Abstract
There is increasing concern regarding the presence of vancomycin-resistant enterococci in domestically farmed animals, which may act as reservoirs and vehicles of transmission for drug-resistant enterococci to humans, resulting in serious infections. In order to assess the potential for the use of monolaurin as a food preservative, it is important to understand both its target and potential mechanisms of resistance. A Tn917 mutant library of Enterococcus faecalis AR01/DGVS was screened for resistance (MIC, >100 μg/ml) to monolaurin. Three mutants were identified as resistant to monolaurin and were designated DGRM2, DGRM5, and DGRM12. The gene interrupted in all three mutants was identified as traB, which encodes an E. faecalis pheromone shutdown protein and whose complementation in trans restored monolaurin sensitivity in all three mutants. DGRM2 was selected for further characterization. E. faecalis DGRM2 showed increased resistance to gentamicin and chloramphenicol (inhibitors of protein synthesis), while no difference in the MIC was observed with the cell wall-active antibiotics penicillin and vancomycin. E. faecalis AR01/DGVS and DGRM2 were shown to have similar rates (30% cell lysis after 4 h) of cell autolytic activity when activated by monolaurin. Differences in cell surface hydrophobicity were observed between the wild type and the mutant, with the cell surface of the parent strain being significantly more hydrophobic. Analysis of the cell wall structure of DGRM2 by transmission electron microscopy revealed an increase in the apparent cell wall thickness and contraction of its cytoplasm. Taken together, these results suggest that the increased resistance of DGRM2 was due to a change in cell surface hydrophobicity, consequently limiting the diffusion of monolaurin to a potential target in the cytoplasmic membrane and/or cytoplasm of E. faecalis.
Monolaurin, a food grade glycerol monoester of lauric acid, has been reported to have the greatest antimicrobial activity of all of the monoglycerides (50). Monolaurin, like any fatty acid ester, is a lipophilic compound and hence its inhibitory activity is probably through interactions with the cytoplasmic membrane. Although the mechanism of antibacterial action of fatty acids and their derivatives is not defined, it has been suggested to involve disruption of the cell membrane permeability barrier and inhibition of amino acid uptake (29, 51). The activity of monolaurin against gram-negative bacteria has been shown to be enhanced when combined with high temperatures (30), freezing (54), acidulants (8, 29, 43, 53), and chelating agents such as EDTA (5), treatments believed to increase the ability of the monoglyceride to access the cytoplasmic membrane (51). Glycerol monolaurate has been shown to inhibit the production of exoenzymes and virulence factors in Staphylococcus aureus (41), to block the induction of vancomycin resistance in Enterococcus faecalis (46), and to modulate T-cell proliferation (62), all of which involve membrane-bound signal transduction systems. Dodecylglycerol (corresponding ether of monolaurin) has been shown to activate the proteolytic enzyme responsible for the activation of autolysin in the cell wall of E. faecium (40, 56, 57) and to inhibit glycerolipid and lipoteichoic acid biosynthesis in Streptococcus mutans (6).
Generally, the activity of fatty acids and their derivatives against bacteria is affected by the presence of starch, proteins such as serum albumin, lipids such as phospholipids, and other surface-active agents such as cholesterol (27, 49). There have been few studies investigating the mechanism(s) of resistance to fatty acids and their derivatives. Lee and Shafer (32) studied the resistance of gonococci to long-chain fatty acids and discovered that resistance was mediated by an efflux pump encoded by farAB (fatty acid resistance). To et al. (55) also reported that the resistance of Listeria monocytogenes to the surfactant benzalkonium chloride was due to efflux pumps.
Enterococci can survive some types of food processing and have been implicated in outbreaks of food-borne illnesses and in the spoilage of processed cooked meat, raw meat, milk, and milk products (1, 14, 28). Enterococci are not important food-borne pathogens, but livestock and poultry can serve as reservoirs for drug-resistant strains of Enterococcus, which may then enter the human food chain and cause serious infections (4, 14, 15, 37, 44). There is increasing concern about the emergence of multiple-antibiotic-resistant enterococci and the presence of vancomycin-resistant enterococci in nonhuman reservoirs (1, 14, 18, 20, 52). In order to assess the utility of monolaurin as a food preservative against enterococci, it was important to determine its cellular target(s) and potential mechanisms of resistance to this compound.
In this communication, we report on the isolation and characterization of monolaurin-resistant E. faecalis mutants. We propose that monolaurin resistance in these mutants is mediated by changes in their cell surface hydrophobicity limiting the access of monolaurin to a potential target in the cytoplasmic membrane and/or in the cytoplasm of the bacterium.
MATERIALS AND METHODS
Chemical stocks.
Unless otherwise stated, all of the chemicals used were purchased from Sigma Chemical Co., St. Louis, MO, and stock solutions were filter sterilized (0.2 μm Supor Acrodisc; Gelman Sciences, Ann Arbor, MI). Monolaurin and Lauricidin (90% lauric acid, 8% myristic acid, 2% capric acid; Med-Chem Labs) were prepared as stock solutions of 50 mg/ml in 95% ethanol. Stock solutions of monolaurin, Lauricidin, and antibiotics were stored at −20°C until required.
Media, bacterial strains, and plasmids.
The media (Difco, Fort Richard Laboratories Ltd., Auckland, New Zealand) used in the present study were prepared according to the manufacturer's specifications. The bacterial strains and plasmids used in the present study are listed in Table 1. For routine cultivation, E. faecalis and Escherichia coli strains were propagated for 24 h at 37°C in brain heart infusion (BHI) and Luria broth, respectively. Strains in regular use were subcultured on either Luria agar or BHI agar every 2 weeks and maintained at 4°C. Stock cultures were stored at −80°C in 10% skim milk containing 20% glycerol.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli MC1061 | F−araD139 (ara-leu)7696 galE15 galK16 (lac)X74 rspL (Strr) hsdR2 (rk− mk+) mcrA mcrB1 | 7 |
| E. faecalis | ||
| AR01/DGVS | AR01/DG cured of pJM02 (Vmr Emr) Tcr Bcr | 36 |
| DGRM2 | AR01/DGVS traB::Tn917 Emr Tcr Bcr Mlr | 36 |
| DGRM5 | AR01/DGVS traB::Tn917 Emr Tcr Bcr Mlr | 36 |
| DGRM12 | AR01/DGVS traB::Tn917 Emr Tcr Bcr Mlr | 36 |
| AR01/DGVS/pAM401 | AR01/DGVS harboring pAM401 Tcr Bcr Cmr | This study |
| DGRM2/pAMCL6 | DGRM2 harboring pAMCL6 Emr Tcr Bcr Spr Mls | This study |
| DGRM5/pAMCL6 | DGRM5 harboring pAMCL6 Emr Tcr Bcr Spr Mls | This study |
| DGRM12/pAMCL6 | DGRM12 harboring pAMCL6 Emr Tcr Bcr Spr Mls | This study |
| JH2-2 | Glycopeptide-susceptible strain commonly used for gene transfer experiments with Enterococcus | 22 |
| JH2-7349 | JH2-2 harboring pPIT7349 Emr | This study |
| JH2-7013 | JH2-2 harboring pPIT7013 Emr | This study |
| Plasmids | ||
| pTV1-OK | repA(Ts)-pWV01Ts aphA3 Tn917 Kmr Emr | 19 |
| pCL1921 | Low-copy-number vector; Str Spr, 4.6 kb | 33 |
| pCL6 | pCL1921 harboring 6.8-kb insert from AR01/DGVS containing traB; Str Spr | This study |
| pAM401 | E. coli-E. faecalis shuttle vector; Cmr Tcr, 10.4 kb | 61 |
| pAMCL6 | pAM401 harboring pCL1921 and 6.8-kb insert from AR01/DGVS containing traB; Tcr | This study |
| pPIT7349 | pPD1::Tn917 derivative with insertion in traB | 16 |
| pPIT7013 | pPD1::Tn917 derivative with insertion in orfY | 16 |
Effect of monolaurin on the growth of E. faecalis AR01/DGVS.
An overnight culture of E. faecalis AR01/DGVS was diluted to an optical density at 595 nm (OD595) of 0.01 in BHI, and 100-μl volumes were dispensed into the wells of a flat-bottom 96-well microtiter plate (Nalgene Nunc GmbH & Co. KG, Wiesbaden, Germany). The stock solution of monolaurin was diluted to attain final concentrations of 800, 400, 200, 100, 50, 25, 10, 5, and 1 μg/ml in BHI, and 100 μl was added to each well when the bacterial cells reached an OD595 of approximately 0.4. Control wells received 100 μl of broth or 100 μl of 0.1% ethanol, and all tests were conducted in triplicate. The cultures were incubated at 37°C for 24 h in a plate reader (Multiskan Ascent Microtiter Plate Reader; LabSystems, Vantaa, Finland) with absorbance readings (595 nm) taken every 2 h. The MIC was defined as the lowest concentration of monolaurin to show complete inhibition of bacterial growth (OD595, <0.1) after 24 h of incubation at 37°C.
Transposon mutagenesis and isolation of monolaurin-resistant mutants.
To identify genes involved in the development of resistance to monolaurin in E. faecalis, an E. faecalis AR01/DGVS Tn917 mutant library (36) was screened for resistance by the following protocol. A Lauricidin stock solution was diluted to 500 μg/ml in BHI/Erm10 (BHI containing 10 μg/ml erythromycin), and 40 μl was added to the wells of microtiter plates containing 100 μl of BHI/Erm10. A 60-μl volume of an overnight culture of each mutant strain was added to each well, and the OD595 was read at 0 h with the plate reader. Following 24 h of incubation at 37°C, the absorbance was read again and the change in OD595 (ΔOD) was calculated by subtracting the absorbance value at time zero from that at 24 h.
Genomic DNA extraction, transformation, and genetic techniques.
E. faecalis chromosomal DNA was obtained as described previously (35). Transformation of E. faecalis with glycine-grown cells was performed as described by Shepard and Gilmore (48). DNA manipulations were carried out according to standard molecular biology protocols (47). Purified plasmid DNA was prepared with a QIAprep spin miniprep kit (QIAGEN, Hilden, Germany) for high-copy plasmid extraction or a plasmid midi kit (QIAGEN) for low-copy vectors. Restriction endonucleases, ligases, and polymerases were used according to the manufacturer's instructions. PCR were performed in accordance with the manufacturer's instructions, by using the PCR program described previously (31). Radiolabeled PCR products and plasmids were prepared by incorporation of [α-32P]dCTP-labeled deoxynucleotides with Ready-to-Go DNA labeling beads (Amersham, Buckinghamshire, England). Southern transfer and hybridization were performed as previously described (35).
Mapping of transposon inserts.
The presence of single Tn917 insertions was determined by Southern blot hybridization of HindIII-digested (Roche, Mannheim, Germany) genomic DNA and a radioactively labeled, HindIII-digested pTV1-OK probe (47).
The sites of Tn917 insertions in the selected monolaurin-resistant mutants were mapped by inverse PCR with Tn917-derived primers ErmP2 (5′-TACAAATTCCTCGTAGGC-3′) and HindIII (5′-GACATTATAAGCCGCTGTCG-3′). Total DNA from Tn917 mutants was digested with HindIII and self-ligated with T4 DNA ligase (Roche), and an inverse PCR was carried out with an Expand Long Template PCR system (Roche) under the conditions recommended by the manufacturer.
Nucleotide sequencing and sequence analysis.
PCR products and plasmids were sequenced directly. Sequencing reactions were carried out with a PRISM ready reaction DyeDeoxy terminator cycle sequencing kit (Applied Biosystems Inc., Warrington, United Kingdom) and a model ABI377 automated DNA sequencer (Applied Biosystems). The nucleotide sequences were assembled with Seqman (DNASTAR, Inc.). Sequence analyses were carried out with Editseq (DNASTAR, Inc.) and the programs BLASTN, BLASTP, BLASTX, and CDD (National Center for Biotechnology Information, Los Alamos, NM), available via the internet.
Cloning of the traB gene and plasmid construction for complementation studies.
The traB gene of E. faecalis AR01/DGVS was cloned as a 6.8-kb EcoRI (Roche) fragment into pCL1921, creating plasmid pCL6. The resulting construct was electroporated into E. coli, and blue/white selection was used to screen the transformants. The DNA insert of randomly selected transformants was sequenced to confirm the presence of the traB gene. To enable complementation, plasmid pAMCL6 was constructed by ligating pCL6 into E. coli-E. faecalis shuttle vector pAM401. E. coli transformants were selected for tetracycline resistance and chloramphenicol sensitivity. The size of pAMCL6 was confirmed by enzyme digestion and gel electrophoresis. pAMCL6 was electroporated into E. faecalis, and its presence was confirmed by enzyme digests and PCR.
The traB::Tn917 mutants were originally selected on the basis of their increased resistance to monolaurin compared to the E. faecalis parent strain. It was therefore necessary to determine if complementation of the traB mutants with the traB gene would restore their sensitivity to monolaurin. Plasmids extracted from the complemented E. faecalis mutants were used as template DNA for amplification of the traB gene with the TraBF22 (5′-CGGAGAGACACCGTCAGGGG-3′) and TraBR1121 (5′-CCTATAGCTCCTCCTAAATT-3′) primers. The MICs of monolaurin and other selected antimicrobials (i.e., vancomycin, gentamicin, penicillin, and chloramphenicol) for E. faecalis strains were established as described above.
E. faecalis JH2-7349.
To further characterize our traB mutant, E. faecalis DGRM2, the MICs and cell surface hydrophobicity of DGRM2 were compared to those of another traB mutant, E. faecalis JH2-7349. The controls included were JH2-2 (no pPD1 plasmid) and JH2-7013 (a non-traB mutant). E. faecalis strains JH2-7349 and JH2-7013 were obtained by electroporating plasmids pPIT7349 and pPIT7013, respectively (provided by Shuhei Fujimoto [16]) into E. faecalis JH2-2.
Autolysis assay.
Cell autolysis was assayed by modification of the method of de Jonge et al. (12). Cells of E. faecalis AR01/DGVS and DGRM2 were grown overnight in 20 ml of THBG (Todd-Hewitt broth containing 2% glucose) and THBG/Erm10 (THBG containing 10 μg/ml erythromycin), respectively. One milliliter of overnight culture was used to inoculate THBG or THBG/Erm10, which was incubated at 37°C until an OD600 of 0.3 was reached, at which point the cells were chilled on ice for 15 min. The cells were washed twice in ice-cold MilliQ water and resuspended in 50 mM Tris-HCl (pH 8.0) to an OD600 of 1.0. The cell suspensions were incubated at 37°C for 6 h, and autolysis was determined by reading the OD600 at regular intervals. Two concentrations of monolaurin were tested for the ability to induce autolysis, 50 μg/ml (AR01/DGVS) and 100 μg/ml (DGRM2). Also included were 0.01% Triton X-100 and MilliQ water as positive and negative controls, respectively.
Fatty acid analysis.
The fatty acid compositions of E. faecalis AR01/DGVS and DGRM2 cells and cell membranes were established when the bacteria were grown in the presence (50 μg/ml) or absence of monolaurin. Overnight cultures of E. faecalis AR01/DGVS and DGRM2 were grown in BHI and BHI/Erm10, respectively.
For the preparation of whole-cell samples, bacterial cells were collected by centrifugation (7,000 × g, 15 min at 4°C). The bacterial cells were washed three times in ice-cold MilliQ water, and cell pellets (0.5 g wet weight) were stored at −20°C until required.
For the preparation of cell membranes, cells (5 g, wet weight) were resuspended in 5 ml of 50 mM morpholinepropanesulfonic acid (MOPS) buffer (pH 7.5) containing 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1.2 mg of lysozyme, and 2,500 U of mutanolysin. Following 60 min of incubation at 37°C with gentle stirring, 15 mM magnesium chloride containing 2 mg of DNase was added to the suspensions. The suspensions were incubated for a further 15 min at 37°C and then lysed by two passages through a French pressure cell at 20,000 lb/in2. The crude lysate was centrifuged at 10,000 × g for 20 min at 4°C to remove unbroken cells. The supernatant was centrifuged for 1 h at 180,000 × g to harvest the cell membranes. The membranes were washed and resuspended in 1 ml of 50 mM potassium phosphate buffer (pH 7.2) containing 2 mM magnesium chloride, 0.5 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride. Samples were stored at −20°C prior use.
To extract lipids, the samples (cells and cell membranes) were transferred to a Kimax tube and the final volume was adjusted to 4.5 ml with MilliQ water. The internal standard (tridecanoic acid; Nu-Check Prep Inc., Elysian, MN), 60 μl of a 10-mg/ml solution in 1:1 (vol/vol) CHCl3-methanol (MeOH) (GR for analysis and LiChrosolv; Merck, Darmstadt, Germany) was added to the tubes (series A tubes). To each sample, 12 ml of CHCl3 and 6 ml of MeOH were added to give a final medium composition of 12:6:4.5 (vol/vol) CHCl3-MeOH-water. The suspensions were shaken at room temperature (120 cycles/min, 30 min) and centrifuged (3,000 × g, 10 min). The organic layer was transferred into another Kimax tube (series B tubes), which was centrifuged (1,000 × g, 10 min) to remove any particulate at the surface of the organic layer. The organic layer was transferred to another 20-ml Kimax tube (series C tubes). Series A tubes were reextracted in 2:1 (vol/vol) CHCl3-MeOH. The organic layer was transferred to series B tubes and centrifuged as described above. The combined organic layer was dried under a constant stream of nitrogen gas (oxygen free) while in a heating block at 30°C.
To series C tubes were added 300 μl of toluene and 1.4 ml of 14% BF3-MeOH. The tubes were heated at 100°C for 2 h and then left to cool to room temperature. A saturated NaCl solution (6 ml) (NaCl AR grade; BDH, Poole, England) was added to each tube, which was shaken and then centrifuged (1,000 × g, 10 min). An aliquot of the top layer was transferred to a 2-ml autosampler vial containing a 250-μl insert for gas chromatography (GC) analysis.
Analysis of fatty acid methyl esters (FAMEs) was carried out on an Agilent 6890N gas chromatograph equipped with an Agilent 7683 series autosampler (Agilent Technologies Inc., Wilmington, DE). The injections were carried out in split mode (40:1) at 250°C. A flame ionization detector at 260°C was used with gas flow rates of air at 300 ml/min, hydrogen at 30 ml/min, and nitrogen at 30 ml/min. For quantitative analysis, separation was carried out on a BPX70 capillary column (50-m length, 330-μm inside diameter, 0.25-μm film thickness; SGE Analytical Products, Melbourne, Australia) with hydrogen gas (2 ml min−1, 33 cm s−1, constant-flow mode). The GC oven was initially held at 35°C for 5 min, increased to 205°C at 2.5°C min−1, and then increased to 230°C at 10°C min−1. For confirmation of peak FAME identities, separation was also carried out on a BPX5 capillary column (30-m length, 250-μm inside diameter, 0.25-μm film thickness; SGE Analytical Products, Melbourne, Australia) with hydrogen gas (1.2 ml/min, 36 cm/s, constant-flow mode). The GC oven started at 60°C and was then increased to 240°C at 2°C/min and held for 20 min. Data integration and computation were performed with HP Chemstation software (Hewlett-Packard).
Identification of fatty acids was carried out with authentic standards, and the retention times were compared on two columns with differing polarities (see above). The authentic standards included a FAME mixture (FAMEQ005) and a set of C18:1 isomers (6-cis-octadecenoic acid methyl ester, 6-trans-octadecenoic acid methyl ester, 11-cis-octadecenoic acid methyl ester, and 11-trans-octadecenoic acid methyl ester; Nu-Check Prep Inc., Elysian, MN).
Cell surface hydrophobicity.
The cell surface hydrophobicity of E. faecalis AR01/DGVS and DGRM2 was assayed by the MATH (microbial adhesion to hydrocarbons) assay as described by Reifsteck et al. (42), with slight modifications. Bacteria were first washed three times in ice-cold MilliQ water and finally resuspended in phosphate wash solution to an OD500 of 0.5. A 4.8-ml volume of each bacterial suspension was mixed with 0.8 ml of n-hexadecane in a glass tube and vigorously shaken for 1 min. After the preparations rested for 30 min, the OD500 values of the aqueous phase were determined. The affinity of bacteria for the solvent was evaluated by the formula % adherence = (1 − A/A0) × 100, where A0 is the OD500 of the bacterial suspension before mixing and A is the OD after mixing.
Transmission electron microscope (TEM) analysis of E. faecalis cells.
To visualize differences occurring in the cell wall structure between E. faecalis AR01/DGVS and DGRM2 grown in the presence of monolaurin (AR01/DGV, 50 μg/ml; DGRM2, 100 μg/ml) or its absence, cells were examined by TEM. E. faecalis strains AR01/DGVS and DGRM2 were grown overnight in the presence or absence of monolaurin. Bacterial cells were washed in 0.1 M phosphate-buffered saline (pH 7.0) and fixed with 3% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.0) with 3 mg/ml ruthenium red. After 2 h on a rotator (2 rpm) at room temperature, the samples were washed three times with the same buffer and postfixed with 2% osmium tetroxide in 0.1 M sodium cacodylate buffer (pH 7.0) containing ruthenium red. After 1 h at room temperature, the samples were washed three times in the same buffer and the cells were embedded in 3% agarose. The samples were then loaded into a tissue processor, dehydrated with increasing concentrations of ethanol, and finally embedded in Quetol 651 resin. The samples were visualized with a Philips CM100 TEM (FEI/Philips, Eindhoven, The Netherlands).
Nucleotide sequence accession number.
The DNA sequence of traB from E. faecalis AR01/DGVS, located on pJM01, has been deposited in GenBank under accession number EF035487.
RESULTS
Effect of monolaurin on the growth of E. faecalis AR01/DGVS.
The Enterococcus strain used in this study was derived from E. faecalis AR01/DG isolated from a dog with mastitis. AR01/DG harbors two plasmids carrying antibiotic resistance genes, pJM01 (tetracycline and bacitracin resistance) and pJM02 (vancomycin and erythromycin resistance). E. faecalis AR01/DGVS was obtained by curing AR01/DG of plasmid pJM02 (36). Plasmid pJM01 is 72 kb in size and has a frequency of conjugation of 7.28 × 10−3 in broth mating (36).
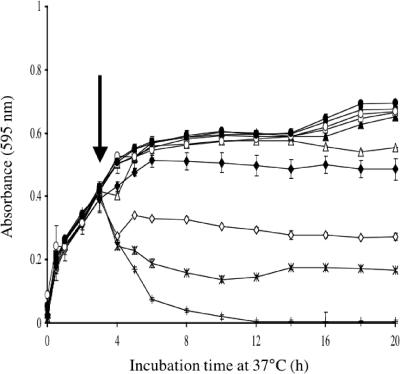
The MIC of monolaurin for E. faecalis AR01/DGVS was 100 μg/ml (Table 2), but 200 μg/ml was required to inhibit exponentially growing cells (Fig. 1). Monolaurin added to exponentially growing cells at high concentrations caused a rapid decrease in OD595 suggestive of cell lysis (Fig. 1). AR01/DGVS mutants resistant to monolaurin were isolated in an attempt to identify its site of action and potential mechanisms of resistance to this compound.
TABLE 2.
Phenotypic characterization of the E. faecalis strains used in this study
| Strain | Genotype | % Hydrophobicity | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|---|---|
| Monolaurin | Gentamicin | Chloramphenicol | Penicillin | Vancomycin | |||
| JH2-2 | Wild type | 95 | 100 | 50 | 7.5 | 2.5 | 2.5 |
| JH2-7013 | Tn917::orfY | 92 | 100 | 50 | 7.5 | 2.5 | 2.5 |
| JH2-7349 | Tn917::traB | 69 | 300 | 100 | 15 | 2.5 | 2.5 |
| AR01/DGVS | Wild type | 100 | 100 | 50 | 7.5 | 2.5 | 5 |
| DGRM2 | Tn917::traB | 65 | 300 | 100 | 15 | 2.5 | 5 |
| DGRM5 | Tn917::traB | 65 | 300 | 100 | 15 | 2.5 | 5 |
| DGRM12 | Tn917::traB | 70 | 300 | 100 | 15 | 2.5 | 5 |
FIG. 1.
Effect of monolaurin on the growth of E. faecalis AR01/DGVS. Monolaurin was added when the OD595 reached 0.4 to final concentrations of 0 μg/ml (•), 1 μg/ml (○), 5 μg/ml (▪), 10 μg/ml (□), 25 μg/ml (▴), 50 μg/ml (▵), 100 μg/ml (♦), 200 μg/ml (⋄), 400 μg/ml (×), and 800 μg/ml (+). Each symbol represents the mean of triplicate samples. Error bars represent standard deviation. The vertical arrow indicates the point at which monolaurin was added.
Isolation of monolaurin-resistant mutants of E. faecalis AR01/DGVS.
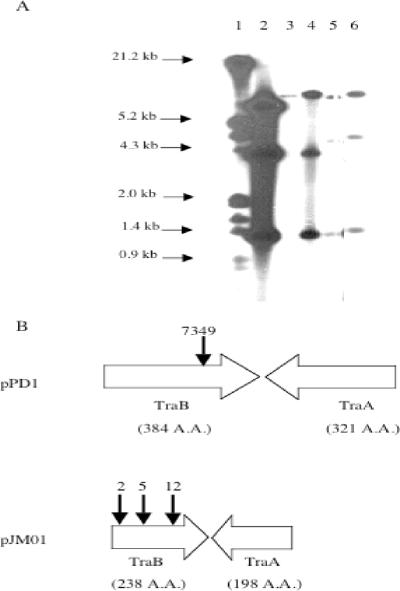
To identify genes involved in the development of resistance to monolaurin in E. faecalis, an E. faecalis AR01/DGVS Tn917 mutant library (36) was screened for resistance to monolaurin. Eight thousand Tn917 insertion mutants of E. faecalis AR01/DGVS were screened for the ability to grow in BHI/Erm10 containing 100 μg/ml Lauricidin. Lauricidin was used in the initial screening because the cost of large amounts of monolaurin was prohibitive. A cell inoculum was added (30%, wt/vol), and Lauricidin was present at the time of inoculation. The average increase in OD595 achieved after 24 h by the 8,000 mutants was 0.355 ± 0.001. Three mutants, designated DGRM2, DGRM5, and DGRM12, grew to OD595s of 0.713, 0.591, and 0.640, respectively. All three monolaurin-resistant mutants had a hybridization profile indicative of a single chromosomal insertion of Tn917 (two bands of variable size and one common fragment of 1.3 kb, internal to Tn917) (Fig. 2A). No hybridization of the pTV1-OK probe with DNA from E. faecalis AR01/DGVS was observed. Inverse PCR was used to amplify the DNA sequence flanking the left variable arm of Tn917. The PCR products were sequenced, and one open reading frame (ORF) was identified that encodes a protein having 67% identity to TraB, a pheromone shutdown protein, from E. faecalis V583. Figure 2B indicates the sites of Tn917 insertions in the traB::Tn917 mutants.
FIG. 2.
Sites of Tn917 insertions. (A) Autoradiogram from a Southern blot analysis of the restriction digests of total DNA from E. faecalis strains following hybridization with 32P-labeled pTV1-OK. Lane 1, lambda DNA digested with HindIII and EcoRI; lane 2, HindIII-digested pTV1-OK; lane 3, HindIII-digested E. faecalis AR01/DGVS. HindIII-digested E. faecalis mutants: lane 4, DGRM2; lane 5, DGRM5; lane 6, DGRM12. (B) Downward-pointing arrows indicate the sites of Tn917 insertions for E. faecalis strains as follows: 2, DGRM2; 5, DGRM5; 12, DGRM12; 7349, JH2-7349. Tn917 insertions for strains DGRM2, DGRM5, and DGRM12 were located within the traB gene of pJM01, while a Tn917 insertion for strain JH2-7349 was located within the traB gene of pPD1. The number of amino acids (A.A.) for each ORF is in parentheses.
Complementation of traB::Tn917 mutants.
When pAMCL6, containing functional traB, was electroporated into E. faecalis DGRM2, DGRM5, and DGRM12, susceptibility to monolaurin was restored (MIC of 100 μg/ml), demonstrating that the Tn917 insertion in the traB gene was responsible for the observed phenotype (data not shown).
Characterization of monolaurin-resistant mutants.
For the characterization of our E. faecalis traB mutant strains, we decided to use for comparison an E. faecalis strain harboring a pheromone-responsive plasmid carrying an inactivated (characterized) traB gene. For this purpose, we generated E. faecalis strain JH2-7349 carrying a pPD1 (pheromone-responsive plasmid) derivative, which had an inactivated traB gene because of a Tn917 insertion (16). The second pPD1 derivative, pPIT7013, had a Tn917 insert mapped in orfY and was used as a control to establish if any of the effects observed were due to the presence of Tn917 on plasmid pPD1 (16).
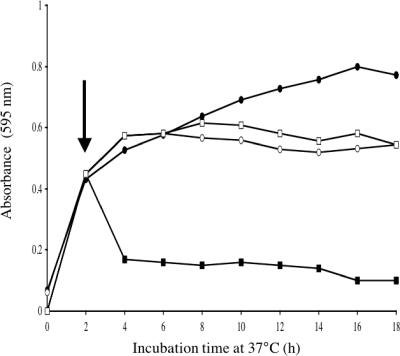
The MIC of monolaurin for strains DGRM2, DGRM5, DGRM12, and JH2-7349 was 300 μg/ml, while for AR01/DGVS, JH2-2, and JH2-7013 it was 100 μg/ml (Table 2). In the absence of monolaurin, the growth rate of DGRM2 was comparable to that of the wild-type strain (Fig. 3). The addition of 200 μg/ml monolaurin to actively growing cells resulted in an immediate decrease in OD595 and inhibition of growth of E. faecalis AR01/DGVS but had no effect on the growth of DGRM2 (Fig. 3). On the basis of the observation that monolaurin induced cell lysis at high concentrations (Fig. 1), the autolytic activities of the wild type and the mutant were compared when they were grown in the presence of monolaurin, and they were found to be identical (30% cell lysis after 4 h), suggesting that the increased resistance of DGRM2 to monolaurin was not linked to altered autolytic activity (data not shown).
FIG. 3.
Growth curves of E. faecalis strains grown in the presence (▪, □) or absence (•, ○) of monolaurin. Growth curves of E. faecalis strains AR01/DGVS (▪, •) and DGRM2 (□, ○) are shown. Each symbol represents the mean of triplicate samples. Error bars represent standard deviations. The vertical arrow indicates the point at which monolaurin was added.
The resistance of E. faecalis AR01/DGVS and the mutants DGRM2, DGRM5, and DGRM12 to other antimicrobial compounds was investigated to establish if the resistance to monolaurin also conferred resistance to antimicrobial compounds with diverse targets. The MICs of gentamicin and chloramphenicol for DGRM2, DGRM5, and DGRM12 were twofold higher than for AR01/DGVS (Table 2). An identical pattern of change in sensitivity to gentamicin and chloramphenicol was observed between JH2-2 and JH2-7349. The MICs of gentamicin and chloramphenicol for JH2-2 and JH2-7013 (transposon inserted in a non-traB gene) were identical. No differences in the MICs of the cell wall-targeting antibiotics penicillin and vancomycin were observed between the parent and mutant strains (Table 2).
The cell surfaces of E. faecalis DGRM2, DGRM5, DGRM12, and JH2-7349 were much more hydrophilic than those of E. faecalis AR01/DGVS, JH2-2, or JH2-7013 (Table 2). The fatty acid composition of E. faecalis AR01/DGVS and DGRM2 cells was analyzed when the bacterial cells were grown in the presence or absence of monolaurin. Myristic (C14:0), palmitic (C16:0), palmitoleic (C16:1Δ9cis), C16:1 isomer, stearic (C18:0), and vaccenic (C18:1) acids were identified as the major fatty acids in the E. faecalis strains (Table 3). The fatty acid profiles of AR01/DGVS and DGRM2 were similar for cultures grown in either the presence or the absence of monolaurin (Table 3). The addition of monolaurin had a minor influence on the total level of fatty acids, which increased on average by 21% (27.5% for AR01/DGVS and 14.6% for DGRM2), but had a major impact on the fatty acid ratios, with lauric acid accounting for 50% of the total fatty acid in both AR01/DGVS and DGRM2 (Table 3). The presence of monolaurin changed the cellular saturated-to-unsaturated fatty acid ratio from 1.4 (averages of 1.26 and 1.54) to 4.35 (averages of 4.26 and 4.43) (Table 3).
TABLE 3.
Fatty acid composition of E. faecalis cells
| Strain, condition | Total fatty acids (μg/g [wet wt]) | SFA/UFA ratiog | Fatty acid (%)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| C12:0 | C14:0 | C16:0 | C16:1 isomer | C16:1Δ9cis | C18:0 | C18:1 | |||
| WTa,b | 1,476 (13.3)e | 1.26 | 4.7 (11.1) | 43.1 (11.8) | 1.6 (15.4) | 4.5 (14.1) | 7.9 (9.6) | 38.0 (16.6) | |
| WT, MLc,d | 1,883 (9.4) | 49.7 (11.8) | 2.7 (6.7) | 26.6 (6.9) | 0.4 (5.5) | 1.1 (4.5) | 11.5 (8.7) | 7.9 (5.2) | |
| WT, ML | 947f | 4.26 | 5.4 | 52.8 | 0.9 | 2.3 | 22.8 | 15.8 | |
| DGRM2a,b | 1,610 (14.3) | 1.54 | 5.8 (15.4) | 47.0 (16.3) | 1.6 (14.4) | 5.5 (17.0) | 7.8 (20.7) | 32.2 (9.7) | |
| DGRM2, MLc,d | 1,845 (0.8) | 51.6 (0.9) | 3.8 (1.1) | 28.6 (0.4) | 0.5 (4.7) | 1.6 (1.4) | 7.2 (1.4) | 6.9 (2.5) | |
| DGRM2, ML | 893f | 4.43 | 7.8 | 59.0 | 1.0 | 3.3 | 14.8 | 14.2 | |
WT, wild-type E. faecalis AR01/DGVS.
Average for two cultures, two extractions per culture, duplicate FAME analyses (n = 8).
ML, grown in the presence of 50 μg/ml monolaurin.
Average for one culture, two extractions, duplicate FAME analyses (n = 4).
Values in parentheses are coefficients of variation (%).
Total fatty acids, excluding lauric acid.
Ratio of saturated fatty acids (SFA) to unsaturated fatty acids (UFA).
The fatty acid analysis of E. faecalis AR01/DGVS and DGRM2 cell membranes revealed the same pattern, i.e., no difference in profiles, irrespective of whether cells were grown in the presence or absence of monolaurin (Table 4). However, it did identify C16:0 and C18:1 as the major saturated and unsaturated fatty acids, respectively (Table 4). Addition of monolaurin to test cultures resulted in the incorporation of lauric acid into the membranes, which was accompanied by a similar decrease in C18:1 levels (Table 4). On average, lauric acid accounted for 10% of the membranes fatty acids (Table 4).
TABLE 4.
Fatty acid composition of E. faecalis cell membranes
| Fatty acid | Fatty acid composition of cell membranes (%)
|
|||
|---|---|---|---|---|
| WTa | WT, MLb | DGRM2 | DGRM2, ML | |
| C12:0 | 0.3 | 11.7 | 0.2 | 9.2 |
| C14:0 | 4.0 | 4.8 | 5.2 | 5.4 |
| C16:0 | 37.0 | 37.9 | 38.0 | 37.1 |
| C16:1 isomer | 1.8 | 0.9 | 1.9 | 1.5 |
| C16:1Δ9cis | 5.0 | 4.2 | 6.8 | 5.6 |
| C18:0 | 6.5 | 7.4 | 4.4 | 6.2 |
| C18:1n9c | 3.5 | 7.6 | 2.0 | 4.2 |
| C18:1 | 41.6 | 25.0 | 41.0 | 30.4 |
| C18:2 | 0.3 | 0.5 | 0.5 | 0.4 |
WT, wild type.
ML, grown in the presence of 50 μg/ml monolaurin.
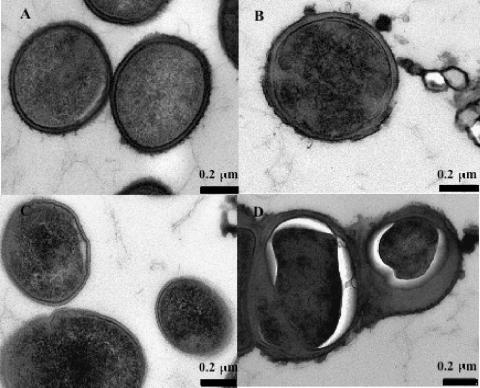
TEM showed no obvious differences between the cell walls of E. faecalis AR01/DGVS and DGRM2 grown in the absence of monolaurin (Fig. 4A and C). However, when grown in the presence of monolaurin, DGRM2 showed an apparent increase in the thickness of the cell wall (Fig. 4D). No changes were noted in the cell cytoplasm of E. faecalis AR01/DGVS; by contrast, E. faecalis DGRM2 showed contraction of its cytoplasm (Fig. 4D). E. faecalis DGRM2 cells were also observed to form small clumps of cells when grown in the presence of monolaurin (data not shown).
FIG. 4.
TEM of E. faecalis AR01/DGVS (A and B) and DGRM2 (C and D) grown either in the absence (A and C) or in the presence (B and D) of monolaurin.
DISCUSSION
Although studies on the antimicrobial properties of fatty acids and their derivatives, or surface-active anionic detergents, date back to 1899 (10), there is little information on the mechanisms of resistance to such compounds. Because of the potential spread of vancomycin-resistant enterococci from animal to humans through the food chain (4, 14, 37, 44), E. faecalis was used as a model to study bacterial resistance to monolaurin. In preliminary studies on the inhibitory effect of monolaurin on E. faecalis AR01/DGVS growth, it was observed that monolaurin induced cell lysis immediately following its addition to actively growing cells. This observation was consistent with reports that monolaurin stimulates the autolytic peptidoglycan hydrolase of Enterococcus faecium ATCC 9790 through activation of a latent proteinase (56, 57).
In order to study the potential target of and resistance mechanisms to monolaurin in E. faecalis, a library of Tn917 E. faecalis AR01/DGVS mutants was generated and screened for monolaurin resistance (MIC, >100 μg/ml). Three monolaurin-resistant mutants were isolated which were shown to have single Tn917 insertions in the same ORF. Sequence analysis of this ORF by BLAST revealed no similarity at the nucleotide level; however, the translated protein sequence showed 67% similarity to TraB of E. faecalis V583/pTEF1, a pheromone shutdown protein (11). TraB is a membrane-bound protein involved in reducing the amount of endogenous pheromone secreted by or associated with the surface of donor cells, and/or inducing secretion of the inhibitor peptide (9, 11). Analysis of the protein sequence revealed that it had a molecular mass of 26,847 Da and was composed of 238 amino acids of which 105 were hydrophobic. The Conserved Domain Database (NCBI) identified the presence of a conserved domain (COG1916) that includes three or four transmembrane loops (9). This domain, also found in other TraB (pAD1 and pPD1) proteins and PrgY-related proteins, can be found in a range of different organisms, including bacteria, archaea, plants, and mammals (9). Further sequencing of the 6.8-kb insert revealed the presence of traA, a key regulator of the pheromone response, and res97, an E. faecalis resolvase. Previous studies in our laboratory had demonstrated that both traA and traB were present on pJM01, an uncharacterized pheromone-responsive plasmid carried by E. faecalis AR01/DGVS which also harbors bacitracin and tetracycline resistance genes (36). Others have also reported traB and traA genes on pheromone-responsive plasmids in E. faecalis (2, 13, 39, 45, 60).
To confirm that monolaurin resistance was due to inactivation of the traB gene, complementation of the E. faecalis mutants DGRM2, DGRM5, and DGRM12 was carried out. In all three monolaurin-resistant mutants, sensitivity to monolaurin was restored by complementation with traB in trans, suggesting that the traB mutation alone conferred resistance to monolaurin. Since all three mutants were shown to be interrupted in traB, a single mutant, E. faecalis DGRM2, was selected for further characterization. E. faecalis mutants resistant to monolaurin also showed increased resistance to gentamicin and chloramphenicol, suggesting a lack of penetration by these compounds and thus an altered cytoplasmic membrane. This observation is supported by other studies where it was shown that dodecylglycerol (corresponding ether of monolaurin) inhibited glycerolipid and lipoteichoic acid biosynthesis in S. mutans (6).
Fatty acids and their derivatives have been reported to target the cytoplasmic membrane (17, 24-27), and it was therefore possible that the increased resistance of the traB mutant was linked to changes in the composition of the cytoplasmic membrane. However, there was no difference in the fatty acid composition (percentage of each identified fatty acid) between E. faecalis AR01/DGVS and DGRM2, irrespective of the growth conditions. We did observe a change in the fatty acid profile (ratio of saturated to unsaturated fatty acids) between AR01/DGVS and DGRM2 when they were grown in the presence of monolaurin, with the ratio of saturated to unsaturated fatty acids increasing in both cases. The increase in saturated fatty acid (C14:0, C16:0, and C18:0) and the decrease in unsaturated fatty acid (C18:1) suggest that AR01/DGVS and DGRM2 were adapting to the presence of monolaurin by attempting to make the cell membrane more rigid and therefore potentially less permeable to monolaurin. Rigidification of the membrane has been suggested as a mechanism of resistance for membrane-active compounds such as nisin (34, 38, 55). It should be noted that when the cells were grown in the presence of monolaurin, lauric acid was incorporated into the cell membranes, resulting in changes in the fatty acid profile of the E. faecalis strains. Juneja and Davidson (23) reported that growing L. monocytogenes in the presence of exogenous fatty acids resulted in the incorporation of and consequently an increase in the percentage of these particular fatty acids in the cell membrane. This suggests that the incorporation of lauric acid into the cell membrane was responsible for the fatty acid profile changes observed and that this incorporation is eventually toxic to the cells.
The cell surface of the parent strain was hydrophobic, while that of the monolaurin-resistant mutant was more hydrophilic in nature. Monolaurin, a hydrophobic compound, would be less able to penetrate a highly hydrophilic cell surface; thus, it is possible that the resistance of DGRM2 to monolaurin was a direct consequence of its low cell surface hydrophobicity. It has been reported that the cell surfaces of organic-solvent-tolerant mutants isolated from E. coli were more hydrophilic than those of their parent strain (3). Low cell surface hydrophobicity has been reported to serve as a defensive mechanism which prevents the accumulation of organic solvent molecules in the cytoplasmic membrane (3). The same change in cell surface hydrophobicity was observed in JH2-7349, a traB mutant (16). The lack of difference in cell surface hydrophobicity between JH2-2 and JH2-7013 indicated that the observed changes were not induced by the presence of Tn917. Although the AR01/DGVS and JH2-7349 traB sequences have no homology to each other or any other traB sequence, the degree of predicted amino acid similarity, the presence of the conserved COG1916 domain, and the fact that the two strains had identical patterns of hydrophobicity and permeability to antibiotics provide strong evidence that the functions of these two proteins are similar.
The possibility cannot, however, be excluded that the low cell surface hydrophobicity observed in the traB mutants is not directly linked to the inactivation of traB since JH2-2 also showed high cell surface hydrophobicity in the absence of traB (JH2-2 does not contain a copy of traB). It is possible that Tn917 inactivation of traB had a downstream effect on the expression of another protein such as the aggregation substance. Aggregation substance, a bacterial adhesin, mediates the contact between donor and recipient cells, thus facilitating plasmid conjugation (2). Various studies (21, 58) have reported that the expression of aggregation substance on the cell surface of E. faecalis cells resulted in a significant increase in cell surface hydrophobicity. The role of TraB is to shut down the pheromone response and/or to prevent self-induction, which results in part in the shutting down of aggregation substance production (2). Waters et al. (59) have shown that a domain of the aggregation substance was binding directly to the lipoteichoic acid of the cell wall of E. faecalis. Thus, decreased lipoteichoic acid production in the presence of monolaurin (6) would result in lower levels of aggregation substance at the cell wall of AR01/DGVS, resulting in low cell surface hydrophobicity when the bacteria are grown in the presence of monolaurin. This remains to be further investigated.
There was no significant difference between the cell wall structure of E. faecalis AR01/DGVS and that of DGRM2 viewed by TEM when bacteria were grown in the presence or absence of monolaurin. However, in the presence of monolaurin, the cytoplasm of E. faecalis DGRM2 seemed to have shrunk drastically and the space between the cell membrane and cell wall markedly increased. The effects of monolaurin on E. faecalis DGRM2 cell morphology could be explained by decreased cell surface permeability preventing proper ethanol fixation. This correlates with the findings by Aono and Kobayashi (3), who reported that low cell surface hydrophobicity prevented the access of organic solvent to the cytoplasmic membrane.
In conclusion, monolaurin-resistant mutants of E. faecalis were isolated by transposon mutagenesis. The monolaurin-resistant mutants were disrupted in the pheromone shutdown protein TraB. The characterization of one of these monolaurin-resistant mutants, E. faecalis DGRM2, suggests that its resistance is linked to a decrease in cell surface hydrophobicity that limits the diffusion of monolaurin into the cell. This, in turn, suggests that monolaurin requires access to the cytoplasmic membrane and/or the cytoplasm to exert its antimicrobial activity.
Acknowledgments
This work was supported by a grant from Tatua Cooperative Dairy Company Ltd. and the Foundation for Research, Science, and Technology.
We thank Michelle Leus (Department of Food Science, University of Otago) for the preparation of samples for GC analysis, Richard Easingwood (Department of Anatomy and Structural Biology, University of Otago) for the preparation of samples for TEM analysis, and Shuhei Fujimoto (Department of Microbiology, Graduate School of Medicine, Gunma University, Japan) for providing the pPD1::Tn917 derivatives.
Footnotes
Published ahead of print on 13 July 2007.
REFERENCES
- 1.Aarestrup, F. M., P. Butaye, and W. Witte. 2002. Nonhuman reservoirs of enterococci, p. 55-100. In M. S. Gilmore (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, DC.
- 2.An, F. Y., and D. B. Clewell. 1994. Characterisation of the determinant (traB) encoding sex pheromone shutdown by the haemolysin/bacteriocin plasmid pAD1 in Enterococcus faecalis. Plasmid 31:215-221. [DOI] [PubMed] [Google Scholar]
- 3.Aono, R., and H. Kobayashi. 1997. Cell surface properties of organic solvent-tolerant mutants of Escherichia coli K-12. Appl. Environ. Microbiol. 63:3637-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batesa, J., J. Z. Jordensa, and D. T. Griffiths. 1994. Farm animals as a putative reservoir for vancomycin-resistant enterococcal infection in man. J. Antimicrob. Chemother. 34:507-514. [DOI] [PubMed] [Google Scholar]
- 5.Branen, J. K., and P. M. Davidson. 2004. Enhancement of nisin, lysozyme, and monolaurin antimicrobial activities by ethylenediaminetetraacetic acid and lactoferrin. Int. J. Food Microbiol. 90:63-74. [DOI] [PubMed] [Google Scholar]
- 6.Brissette, J. L., E. A. Cabacungan, and R. A. Pieringer. 1986. Studies on the antibacterial activity of dodecylglycerol. Its limited metabolism and inhibition of glycerolipid and lipoteichoic acid biosynthesis in Streptococcus mutans BHT. J. Biol. Chem. 261:6338-6345. [PubMed] [Google Scholar]
- 7.Casadaban, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138:179-207. [DOI] [PubMed] [Google Scholar]
- 8.Catsara, M., J. P. Danjoy, and R. Seynave. 1987. Temperature and action of LauriLac on multiplication of Salmonella in minced meat. Bull. Acad. Vet. Fr. 60:359. [Google Scholar]
- 9.Chandler, J. R., A. R. Flynn, E. M. Bryan, and G. M. Dunny. 2005. Specific control of endogenous cCF10 pheromone by a conserved domain of the pCF10-encoded regulatory protein PrgY in Enterococcus faecalis. J. Bacteriol. 187:4830-4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark, J. R. 1899. On the toxic effect of deleterious agents on the germination and development of certain filamentous fungi. Bot. Gaz. 28:289. [Google Scholar]
- 11.Clewell, D. B., and G. M. Dunny. 2002. Conjugation and genetic exchange in enterococci, p. 265-300. In M. S. Gilmore (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, DC.
- 12.de Jonge, B. L. M., H. de Lencastre, and A. Tomas. 1991. Suppression of autolysis and cell wall turnover in heterogeneous Tn551 mutants of a methicillin-resistant Staphylococcus aureus strain. J. Bacteriol. 173:1105- 1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunny, G., C. Funk, and J. Adsit. 1981. Direct stimulation of the transfer of antibiotic resistance by sex pheromones in Streptococcus faecalis. Plasmid 6:270-278. [DOI] [PubMed] [Google Scholar]
- 14.Franz, C. M. A. P., W. H. Holzapfel, and M. E. Stiles. 1999. Enterococci at the crossroads of food safety. Int. J. Food Microbiol. 47:1-24. [DOI] [PubMed] [Google Scholar]
- 15.Franz, C. M., A. B. Muscholl-Silberhorn, N. M. Yousif, M. Vancanneyt, J. Swings, and W. H. Holzapfel. 2001. Incidence of virulence factors and antibiotic resistance among enterococci isolated from food. Appl. Environ. Microbiol. 67:4385-4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimoto, S., H. Tomita, E. Wakamatsu, K. Tanimoto, and Y. Ike. 1995. Physical mapping of the conjugative bacteriocin plasmid pPD1 of Enterococcus faecalis and identification of the determinant related to the pheromone response. J. Bacteriol. 177:5574-5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galbraith, H., and T. B. Miller. 1973. Effect of long chain fatty acids on bacterial respiration and amino acid uptake. J. Appl. Bacteriol. 36:659-675. [DOI] [PubMed] [Google Scholar]
- 18.Gambarotto, K., M.-C. Ploy, F. Dupron, M. Giangiobbe, and F. Denis. 2001. Occurrence of vancomycin-resistant enterococci in pork and poultry products from a cattle-rearing area of France. J. Clin. Microbiol. 39:2354-2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutierrez, J. A., P. J. Crowley, D. P. Brown, J. D. Hillman, P. Youngman, and A. S. Bleiweis. 1996. Insertional mutagenesis and recovery of interrupted genes of Streptococcus mutans by using transposon Tn917: preliminary characterisation of mutants displaying acid sensitivity and nutritional requirements. J. Bacteriol. 178:4166-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heuer, O. E., A. M. Hammerum, P. Collignon, and H. C. Wegener. 2006. Human health hazard from antimicrobial-resistant enterococci in animals and food. Clin. Infect. Dis. 43:911-916. [DOI] [PubMed] [Google Scholar]
- 21.Hirt, H., S. L. Erlandsen, and G. M. Dunny. 2000. Heterologous inducible expression of Enterococcus faecalis pCF10 aggregation substance asc10 in Lactococcus lactis and Streptococcus gordonii contributes to cell hydrophobicity and adhesion to fibrin. J. Bacteriol. 182:2299-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juneja, V. K., and P. M. Davidson. 1993. Influence of altered fatty acid composition on resistance of Listeria monocytogenes to antimicrobials. J. Food Prot. 50:302-305. [DOI] [PubMed] [Google Scholar]
- 24.Kabara, J. J. 1978. Fatty acids and derivatives as antimicrobial agents—a review, p. 1-14. In J. J. Kabara (ed.), Symposium on the pharmacological effect of lipids. The American Oil Chemists' Society, Champaign, IL.
- 25.Kabara, J. J. 1993. Medium-chain fatty acids and esters, p. 307-342. In P. M. Davidson and A. H. Branen (ed.), Antimicrobials in foods. Academic Press, Inc., New York, NY.
- 26.Kabara, J. J., D. M. Swieczkowski, A. J. Conley, and J. P. Truant. 1972. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 2:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kabara, J. J., R. Vrable, and M. S. F. Lie Ken Jie. 1977. Antimicrobial lipids: natural and synthetic fatty acids and monoglycerides. Lipids 12:753-759. [DOI] [PubMed] [Google Scholar]
- 28.Kalra, M. S., G. Kaur, A. Singh, and R. S. Kahlon. 1987. Studies on Streptococcus faecalis enterotoxin. Acta Microbiol. Pol. 36:83-92. [PubMed] [Google Scholar]
- 29.Kato, N., and I. Shibasaki. 1976. Combined effect of citric and polyphosphoric acid on the antibacterial activity of monoglycerides. J. Antibact. Antifung. Agents 4:254-261. [Google Scholar]
- 30.Kato, N., and I. Shibasaki. 1975. Enhancing effect of fatty acids and their esters on the thermal destruction of E. coli and Pseudomonas aeruginosa. J. Ferment. Technol. 53:802. [Google Scholar]
- 31.Keis, S., C. F. Bennett, V. K. Ward, and D. T. Jones. 1995. Taxonomy and phylogeny of industrial solvent-producing clostridia. Int. J. Syst. Bacteriol. 45:693-705. [DOI] [PubMed] [Google Scholar]
- 32.Lee, E. H., and W. M. Shafer. 1999. The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol. Microbiol. 33:839-845. [DOI] [PubMed] [Google Scholar]
- 33.Lerner, C. G., and M. Inouye. 1990. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 18:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loughlin, M. F., M. V. Jones, and P. A. Lambert. 2002. Pseudomonas aeruginosa cells adapted to benzalkonium chloride show resistance to other membrane-active agents but not to clinically relevant antibiotics. J. Antimicrob. Chemother. 49:631-639. [DOI] [PubMed] [Google Scholar]
- 35.Manson, J. M., S. Keis, J. M. B. Smith, and G. M. Cook. 2003. A clonal lineage of VanA-type Enterococcus faecalis predominates in vancomycin-resistant enterococci isolated in New Zealand. Antimicrob. Agents Chemother. 47:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manson, J. M., S. Keis, J. M. B. Smith, and G. M. Cook. 2004. Acquired bacitracin resistance in Enterococcus faecalis is mediated by an ABC transporter and a novel regulatory protein, BcrR. Antimicrob. Agents Chemother. 48:3743-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Messi, P., E. Guerrieri, S. de Niederhäusern, C. Sabia, and M. Bondi. 2006. Vancomycin-resistant enterococci (VRE) in meat and environmental samples. Int. J. Food Microbiol. 107:218-222. [DOI] [PubMed] [Google Scholar]
- 38.Ming, X., and M. A. Daeschel. 1993. Nisin resistance of foodborne bacteria and the specific resistance responses of Listeria monocytogenes. J. Food Prot. 56:944-948. [DOI] [PubMed] [Google Scholar]
- 39.Nakayama, J., K. Yoshida, H. Kobayashi, A. Isogal, D. B. Clewell, and A. Suzuki. 1995. Cloning and characterisation of a region of Enterococcus faecalis plasmid pPD1 encoding pheromone inhibitor (ipd), pheromone sensitivity (traC), and pheromone shutdown (traB) genes. J. Bacteriol. 177:5567-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pooley, H. M., and G. D. Shockman. 1969. Relationship between the latent form and the active form of the autolytic enzyme of Streptococcus faecalis. J. Bacteriol. 100:617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Projan, S. J., S. Brown-Skrobot, P. M. Schlievert, F. Vandenesch, and R. P. Novick. 1994. Glycerol monolaurate inhibits the production of beta-lactamase, toxic shock syndrome toxin-1, and other staphylococcal exoproteins by interfering with signal transduction. J. Bacteriol. 176:4204-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reifsteck, F., S. Wee, and B. J. Wilkinson. 1987. Hydrophobicity-hydrophilicity of staphylococci. J. Med. Microbiol. 24:65-73. [DOI] [PubMed] [Google Scholar]
- 43.Robach, M. C., C. Hickey, and E. C. To. 1981. Comparison of antimicrobial actions of monolaurin and sorbic acid. J. Food Safety 3:89-98. [Google Scholar]
- 44.Robredo, B., K. V. Singh, F. Baquero, B. E. Murray, and C. Torres. 2000. Vancomycin-resistant enterococci isolated from animals and food. Int. J. Food Microbiol. 54:197-204. [DOI] [PubMed] [Google Scholar]
- 45.Ruhfel, R. E., D. A. Manias, and G. M. Dunny. 1993. Cloning and characterisation of a region of the Enterococcus faecalis conjugative plasmid, pCF10, encoding a sex pheromone-binding function. J. Bacteriol. 175:5253-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruzin, A., and R. P. Novick. 1998. Glycerol monolaurate inhibits induction of vancomycin resistance in Enterococcus faecalis. J. Bacteriol. 180:182-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 48.Shepard, B. D., and M. S. Gilmore. 1995. Electroporation and efficient transformation of Enterococcus faecalis grown in high concentrations of glycine. Methods Mol. Biol. 47:217-226. [DOI] [PubMed] [Google Scholar]
- 49.Sheu, C. W., and E. Freeze. 1973. Lipopolysaccharide layer protection of gram-negative bacteria against inhibition by long-chain fatty acids. J. Bacteriol. 115:869-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shibasaki, I. 1982. Recent trends in the development of food preservatives. J. Food Safety 4:35-78. [Google Scholar]
- 51.Shibasaki, I., and N. Kato. 1978. Combined effects on antibacterial activity of fatty acids and their esters against gram-negative bacteria, p. 1-24. In J. J. Kabara (ed.), Pharmacological effect of lipids. American Oil Chemists Society, Champaign, IL.
- 52.Simonsen, G. S., H. Haaheim, K. H. Dahl, H. Kruse, A. Lovseth, O. Olsvik, and A. Sundsfjord. 1998. Transmission of VanA-type vancomycin-resistant enterococci and vanA resistance elements between chicken and humans at avoparcin-exposed farms. Microb. Drug Resist. 4:313-318. [DOI] [PubMed] [Google Scholar]
- 53.Smith, J. L., and S. A. Palumbo. 1980. Inhibition of aerobic and anaerobic growth of Staphylococcus aureus in a model sausage system. J. Food Safety 2:221-233. [Google Scholar]
- 54.Takano, M., A. B. Symbol, M. Yasin, and I. Shibasaki. 1979. Bactericidal effect of freezing with chemical agents. J. Food Sci. 44:112-115. [Google Scholar]
- 55.To, M. S., S. Favrin, N. Romanova, and M. W. Griffiths. 2002. Postadaptational resistance to benzalkonium chloride and subsequent physicochemical modifications of Listeria monocytogenes. Appl. Environ. Microbiol. 68:5258-5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ved, H. S., E. Gustow, and R. A. Pieringer. 1984. The involvement of the proteinase of Streptococcus faecium ATCC 9790 in the stimulation of its autolysin activity by dodecylglycerol. J. Biol. Chem. 259:8122-8124. [PubMed] [Google Scholar]
- 57.Ved, H. S., E. Gustow, V. Mahadevan, and R. A. Pieringer. 1984. Dodecylglycerol. A new type of antibacterial agent which stimulates autolysin activity in Streptococcus faecium ATCC 9790. J. Biol. Chem. 259:8115-8121. [PubMed] [Google Scholar]
- 58.Waters, C. M., and G. M. Dunny. 2001. Analysis of functional domains of the Enterococcus faecalis pheromone-induced surface protein aggregation substance. J. Bacteriol. 183:5659-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waters, C. M., H. Hirt, J. K. McCormick, P. M. Schlievert, C. L. Wells, and G. M. Dunny. 2004. An amino-terminal domain of Enterococcus faecalis aggregation substance is required for aggregation, bacterial internalization by epithelial cells and binding to lipoteichoic acid. Mol. Microbiol. 52:1159-1171. [DOI] [PubMed] [Google Scholar]
- 60.Weaver, K. E., and D. B. Clewell. 1990. Regulation of the pAD1 sex pheromone response in Enterococcus faecalis: effects of host strain and traA, traB, and C region mutants in expression of an E region pheromone-inducible lacZ fusion. J. Bacteriol. 172:2633-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wirth, R., F. Y. An, and D. B. Clewell. 1986. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J. Bacteriol. 165:831-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Witcher, K. J., R. P. Novick, and P. M. Schlievert. 1996. Modulation of immune cell proliferation by glycerol monolaurate. Clin. Diagn. Lab. Immunol. 3:10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]