Abstract
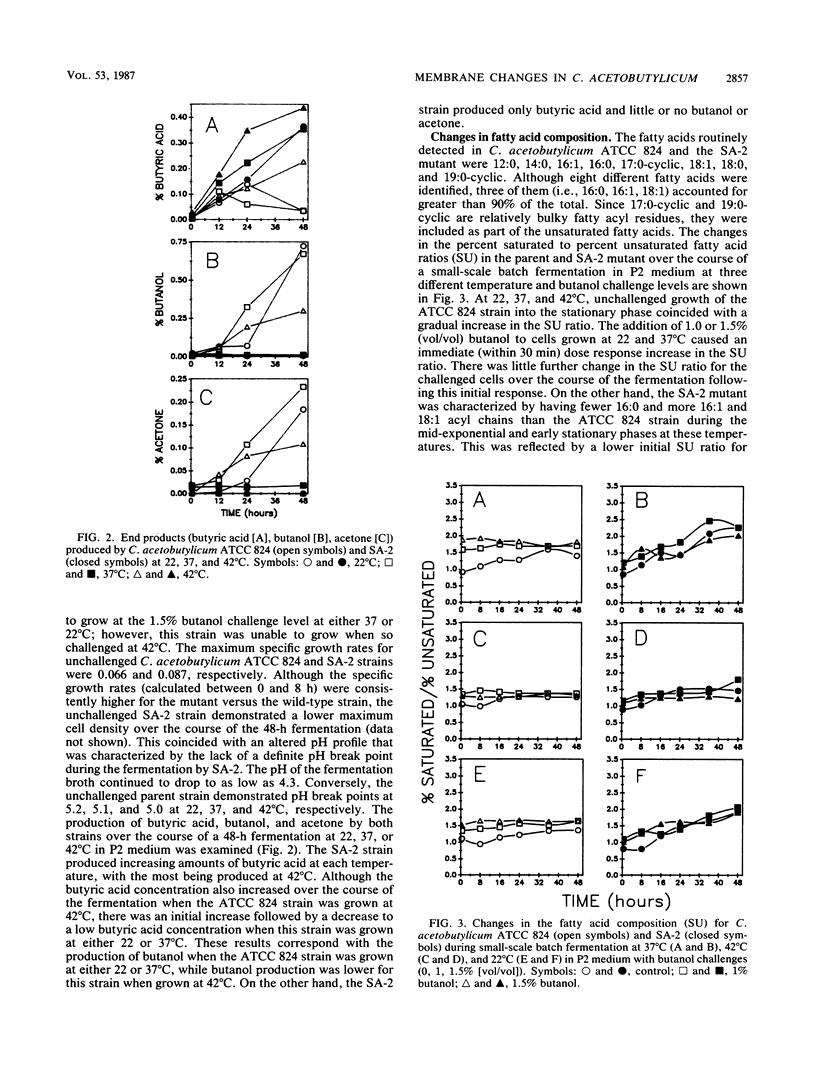
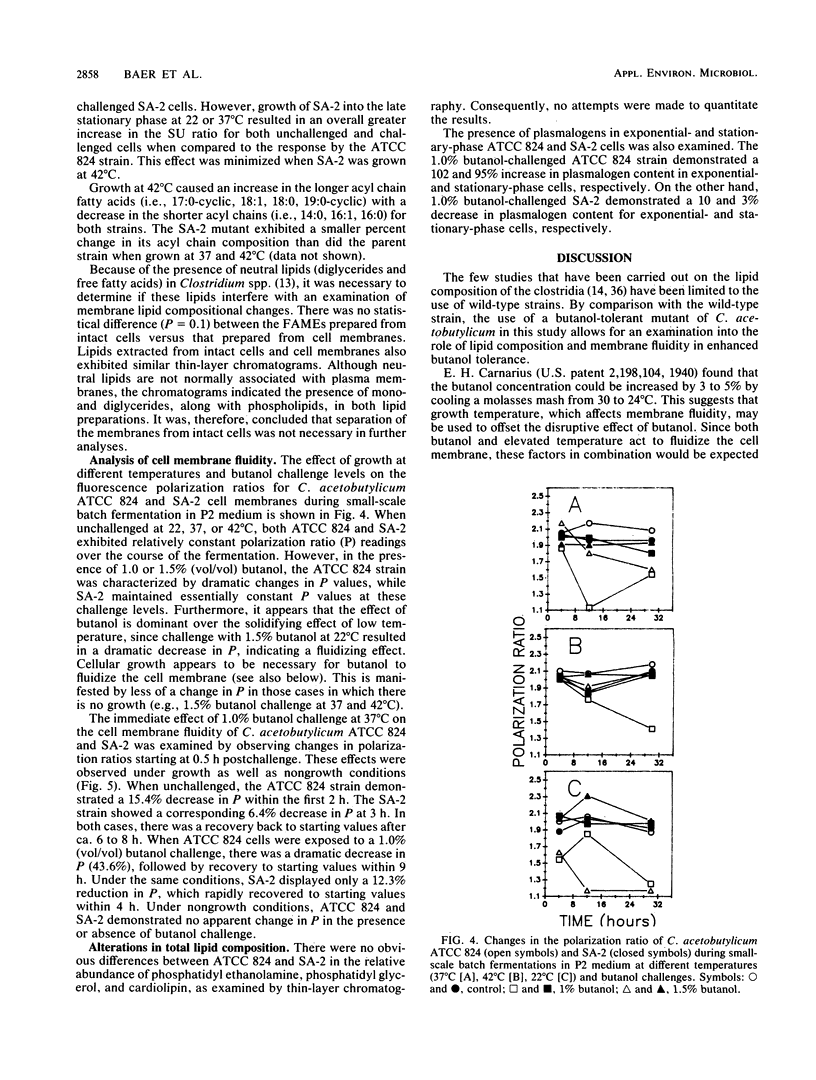
The effect of butanol challenge (0, 1.0, 1.5% [vol/vol]) and growth temperature (22, 37, 42°C) on the membrane composition and fluidity of Clostridium acetobutylicum ATCC 824 and a butanol-tolerant mutant, SA-2, was examined in chemically defined medium. Growth of strain ATCC 824 into the stationary phase coincided with a gradual increase in the percent saturated to percent unsaturated (SU) fatty acid ratio. When challenged with butanol at 22 and 37°C, ATCC 824 demonstrated an immediate (within 30 min) dose-response increase in the SU ratio. This strain showed little additional change over a 48-h fermentation. Compared with ATCC 824, growth of SA-2 into the late stationary phase at 22 or 37°C resulted in an overall greater increase in the SU ratio for both unchallenged and challenged cells. This effect was minimized when SA-2 was challenged at 42°C, probably due to the combination of the membrane fluidizing effect of butanol and the elevated temperature. Growth at 42°C resulted in an increase in longer acyl chain fatty acids at the expense of shorter acyl chains for both strains. The membrane fluidity exhibited by SA-2 remained essentially constant at various butanol challenge and temperature combinations, while that for the ATCC 824 strain increased with increasing butanol challenge. By synthesizing an increased amount of saturated fatty acids, the butanol-tolerant SA-2 strain has apparently developed a mechanism for maintaining a more stable membrane environment. Growth of the microorganism is necessary for butanol to fluidize the membrane. Incorporation of exogenous fatty acids (18:1) did not significantly improve the butanol tolerance of either strain. Since SA-2 was able to produce only trace amounts of either butanol or acetone, increased tolerance to butanol does not necessarily coincide with greater solvent yields in this strain.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BROQUIST H. P., SNELL E. E. Biotin and bacterial growth. I. Relation to aspartate, oleate, and carbon dioxide. J Biol Chem. 1951 Jan;188(1):431–444. [PubMed] [Google Scholar]
- CHEN R. F., BOWMAN R. L. FLUORESCENCE POLARIZATION: MEASUREMENT WITH ULTRAVIOLET-POLARIZING FILTERS IN A SPECTROPHOTOFLUOROMETER. Science. 1965 Feb 12;147(3659):729–732. doi: 10.1126/science.147.3659.729. [DOI] [PubMed] [Google Scholar]
- FINN R. K., NOWREY J. E. A note on the stability of clostridia when held in continuous culture. Appl Microbiol. 1959 Jan;7(1):29–32. doi: 10.1128/am.7.1.29-32.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwin J. L., Cronan J. E., Jr Thermal modulation of fatty acid synthesis in Escherichia coli does not involve de novo enzyme synthesis. J Bacteriol. 1980 Mar;141(3):1457–1459. doi: 10.1128/jb.141.3.1457-1459.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero A. A., Gomez R. F., Roberts M. F. Ethanol-induced changes in the membrane lipid composition of Clostridium thermocellum. Biochim Biophys Acta. 1982 Dec 8;693(1):195–204. doi: 10.1016/0005-2736(82)90487-4. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Buttke T. M. Effects of alcohols on micro-organisms. Adv Microb Physiol. 1984;25:253–300. doi: 10.1016/s0065-2911(08)60294-5. [DOI] [PubMed] [Google Scholar]
- Johnston N. C., Goldfine H. Lipid composition in the classification of the butyric acid-producing clostridia. J Gen Microbiol. 1983 Apr;129(4):1075–1081. doi: 10.1099/00221287-129-4-1075. [DOI] [PubMed] [Google Scholar]
- KUTZENOK A., ASCHNER M. Degenerative processes in a strain of Clostridium butylicum. J Bacteriol. 1952 Dec;64(6):829–836. doi: 10.1128/jb.64.6.829-836.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuller G. K., Goldfine H. Phospholipids of Clostridium butyricum. V. Effects of growth temperature on fatty acid, alk-1-enyl ether group, and phospholipid composition. J Lipid Res. 1974 Sep;15(5):500–507. [PubMed] [Google Scholar]
- Khuller G. K., Goldfine H. Replacement of acyl and alk-1-enyl groups in Clostridium butyricum phospholipids by exogenous fatty acids. Biochemistry. 1975 Aug 12;14(16):3642–3647. doi: 10.1021/bi00687a020. [DOI] [PubMed] [Google Scholar]
- Lenaz G., Bertoli E., Curatola G., Mazzanti L., Bigi A. Lipid protein interactions in mitochondria. Spin and fluorescence probe studies on the effect of n-alkanols on phospholipid vesicles and mitochondrial membranes. Arch Biochem Biophys. 1976 Jan;172(1):278–288. doi: 10.1016/0003-9861(76)90077-1. [DOI] [PubMed] [Google Scholar]
- Lentz B. R., Moore B. M., Barrow D. A. Light-scattering effects in the measurement of membrane microviscosity with diphenylhexatriene. Biophys J. 1979 Mar;25(3):489–494. doi: 10.1016/S0006-3495(79)85318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Blaschek H. P. Butanol Production by a Butanol-Tolerant Strain of Clostridium acetobutylicum in Extruded Corn Broth. Appl Environ Microbiol. 1983 Mar;45(3):966–973. doi: 10.1128/aem.45.3.966-973.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfouz M. M., Smith T. L., Kummerow F. A. Effect of dietary fats on desaturase activities and the biosynthesis of fatty acids in rat-liver microsomes. Lipids. 1984 Mar;19(3):214–222. doi: 10.1007/BF02534800. [DOI] [PubMed] [Google Scholar]
- Martin C. E., Thompson G. A., Jr Use of fluorescence polarization to monitor intracellular membrane changes during temperature acclimation. Correlation with lipid compositional and ultrastructural changes. Biochemistry. 1978 Aug 22;17(17):3581–3586. doi: 10.1021/bi00610a025. [DOI] [PubMed] [Google Scholar]
- Overath P., Träuble H. Phase transitions in cells, membranes, and lipids of Escherichia coli. Detection by fluorescent probes, light scattering, and dilatometry. Biochemistry. 1973 Jul 3;12(14):2625–2634. doi: 10.1021/bi00738a012. [DOI] [PubMed] [Google Scholar]
- Pang K. Y., Chang T. L., Miller K. W. On the coupling between anesthetic induced membrane fluidization and cation permeability in lipid vesicles. Mol Pharmacol. 1979 May;15(3):729–738. [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as fluorescent probes: synthetic phospholipid membrane studies. Biochemistry. 1977 Mar 8;16(5):819–828. doi: 10.1021/bi00624a002. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Miljanich G. P., Dratz E. A. Phospholipid lateral phase separation and the partition of cis-parinaric acid and trans-parinaric acid among aqueous, solid lipid, and fluid lipid phases. Biochemistry. 1979 May 1;18(9):1707–1716. doi: 10.1021/bi00576a012. [DOI] [PubMed] [Google Scholar]
- Stewart J. C. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980 May 1;104(1):10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- Vollherbst-Schneck K., Sands J. A., Montenecourt B. S. Effect of butanol on lipid composition and fluidity of Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol. 1984 Jan;47(1):193–194. doi: 10.1128/aem.47.1.193-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


