges.
Asterisks indicate values that are significantly different (P < 0.01).
ND, not detected (the product cycle threshold values were the same as the background detection levels).
The results show that ManR is needed for induction of mpt transcription by glucose in L. innocua, as has previously been demonstrated in L. monocytogenes (7). Interestingly, the mpt mRNA levels are reduced at least 1,000-fold (to the background level of mRNA detection) in the lin0142 knockout strain, G7, in which lin0142 transcription is abolished due to insertion of Tn917 into the lin0142 promoter (26). The combined results indicate that ManR and Lin0142 are required for the basal, yet significant level of mpt transcription that occurs in the absence of glucose. Some mpt transcription may still occur in the absence of glucose because this sugar is the preferred sugar in Listeria.
Roles of conserved histidines in PRD-I and -II of ManR in regulation of mpt transcription by glucose.
Sequence alignments of L. innocua ManR and other regulatory protein PRDs are presented in Fig. 2. The alignments show that His-506 in PRD-I and His-871 in PRD-II of ManR are highly conserved along with histidines that are important in regulation of the activity of proteins such as GlcT, LevR, LicT, and MtlR (14, 20, 21, 25).
FIG. 2.
Sequence alignment of transcriptional activator (LevR, ManR, LicR, and MtlR) and antiterminator (BglG, LicT, SacT, SacY, and GlcT) PRDs. The BglG sequence is from E. coli (accession no. NP_418179), the ManR sequence is from L. innocua CLIP11262 (http://genolist.pasteur.fr/ListiList), and the other sequences are from B. subtilis (http://genolist.pasteur.fr/SubtiList). Conserved histidines are in bold type and capitalized. The PRD sequences of the Lin11 and CLIP11262 ManR proteins differ only at position 895, where there are glutamate and aspartate residues, respectively.
To determine if these histidines are needed for ManR activity, strains designated manR-H506A, manR-H871A, and manR-H506A/H871A were constructed, in which one or both residues were replaced with alanine. The manR mutant alleles were created in pKSV7 using SOE PCR (Table 1) and were introduced as single copies into the L. innocua Lin11 chromosome by homologous recombination. The JXD1 manR deletion strain was used to construct the manR-H871A strain. Another manR deletion strain, JXD2, in which the N-terminal 161 amino acids and C-terminal 41 amino acids of ManR are preserved, was used to construct strains manR-H506A and manR-H506A/H871A. In all cases, mutant genes were sequenced in their entirety and confirmed to differ from the wild type only at the substitution sites.
The effects of substitution mutations were assessed by measuring the levels of mRNAs for the mpt, lin0142, rpoN, and manR genes by the methods described above (Table 2). In the manR-H506A strain, the mpt mRNA level was the same as the level in Lin11 in the absence of glucose but, unlike the wild-type level, did not increase in the presence of the sugar. In the manR-H871A strain, the mpt mRNA level increased 51.5-fold compared to the level in Lin11 in the absence of glucose. However, a wild-type level of mpt mRNA still was observed in the manR-H871A strain in glucose medium. In the manR-H506A/H871A double mutant, the mpt mRNA level was 8.6-fold higher than the level in Lin11 in the medium without glucose and was the same as the Lin11 basal level in glucose medium. The data generally indicate that His-506 is required for the activation of ManR and induction of mpt transcription in the presence of glucose. His-871 is needed for the inactivation of ManR and a reduction in mpt transcription in the absence of glucose but is not needed for ManR activation in glucose medium. Note that the mRNA levels for the lin0142, rpoN, and the manR mutant genes were unaffected in all strains.
Alterations in mpt transcription are unlikely to be caused by changes in the levels of mutant ManR proteins in the strains. The protein synthesis rates should not be affected because the mutation sites are far removed from the 5′ ends of the transcripts and the manR mRNA levels are the same as the wild-type levels (Table 2). For the relatively inactive proteins that contain the H506A substitution, the mpt transcript levels did not fall below the uninduced level observed in Lin11, suggesting that this substitution does not strongly destabilize the protein. If a mutation rendered a protein highly unstable or completely nonfunctional, the level of mpt mRNA would be greatly reduced, as in the manR deletion strain. It also is unlikely that proteins with the H871A substitution accumulate to greater-than-normal levels in cells and thereby elevate mpt transcription. In this regard, the level of mpt mRNA returned to normal in the single-mutant strain grown in the presence of glucose and was equivalent to the basal uninduced level in the double-mutant strain in glucose medium, probably due to the H506A mutation. Instead, the results suggest that ManR specific activity is altered by the alanine substitutions.
Model for mpt regulation.
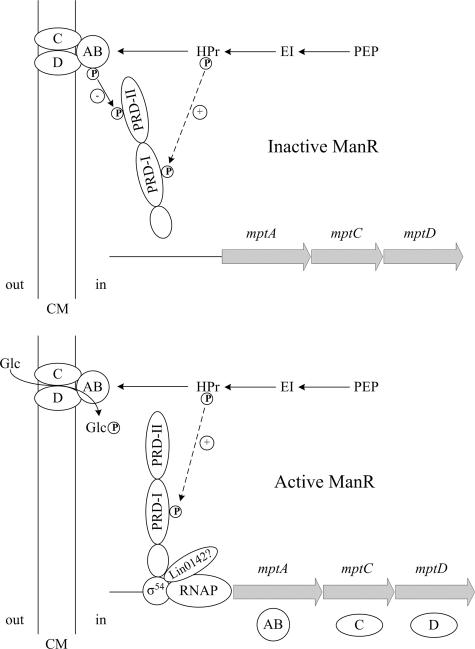
A model for regulation of mpt operon transcription by PTS phosphorylation of ManR is presented in Fig. 3. The model is based on the data described above and on a large number of studies of PTS operon regulation in gram-positive bacteria. Below, these studies are summarized first, with a focus on work conducted with the 38% identical σ54-associated activator LevR, which controls the lev operon in B. subtilis (7, 14), the LevR-like regulator EsuR, which controls the esu operon in Lactobacillus casei (27), and the GlcT antiterminator, which controls the ptsGHI operon in B. subtilis (20).
FIG. 3.
Model for the regulation of ManR activity. In the absence of glucose (Glc), the IIABMan subunit of EIItMan, which is present in the phosphorylated state, transfers its phosphate to His-871 in PRD-II, inactivating ManR and repressing mpt transcription. His-506 in PRD-I also may be phosphorylated by HPr. In the presence of glucose, the phosphoryl group is transferred from IIABMan to the incoming sugar, and ManR is not phosphorylated on His-871. Similarly, fewer ManR molecules are phosphorylated on His-506 in PRD-I by HPr. The monophosphorylated ManR species appears to be active and capable of interacting with RNA polymerase (RNAP), σ54, and perhaps Lin0142 to drive transcription of the mpt operon. CM, cytoplasmic membrane; PEP, phosphoenolpyruvate; EI, enzyme I.
In the absence of inducer transport, the transcription of the lev and esu operons is repressed by EIIB phosphorylation of LevR and EsuR histidines in the PRD-IIs of these proteins (14, 27). The targeted histidine in LevR, His-869, corresponds to His-871 in ManR (Fig. 2). Transcription of the lev operon actually increases to a level above the wild-type induced level when nonphosphorylatable alanine is substituted at this site for unknown reasons (14). In contrast to ManR, His-506 of LevR PRD-I, which corresponds to His-506 in ManR, is not needed for LevR activation by fructose. In the GlcT antiterminator, IIBGlc phosphorylation of His-104 in PRD-I in the absence of glucose strongly inactivates GlcT and represses ptsGHI transcription (20). In contrast, HPr phosphorylation of His-210 in PRD-II in the presence of glucose slightly activates the protein. Note that the order of the PRDs is reversed within the polypeptide chains of antiterminators and activators. As occurs with LevR, replacement of His-104 in GlcT with alanine leads to greater-than-wild-type induction of ptsGHI transcription (20).
Based on our data showing that His-871 must be present in ManR PRD-II for repression of mpt in the absence of glucose, we propose that this site is phosphorylated by IIABMan in the absence of glucose and phosphorylation inactivates the protein, as occurs in the LevR, EsuR, and GlcT regulators (Fig. 3). This idea is bolstered by the observation that the amount of mpt mRNA reaches a greatly elevated level in the H871A single-substitution mutant grown in the absence of glucose (Table 2). When glucose is being transported, we propose that wild-type ManR molecules are not phosphorylated at His-871, and this increases the activity of ManR and mpt transcription, as also occurs with other regulators. In the H871A mutant strain grown in the absence of glucose, the amount of mpt mRNA increases to a level above the level in the wild-type strain during glucose induction because none of the copies of the ManR protein can be phosphorylated at His-871.
mRNA measurements showed that both single- and double-mutant ManR proteins containing the H506A substitution retain only the basal capacity for transcription of the mpt operon in glucose medium (Table 2). Work performed with GlcT indicates that the homologous histidine in this antiterminator can be phosphorylated by HPr (20, 22). Our finding that the mpt mRNA level is reduced ∼6-fold in the H506A/H871A double mutant compared to the H871A single mutant in LB medium lacking glucose suggests that ManR is activated by phosphorylation at His-506. Furthermore, the threefold decline in the mpt mRNA level observed when the H871A mutant was grown in glucose medium, in which phosphorylation at this site should have been reduced, also suggests that ManR is activated by phosphorylation on His-506.
In conclusion, based on the data described above we propose that although the wild-type ManR protein probably is phosphorylated by HPr on His-506 both in the absence and in the presence of glucose (8, 22, 23), the dual-phosphorylated protein that exists in the absence of glucose is inactive, while the single-phosphorylated species that occurs in glucose medium is active (Fig. 3). In other words, the ratio of phosphorylated residues to unphosphorylated residues in ManR determines its activity. The phosphorylation that occurs at His-871 in the absence of glucose appears to exert a dominant effect and down-regulates the activity of the protein. Additional studies are required to clarify why Lin0142 is needed in addition to ManR for activation of RNA polymerase at the σ54-controlled mpt promoter.
Acknowledgments
We acknowledge financial support from the University of Wyoming.
Footnotes
Published ahead of print on 6 July 2007.
REFERENCES
- 1.Barabote, R. D., and M. H. Saier, Jr. 2005. Comparative genomic analyses of the bacterial phosphotransferase system. Microbiol. Mol. Biol. Rev. 69:608-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Zeev, E., L. Fux, O. Amster-Choder, and M. Eisenstein. 2005. Experimental and computational characterization of the dimerization of the PTS-regulation domains of BglG from Escherichia coli. J. Mol. Biol. 347:693-706. [DOI] [PubMed] [Google Scholar]
- 3.Buck, M., M. T. Gallegos, D. J. Studholme, Y. Guo, and J. D. Gralla. 2000. The bacterial enhancer-dependent σ54 (σN) transcription factor. J. Bacteriol. 182:4129-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen, D. P., and R. W. Hutkins. 1994. Glucose uptake by Listeria monocytogenes Scott A and inhibition by pediocin JD. Appl. Environ. Microbiol. 60:3870-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Commichau, F. M., K. Forchhammer, and J. Stulke. 2006. Regulatory links between carbon and nitrogen metabolism. Curr. Opin. Microbiol. 9:167-172. [DOI] [PubMed] [Google Scholar]
- 7.Dalet, K., Y. Cenatiempo, P. Cossart, and Y. Hechard. 2001. A σ54-dependent PTS permease of the mannose family is responsible for sensitivity of Listeria monocytogenes to mesentericin Y105. Microbiology 147:3263-3269. [DOI] [PubMed] [Google Scholar]
- 8.Deutscher, J. R., C. Francke, and P. W. Postma. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70:939-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbreth, S. E., A. K. Benson, and R. W. Hutkins. 2004. Catabolite repression and virulence gene expression in Listeria monocytogenes. Curr. Microbiol. 49:95-98. [DOI] [PubMed] [Google Scholar]
- 10.Glaser, P., L. Frangeul, C. Buchrieser, C. Rusniok, A. Amend, F. Baquero, P. Berche, H. Bloecker, P. Brandt, T. Chakraborty, A. Charbit, F. Chetouani, E. Couve, A. de Daruvar, P. Dehoux, E. Domann, G. Dominguez-Bernal, E. Duchaud, L. Durant, O. Dussurget, K. D. Entian, H. Fsihi, F. Garcia-del Portillo, P. Garrido, L. Gautier, W. Goebel, N. Gomez-Lopez, T. Hain, J. Hauf, D. Jackson, L. M. Jones, U. Kaerst, J. Kreft, M. Kuhn, F. Kunst, G. Kurapkat, E. Madueno, A. Maitournam, J. M. Vicente, E. Ng, H. Nedjari, G. Nordsiek, S. Novella, B. de Pablos, J. C. Perez-Diaz, R. Purcell, B. Remmel, M. Rose, T. Schlueter, N. Simoes, A. Tierrez, J. A. Vazquez-Boland, H. Voss, J. Wehland, and P. Cossart. 2001. Comparative genomics of Listeria species. Science 294:849-852. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg, D. B., J. Stulke, and M. H. Saier, Jr. 2002. Domain analysis of transcriptional regulators bearing PTS regulatory domain. Res. Microbiol. 153:519-526. [DOI] [PubMed] [Google Scholar]
- 12.Herro, R., S. Poncet, P. Cossart, C. Buchrieser, E. Gouin, P. Glaser, and J. Deutscher. 2005. How seryl-phosphorylated HPr inhibits PrfA, a transcription activator of Listeria monocytogenes virulence genes. J. Mol. Microbiol. Biotechnol. 9:224-234. [DOI] [PubMed] [Google Scholar]
- 13.Horton, R. M., Z. L. Cai, S. N. Ho, and L. R. Pease. 1990. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques 8:528-535. [PubMed] [Google Scholar]
- 14.Martin-Verstraete, I., V. Charrier, J. Stulke, A. Galinier, B. Erni, G. Rapoport, and J. Deutscher. 1998. Antagonistic effects of dual PTS-catalysed phosphorylation on the Bacillus subtilis transcriptional activator LevR. Mol. Microbiol. 28:293-303. [DOI] [PubMed] [Google Scholar]
- 15.Milenbachs, A. A., D. P. Brown, M. Moors, and P. Youngman. 1997. Carbon-source regulation of virulence gene expression in Listeria monocytogenes. Mol. Microbiol. 23:1075-1085. [DOI] [PubMed] [Google Scholar]
- 16.Postma, P. W., J. W. Lengeler, and G. R. Jacobson. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57:543-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramnath, M., M. Beukes, K. Tamura, and J. W. Hastings. 2000. Absence of a putative mannose-specific phosphotransferase system enzyme IIAB component in a leucocin A-resistant strain of Listeria monocytogenes, as shown by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Appl. Environ. Microbiol. 66:3098-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramnath, M., S. Arous, A. Gravesen, J. W. Hastings, and Y. Hechard. 2004. Expression of mptC of Listeria monocytogenes induces sensitivity to class IIa bacteriocins in Lactococcus lactis. Microbiology 150:2663-2668. [DOI] [PubMed] [Google Scholar]
- 19.Robichon, D., E. Gouin, M. Debarbouille, P. Cossart, Y. Cenatiempo, and Y. Hechard. 1997. The rpoN (σ54) gene from Listeria monocytogenes is involved in resistance to mesentericin Y105, an antibacterial peptide from Leuconostoc mesenteroides. J. Bacteriol. 179:7591-7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmalisch, M. H., S. Bachem, and J. Stulke. 2003. Control of the Bacillus subtilis antiterminator protein GlcT by phosphorylation. J. Biol. Chem. 278:51108-51115. [DOI] [PubMed] [Google Scholar]
- 21.Stulke, J., M. Arnaud, G. Rapoport, and I. Martin-Verstraete. 1998. PRD—a protein domain involved in PTS-dependent induction and carbon catabolite repression of catabolic operons in bacteria. Mol. Microbiol. 28:865-874. [DOI] [PubMed] [Google Scholar]
- 22.Stulke, J., and W. Hillen. 2000. Regulation of carbon catabolism in Bacillus species. Annu. Rev. Microbiol. 54:849-880. [DOI] [PubMed] [Google Scholar]
- 23.Titgemeyer, F., and W. Hillen. 2002. Global control of sugar metabolism: a Gram-positive solution. Antonie Leeuwenhoek 82:59-71. [PubMed] [Google Scholar]
- 24.van Tilbeurgh, H., and N. Declerck. 2001. Structural insights into the regulation of bacterial signaling proteins containing PRDs. Curr. Opin. Struct. Biol. 11:685-693. [DOI] [PubMed] [Google Scholar]
- 25.van Tilbeurgh, H., D. Le Coq, and N. Declerck. 2001. Crystal structure of an activated form of the PTS regulation domain from the LicT transcriptional antiterminator. EMBO J. 20:3789-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue, J., I. Hunter, T. Steinmetz, A. Peters, B. Ray, and K. W. Miller. 2005. Novel activator of mannose-specific phosphotransferase system permease expression in Listeria innocua, identified by screening for pediocin AcH resistance. Appl. Environ. Microbiol. 71:1283-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yebra, M. J., R. Viana, V. Monedero, J. Deutscher, and G. Perez-Martinez. 2004. An esterase gene from Lactobacillus casei cotranscribed with genes encoding a phosphoenolypyruvate:sugar phosphotransferase system and regulated by a LevR-like activator and σ54 factor. J. Mol. Microbiol. Biotechnol. 8:117-128. [DOI] [PubMed] [Google Scholar]