Abstract
Male rats received normal chow or high-fat diets rich in dextrose (HFD) or sucrose (HFS). Half of the rats received 90-day unrestricted access to their diet prior to training, whereas the other half were food restricted throughout the study. We evaluated the effects of these dietary manipulations on discrimination and reversal performance and on post-training levels of brain-derived neurotrophic factor (BDNF) in the prefrontal cortex and the ventral and dorsal hippocampus. Neither diet nor restriction condition affected discrimination acquisition. However, prior unrestricted access to the HFD diet impaired discrimination reversal learning and reduced BDNF in the prefrontal cortex and ventral hippocampus. Also, rats given the HFD diet responded more than controls to the previously rewarded cue at the outset of discrimination reversal. The results suggest that consumption of the HFD diet may have had enduring effects on learning processes, some of which may contribute to the control of intake regulation.
Keywords: diet, macronutrient, rat, memory, inhibition, obesity, dextrose
1. Introduction
The typical diet in modern industrialized western societies is energy-rich containing high level of saturated fats and processed sugars Chronic consumption of a this type of diet has long been associated with a variety of heath problems including high blood pressure, Type II diabetes, cardiovascular disease [1]. Recent research also suggests that these diets may have detrimental consequences on cognitive ability. For instance, in humans, a diet high in saturated fat is considered a significant risk factor for the development of Alzheimer's disease and dementia [2-5]. Furthermore, cognitive impairments have also been associated with the effects of certain high-carbohydrate diets on glucoregulation [6, 7].
Rodents maintained on energy-rich diets containing high levels of saturated fat also show impaired learning and memory performance [8-10]. Recent evidence indicates that such deficits may be related to the effects of these diets on the regulation of brain-derived neurotrophic factor (BDNF) in the hippocampus [11-13], a brain area that has long been implicated as a substrate for learning and memory [14].
BDNF plays an important role in the survival, maintenance and growth of many types of neurons [15, 16] and is expressed abundantly in the hippocampus, hypothalamus, and cerebral cortex. Recent findings have linked reductions in BDNF levels to interference with long-term potentiation (LTP) and neurogenesis [17-19]—two processes that have been described as potential cellular mechanisms for hippocampal-dependent forms of learning and memory [20, 21]. Reduced hippocampal BDNF has also been linked to dietary factors. For example, rats exposed for 60 days to a high-fat diet mixed with sucrose (referred to hereafter as an HFS diet) show impaired hippocampal-dependent spatial learning on the Morris water maze, weakened hippocampal LTP, and lower levels of hippocampal BDNF compared to control rats given normal lab chow [e.g., 11]. These results suggest that a reduction in BDNF produced by intake of the HFS diet may impair learning and memory by interfering with hippocampal functioning.
The present study examined further the effects of diets high in saturated fat and sugar on learning and memory performance and on BDNF levels in the brain. Rather than assessing spatial memory, we studied the effects of these diets on performance in a nonspatial Pavlovian discrimination and reversal learning problem. In this problem, animals are first trained on a simple discrimination where presentation of one conditioned stimulus (CS +) is followed by the delivery of a food unconditioned stimulus (US), and presentation of a different CS (CS−) is not followed by a US. When asymptotic discrimination performance is achieved, the discriminative contingencies are reversed so that US delivery is now signaled by the former CS− and not by the former CS+.
The results of lesion studies show that damage to the hippocampus or to the prefrontal cortex has little effect on acquisition of simple discrimination, but that both types of lesions retard learning when the discriminative contingencies have been reversed [22-27]. If chronic intake of diets high in saturated fat and sugar also impair discrimination reversal performance it would be of interest to know if this impairment is related to levels of hippocampal and prefrontocortical BDNF. The effects of chronic intake of these diets on BDNF in the prefrontal cortex have not been reported previously.
Furthermore, recent evidence points to functional differentiation along the hippocampal dorsal-ventral axis with the dorsal segment more involved with spatial information processing and the ventral segment playing a greater role in processes involved with motivation, reward, and extinction [28-30]. Based on some accounts [31], nonspatial discrimination reversal performance is more likely to depend on the structural integrity of the ventral compared to dorsal hippocampus. Previous reports have not distinguished between ventral and dorsal loci when assessing the effects of diets in saturated fat and sugar on hippocampal BDNF. Thus, one expectation might be that if these diets impair discrimination reversal performance reductions in BDNF levels should be observed in the ventral hippocampus.
The present experiment also compared discrimination and reversal performance for rats maintained on diets that were high in saturated fat and sucrose (HFS), high in saturated fat and dextrose (HFD) or a normal low-fat lab chow diet. Compared to HFS diets, HFD diets are more potent stimulators of insulin release [e.g., 32]. Therefore, prolonged intake of the HFD diet is more likely than an HFS to produce insulin resistance and hyperglycemia—conditions that have been associated with cognitive deficits in rats [33] and humans [34]. This suggests that the HFD diet might have a greater detrimental effect of discrimination-reversal performance compared to the HFS diet. Alternatively, the effects of HFD and HFS diets should be the same, if learning and memory functioning depends only on the amount of saturated fat in the diet.
Each of the three diet groups were further subdivided into restricted and unrestricted access conditions. The main purpose of this manipulation was to try to determine if the effects of HFS and HFD diets on discrimination and reversal learning performance and BDNF levels were dependent on body weight. Rats fed high-fat diets gain considerably more body weight than controls fed regular lab chow. Similar weight differences were also expected to be shown in the present study by rats given the unrestricted HFS and HFD diets compared to their unrestricted chow controls. Under these conditions it would be difficult to determine if any effects learning or BDNF levels are direct results of the dietary manipulation per se or are an indirect consequence of the excess weight gain that is produced by consuming an high-fat diet relative to chow.
For the rats in the restricted access conditions the HFS, HFD, and Chow diets were rationed so that all rats weighed the same (85% of the preexperimental ad libitum body weight) throughout the study. Thus, if a difference among these groups in learning or BDNF levels is obtained, this effect could not be based on differences in body weight. In contrast, rats in the unrestricted access condition were given unlimited access to their designated diet for 90 days. If subsequent performance during discrimination and/or reversal training is impaired relative to chow controls for rats given unrestricted access to HFD and HFS diets, but not for rats given restricted access to their diets, this outcome could be based either on differences in body weight or on the amount of HFD and HFS diets that the rats consumed.
2. Materials and Methods
2.1. Subjects
The subjects were 48 naïve, male Sprague-Dawley albino rats (Harlan Sprague Dawley, Indianapolis, IN). The rats were about 90 days of age upon arrival in the laboratory. Subjects were housed individually in stainless steel cages and maintained on a 12- hr light/dark cycle with lights on at 0700 h. All procedures for the care and treatment of the rats during this experiment were approved by the Purdue Animal Care and Use Committee.
2.2. Diets
A diet high in saturated fat with dextrose as the primary carbohydrate source (HFD diet) (Harlan Teklad, TD.04489) contained the following ingredients (g/kg): 270 g casein, 220.5 g dextrose, 200 g cornstarch, 50 g cellulose, 170 g lard, 15 g safflower oil, 15 g soybean oil. This diet had a caloric density of 4.5 kcal/g, and contained the following percentages of energy from the three macronutrient classes: 38% kcal from carbohydrate, 21% kcal from protein, and 40% kcal from fat. A diet high in saturated fat with sucrose as the primary carbohydrate source (HFS diet) (Harlan Teklad, TD 04490) was identical to the HF diet with the exception that sucrose was substituted for dextrose as the primary carbohydrate source. A standard laboratory rodent chow diet (Purina formula 5001) was used for the control diet. This control diet had a caloric density of 3.0 kcal/g, and contained the following percentages of energy from the three macronutrient classes: 59% kcal from carbohydrates, 28% kcal from protein, and 12% kcal from fat. All three test diets (HFD, HFS, and control) were in powdered form. The diets were presented in glass jars that were fastened inside of the home cage of each rat.
2.3. Apparatus
The training procedures were conducted in eight identical conditioning chambers, constructed of aluminum end walls and clear Plexiglas side walls, measuring 21.6 × 21.6 × 27.9 cm. The floors of each conditioning chamber consisted of stainless steel bars spaced 1.9 cm apart, measuring 0.48 cm in diameter. A recessed food magazine was located in the center of one end wall of each chamber.
The auditory stimuli were produced by a Radio Shack 2800 Hz Piezo Alerting Buzzer (Cat. No. 273-068) located outside the conditioning chamber near the end wall with the food magazine. The buzzer produced either a 5 second tone (CS1) or a 5 second clicker noise (CS2) as conditioned stimuli. A computer controlled infrared monitoring system with two photo beams was used to record food magazine approach and entries. One infrared photo transmitter and one receiver were located on each side wall, approximately 1.27 cm from the end wall containing the food cup; the other transmitter and receptor were located inside the food cup. Responding to conditioned stimuli (CSs) were calculated as the percentage of time the photobeam located inside the food cup was interrupted during the occurrence of a 5 second CS (CS period), minus the percentage of time the photobeams were interrupted during the 5 second period prior to the occurrence of a CS (pre-CS period).
2.4. Pretraining Procedures
Upon arrival in the laboratory, subjects were assigned to one of six groups based on body weight: Group HFS (high fat and high sucrose diet) unrestricted, Group HFS restricted, Group HFD (high fat, high dextrose diet) unrestricted, Group HFD restricted, Chow (standard laboratory chow diet) unrestricted, and Chow restricted. The rats were all given ad libitum access to standard laboratory rodent chow for a 7 day period after arrival to the laboratory. The three “restricted” groups were maintained at 85% of an ad libitum body weight that was established after this 7 day period. All subjects were then given their respective diets following this 7 day assimilation period. The subjects were weighed daily and received food (ad libitum or restricted) and water ad libitum for a period of 90 days. The time period of 90 days was chosen because previous studies have shown cognitive impairments in nonspatial tasks following 3 months exposure to a diet high in saturated fatty acids [9, 10]. One of the animals became sick during this 90 day period and was removed from the study. After 90 days, the subjects in the “ad lib” groups were reduced to 85% of an ad libitum body weight that was established at the end of the 90 day period, and remained at 85% body weight throughout behavioral training and prior to decapitation.
2.5. Discrimination Training Procedures
After all of the subjects were reduced to 85% of their free-feeding weight at the end of the 90 day period, they were given magazine training to habituate them to the apparatus. During magazine training, the rats were placed in the apparatus in six squads of eight rats, with each rat in a squad assigned to a different conditioning box. Assignment to squads and to conditioning boxes was counterbalanced with respect to the 6 experimental groups. All rats received 10 presentations of two sucrose pellets on a fixed-time 60 sec schedule. The rats remained in the apparatus for five additional minutes after the last 2 pellets had been presented. Additional magazine training sessions were held until all of the rats ate the pellets from the magazine. No conditioned stimuli (CSs) were presented during magazine training.
2.6. Procedure for Discrimination Reversal and Latent Discrimination Reversal
After magazine training, all subjects were trained on the discrimination reversal learning task. Training consisted of daily 30 minute sessions, using the same squad and conditioning box assignments as magazine training. Each session consisted of four trials: two presentations of a five- second tone stimulus (CS 1) and two presentations of a five- second clicker stimulus (CS 2). The order of stimulus presentations was randomly generated before each training session. The inter-trial interval was also randomly generated before each session with an average of 445 seconds. For the rats in the first and third squads, CS 1 (tone) was followed by a food reward (US) of two sucrose pellets (45 mg), and CS 2 (clicker) was not followed by the food US. The rats in the second and fourth squads received the opposite contingency (CS 2 rewarded, CS 1 not rewarded).
After asymptotic performance was reached on this discrimination task (16 sessions), the stimulus-reward contingencies were reversed for all six squads. Training under the reversed stimulus-reward contingencies continued for 16 training sessions. At this point, the rats had not exhibited clear reversal of responding (greater responding to current rewarded vs. nonrewarded stimulus). A “latent discrimination” procedure was then employed. Latent discrimination was conducted using the same procedures as discrimination reversal training except that reinforcement was suspended on all trials for both stimuli. Previous studies have shown discriminative learning that was not revealed in performance at the time of reinforced training often emerges in the form of more rapid extinction of responding to the former nonreinforced cue compared to the former reinforced cue when both cues are nonreinforced [35, 36]. The rats were exposed to the nonreinforced latent discrimination procedure for an additional 16 training sessions.
2.7. BDNF Measurements
Two days after behavioral training concluded (after the 16th session of the extinction phase), the rats were sacrificed in 4 squads of 12 rats across a 4 day period. The order of sacrificing was counterbalanced within the 4 squads with respect to the 6 treatment conditions (2 rats from each treatment on each day). The rats were anesthetized with sodium pentobarbital (50 mg/kg) and sacrificed by rapid decapitation. The brains were quickly removed and 2 mm slices encompassing the hippocampus and the prefrontal cortex were removed. Samples of the dorsal and ventral hippocampus and a portion of the prefrontal cortex were dissected (individually, bilaterally) using stainless steel tubing (inside diameter = 2.27 mm). Tissue was immediately quick frozen on dry ice and stored at −80°C until use.
Individual punches from each (left) brain area were homogenized with a motorized pellet pestle (Kontes Glass) in 30 ul of processing buffer (1% SDS, 50 mM NaF, 3.3 mM EGTA, plus protease inhibitors (Protease Inhibitor Cocktail, Sigma)), heated to 95°C for 10 minutes and placed on ice. Analysis of protein content was assayed using the DC Protein Assay (BioRad Laboratories). Samples from 47 animals were split into 6 runs (run=gel/blot set) for processing (2 runs per brain area, 4 gels/blots per run), with each gel containing a sample from an animal from each experimental treatment condition, plus a pre-stained protein standard (BioRad Laboratories, cat# 161-0305) and a positive control (100 ng BDNF, Santa Cruz Biotechnology, cat# sc-4554). Protein samples were electrophoretically resolved on a 12.5% SDS polyacrylamide gel for approximately 45 minutes at 250 V. Resolved proteins were then transferred to a nitrocellulose membrane for 90 minutes at 15 V. Nonspecific binding sites were blocked in blocking solution (5% non-fat dry milk (Bio-Rad Laboratories) in wash buffer: Tris-buffered saline (TBS) with 0.1% Tween-20, Sigma) overnight at 4°C with shaking. Following a quick wash in wash buffer, the blots were incubated with a BDNF primary antibody previously characterized by the manufacturer (Santa Cruz Biotechnologies, cat# sc-20981, 1: 1000) in 20 ml of blocking solution at room temperature for 2 hours with shaking. The blots were then washed in wash buffer (6 × 5 min) and incubated with secondary antibody (Goat anti-rabbit-IgG- horseradish peroxidase (HRP)-linked antibody, Cell Signaling Technology, cat# 7074, 1:1500) in 20 ml of blocking buffer for one hour at room temperature with shaking. After washing in wash buffer (6 × 5 min), bound antibody was detected using chemiluminescence (ECL; Amersham) on Kodak Biomax film.
The density of each BDNF blot was calculated using ImageJ software (version 1.34s, Rasband, National Institues of Health, USA). Western Blot analysis was restricted to the dominant band which corresponded to the molecular weight of the BDNF protein control (see Figure 5). Relative densities were calculated by dividing the BDNF protein density for each rat in the high fat diet groups by the density of the chow control rat (of the same restriction condition) that was on the same Kodak Biomax film. Thus, the relative BDNF protein density for each rat in the chow control group (both restricted and unrestricted) was 1.0. A separate analysis calculated relative BDNF densities by dividing the density for each rat by the positive control (100 ng BDNF, Santa Cruz Biotechnology, cat# sc-4554) that was on the same Kodak Biomax film. This analysis yielded a similar pattern of results. All BDNF data presented used the former calculation of relative BDNF protein densities.
Figure 5.

A sample BDNF Western Blot from the prefrontal cortex, with a BDNF control protein on the far left and one rat from each treatment condition: A = High Fat Dextrose Unrestricted, B = High Fat Dextrose Restricted, C = High Fat Sucrose Unrestricted, D = High Fat Sucrose Restricted, E = Control Unrestricted, F = Control Restricted. Western Blot analysis was restricted to the dominant band corresponding to the molecular weight of the BDNF control protein.
2.8. Statistical Analysis
The data was analyzed using Statistica software package (Statsoft, Inc., Tulsa, OK). Analysis of variance (ANOVA) was used to analyze body weight gain data for the unrestricted groups with Diet (C, HFD, or HFS) as a between subjects factor and Weeks (1-14) as a within subjects factor. ANOVA was used to analyze behavioral data with Diet (C, HFD, or HFS) and Restriction (restricted or unrestricted) as between subjects factors. Training Block (1-12), Trial (1-2 for each session), and Trial Type (rewarded or nonrewarded) were within subjects factors. BDNF levels in the ventral hippocampus, dorsal hippocampus, and prefrontal cortex were analyzed using ANOVA with Diet, Restriction, and Film (11-12 protein samples on each photographic film negative, each from a different rat) as between-subjects factors. Analyses of simple main effects were used to evaluate significant interactions. Alpha level for all statistical comparisons was set at 0.05. The percentage of time the photobeam inside of the food cup was broken during the 5 second stimulus period, minus the percentage of time the photobeam was broken during the 5 second period prior to stimulus onset (pre-CS period) served as the index of appetitive responding
3. Results
3.1. Body Weights
Figure 1 depicts mean cumulative weight gain (grams) for the unrestricted groups that received the respective chow, HFD and HFS diets during the 90-day period prior to the beginning behavioral training. The figure shows that rats given the HFS diet gained slightly more weight than rats given the HFD diet, and that both these groups gained substantially more weight than the rats that were given normal chow. ANOVA found that this pattern of differences yielded a significant Diet X Week interaction (F(26,260) = 4.22, p < .00001). Significant Diet X Week interactions were also obtained when weight gain for the chow group was compared to that of each high-fat group separately (smallest F(13,169) = 3.63, p < .01, for the Chow versus the HFD group comparison). This interaction did not achieve significance when the HFS and the HFD groups were directly compared (Diet X Week interaction, F(13,169) = 1.18, p = .30).
Figure 1.
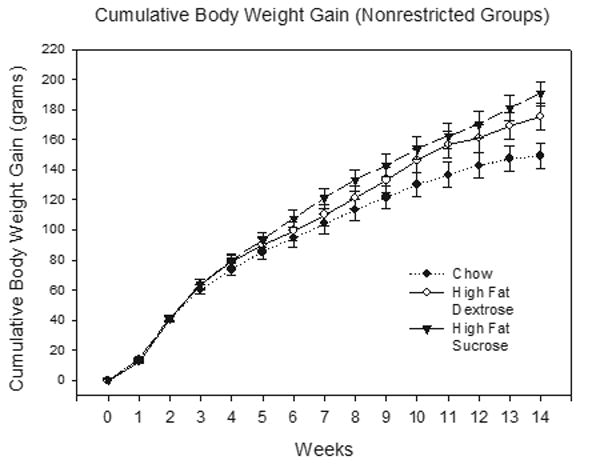
Mean cumulative body weight gain ± S.E.M. for the unrestricted groups prior to behavioral training.
3.2. Discrimination Training
Figure 2 depicts the mean appetitive responding for rats given the Chow (left panel), HFD (center panel) and HFS (right panel) diets on rewarded CS+ (filled circles) and nonrewarded CS− trials (open circles) during each eight-trial block of discrimination training (nonrestricted groups in Figure 2a, restricted groups in Figure 2b). As can be seen in Figure 2, for each diet treatment group, the rats came to exhibit more appetitive responding on CS+ compared to CS− trials. No differences in discrimination performance among the three types of diets or between the restricted or unrestricted access groups were evident.
Figure 2.
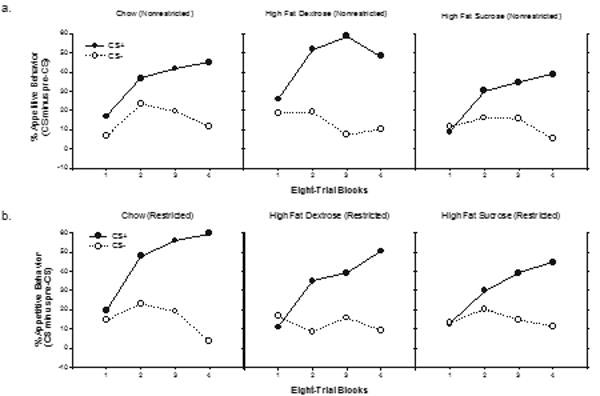
Mean appetitive responding (CS minus pre-CS) ± S.E.M. to both stimuli (CS+ and CS−) during the discrimination acquisition phase for the unrestricted (Figure 2a) and the restricted (Figure 2b) groups (8 trial blocks).
An overall ANOVA with Diet and Restriction Condition as between-subjects factor, and with Trial type (CS+ vs CS−) and Block as within-subjects factors, obtained significant main effect of Trial type (F(1,37) = 90.45, p < .00001) and a significant Trial type X Block interaction (F(3,111) = 50.78, p < .0001). However, neither of these factors interacted significantly with either Diet or Restriction (largest F (2, 37) = 1.93, p = .16, for the Diet × Trial type interaction) nor did the main effects of Diet or Restriction achieve significance (Fs < 1). The results of discrimination training showed that all groups learned to respond significantly more on CS+ compared to CS− trials and that the magnitude of this difference did not vary significantly as a function of type of diet or prior dietary restriction condition.
3.3. Discrimination Reversal and Latent Discrimination Reversal
The results of reinforced discrimination reversal training and of subsequent nonreinforced latent discrimination reversal testing are presented in Figures 3a and 3b. Figure 3a depicts responding (CS minus Pre CS) to both the former CS+ and former CS− for the rats that were given unrestricted access to lab chow (leftmost panel), the HFD diet (center panel) and the HFS diet (rightmost panel). Figure 3b depicts the same data for the rats that were given restricted access to each diet. Within in each panel, the data presented to the left of the dashed line depicts performance during reinforced reversal training, whereas the data presented on the right of the dashed line depicts latent discrimination reversal performance. The data depicted in Figure 3a show that across both the reinforced and latent discrimination phases of the experiment, rats given nonresticted access to the HFD diet (center panel) exhibited the poorest performance compared to both the Chow and HFS groups. Figure 3b shows that this pattern of results was not exhibited by rats that received restricted access to these diets.
Figure 3.
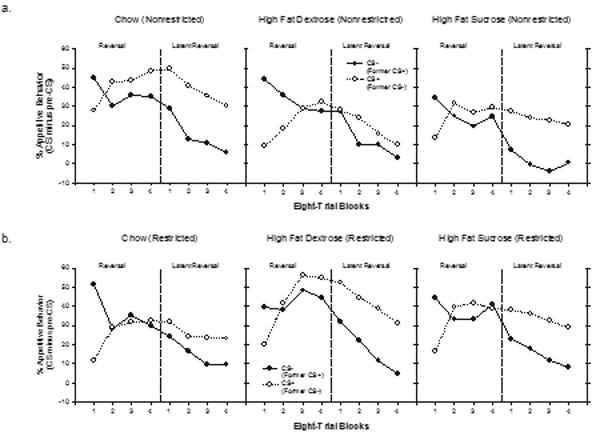
Mean appetitive responding (CS minus pre-CS) ± S.E.M. to both stimuli (CS+ and CS−) during the discrimination reversal phase (left side of each section) and the latent reversal, i.e., extinction phase (right side of each section) for the unrestricted (Figure 3a) and the restricted (Figure 3b) groups.
Separate ANOVAs with Diet and Restriction Condition as between-subjects factors, and with Trial type (CS+ vs CS−) and Block as within-subjects factors were used to evaluate the data from the respective reinforced discrimination reversal and the latent discrimination reversal phases. For reinforced discrimination reversal training, the analysis obtained a significant main effect of Trial type (F(1,37) = 4.51, p < .05) and a significant Trial type X Block interaction (F (3,111) = 48.22, p < .0001). As a means of assessing whether or not the rats learned to respond based on the reversed stimulus-reward contingencies, performance on the last block of reinforced discrimination reversal training was analyzed separately for all rats. This overall analysis yielded a significant main effect of Trial Type (F(1, 37) = 4.66, p < .05) that did not vary significantly as a function of either Diet or Restriction condition (largest F < 1). Although the results of this analysis indicated that reversal learning was achieved when performance was assessed collapsed across all diet and restriction conditions, additional analyses were conducted to determine if the effect of Trial type was significant on the last block of reinforced reversal training within each combination of the levels of Diet and Restriction. These analyses found no significant effect of trial type for any combination of diet and restriction condition. Reversal performance approached significance only for the rats in the unrestricted Chow condition (largest F(1, 6) = 4.19, p > .08). Thus, reversal learning, defined as significantly more responding to the former CS− relative to the former CS+ was not observed for any of the six treatment conditions on the last block of reinforced discrimination reversal training.
Consistent with the results of previous studies that used the latent discrimination strategy [e.g., 35, 36], clear evidence of reversal learning emerged when neither CS+ or CS− was followed by reinforcement. ANOVA obtained a significant main effect of Block (F(3,111) = 34,87, p < .01) and a significant main effect of Trial type (F(1,37) = 58.59, p < .01). Neither of these factors interacted significantly with either Diet or Restriction, nor did the main effects of Diet or Restriction achieve significance (all Fs < 1.5); however, the Diet X Restriction X Trial Type interaction was significant (F(2,37) = 3.65, p < .05). Separate analyses of the Restriction X Trial Type interaction for each diet group separately yielded a significant interaction for only the HFD group (F(1,11) = 5.68, p < .05.). The main effect of Trial Type was significant for the HFD restricted group (F(1,5) = 21.53, p < .01) but not for the HFD unrestricted group (F(1,4) = 1.22, p = .33). Moreover, the HFD unrestricted group was the only treatment condition of the six that did not show a significant main effect of Trial Type. In summary, during the latent discrimination reversal phase, significantly more responding to CS+ compared to CS− emerged for all groups except for the group given nonresticted access to the HFD diet.
3.4. Responding to CS− (Former CS+)
Previous reports indicated that rats with hippocampal damage are not only impaired in reversal learning but are also slower to extinguish responding to previously reinforced cues after reinforcement has been suspended [see 37]. We performed an additional a priori analysis to assess whether or not a similar type of deficit would be observed during responding to CS− (the former CS+), at the outset of discrimination reversal training, based on type of diet. A transient effect of Diet that did not interact with Restriction condition was obtained over the first two 8-eight trial blocks of reversal training. Figure 4 shows that the greatest reduction in responding from the first to the second block of CS− trials was exhibited by rats given chow and that a smaller reduction was shown by the rats that ate the HFS diet, with the smallest reduction in CS− responding exhibited by the rats that ate the HFD diet.
Figure 4.
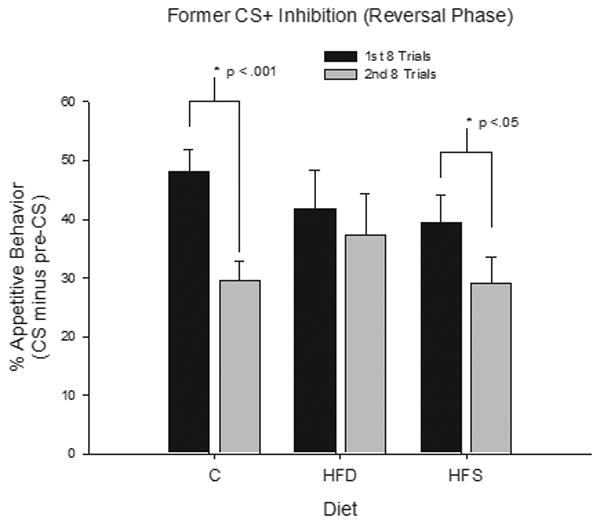
Mean appetitive responding (CS minus pre-CS) ± S.E.M. to the CS− (former CS+) during the discrimination reversal phase for the first 8 trials (black) and the second 8 trials (gray) (restricted and unrestricted groups collapsed together).
Individual ANOVAs compared the performance of the chow group with that of the HFD and HFS groups respectively, over the first two 8-eight trial blocks of reversal training with CS−. Comparison of the rats given chow with those given the HFD diet yielded a significant effect of Block (F(1, 23) = 12.90, p < .01) and a significant Block × Diet interaction (F(1, 23) = 4.53, p < .05). Neither Block nor Diet interacted significantly with Restriction Condition. Analysis of the simple main effects yielded a significant main effect of Block for rats given the Chow diet (F (1, 12) = 21.06, p < .01), but not for rats that ate the HFD diet (F(1, 11) < 1). When rats that received chow were compared with those that received the HFS diet, the main effect of Block was significant (F(1, 26) = 23.42, p < .01) but the Block × Diet interaction failed to achieve significance (F(1, 26) = 2.13, p > .15). In summary, decrease in responding to CS− from the first to the second 8-trial block of reversal training, was significant for rats fed the respective chow and the HFS diets but not for rats that ate the HFD diet. A parallel analysis of responding to the former CS+ revealed that the decrease over last two eight-trial blocks of reinforced discrimination reversal training did not vary significantly as a function of Diet or Restriction condition.
3.5. BDNF Western Blot Analysis
A BDNF Western Blot sample can be seen in Figure 5. Figure 6 represents relative levels of BDNF protein density for the six treatment conditions. As shown in the left panel of Figure 6, Western Blot analysis revealed that BDNF protein levels in the ventral hippocampus and the prefrontal cortex were reduced for rats in the unrestricted HFD group relative to the unrestricted Chow group. These differences were not significant when the unrestricted Chow and unrestricted HFS groups were compared. Furthermore, neither the unrestricted HFD nor the unrestricted HFS groups showed reduced levels of BDNF in the dorsal hippocampus relative to the unrestricted Chow control group. The right panel of Figure shows that for rats that had restricted access to these diets, little difference in BDNF levels were observed when the effects of the Chow diet were compared to the effects of the HFD and the HFS diets, respectively.
Figure 6.
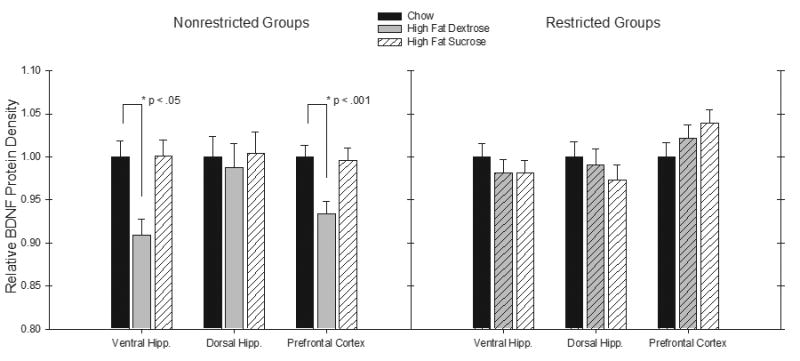
Mean relative BDNF protein density (± S.E.M.) from a Western Blot Analysis in the ventral hippocampus, dorsal hippocampus, and the prefrontal cortex for the unrestricted groups (left) and the restricted groups (right) (Chow = black, High Fat Dextrose = gray, High Fat Sucrose = striped).
An overall ANOVA with Diet and Restriction condition as between-subjects factors and Brain area (i.e., ventral hippocampus, dorsal hippocampus, and prefrontal cortex) as a within-subjects factor yielded a significant Brain area × Restriction interaction (F(2, 80) = 4.27, p < .05). Thus, additional ANOVAs were used to compare the effects of Diet and Brain area on BDNF protein levels for each restriction condition separately. For the unrestricted condition, ANOVA revealed that the main effect of Diet was significant in the ventral hippocampus (F(2,20) = 3.99, p < .05), and the prefrontal cortex (F(2,20) = 4.77, p < .05), but not the dorsal hippocampus (F < 1.0). Analyses of simple main effects were used to individually compare the unrestricted HFD and the unrestricted HFS groups with the unrestricted Chow controls. These analyses found that relative BDNF protein levels in ventral hippocampus (F(1,13) = 6.39, p < .05) and in the prefrontal cortex (F(1,13) = 26.80, p < .001) were significantly reduced for unrestricted HFD group relative to the Chow Group. However, neither of these differences were significant when the unrestricted HFS and the unrestricted Chow Groups were compared (Fs < 1.0). Furthermore, significant differences were not obtained when BDNF levels in the dorsal hippocampus for unrestricted Chow groups were compared to those exhibited by rats given either the unrestricted HFD or unrestricted HFS diets. When these same analyses of BDNF levels were conducted for rats in the Restricted condition, no significant differences based on Brain Area or Diet were obtained when the respective HFD and HFS diets were compared individually with the chow diet.
4. Discussion
In the present experiment, neither type of diet nor dietary restriction condition had significant effects on the acquisition of a simple Pavlovian discrimination problem. In contrast, discrimination reversal performance appeared to depend on both of these variables. Specifically, impaired discrimination-reversal learning was exhibited only by rats that received a 90-day period of unrestricted access to a diet high in saturated fat and dextrose (an HFD diet) prior to the beginning of original discrimination training. No impairments were shown by rats that received restricted access to the HFD diet or by rats that received either unrestricted or restricted access to a diet high in saturated fat and sucrose (HFS).
The effects of type of diet and dietary restriction on BDNF levels in the ventral hippocampus and prefrontal cortex corresponded to the effects of those variables on discrimination reversal performance. That is, reduced BDNF levels were exhibited only by rats that were given prior unrestricted access to the HFD diet—the same group that showed impaired latent discrimination reversal performance. Furthermore, no differences in BDNF levels were found in the dorsal hippocampus as a function of either type of diet or dietary restriction condition.
The results of previous studies indicated that acquisition of a simple Pavlovian discrimination problem does not depend on either the hippocampus or the prefrontal cortex, whereas discrimination reversal performance and extinction of responding to previously reinforced cues are impaired as a consequence of damage to either area. In our study, normal discrimination acquisition, but impaired discrimination reversal was also exhibited by rats that had been given prior unrestricted access to a HFD diet. This outcome suggests that (a) the hippocampus, the prefrontal cortex, or both structures, may be especially sensitive to the detrimental effects of chronic, unrestricted access to high fat diets containing dextrose and; (b) this sensitivity may be related to reductions in BDNF levels in the ventral hippocampus and prefrontal cortex.
As expected, the rats given nonrestricted access to their designated diets weighed more than rats that had restricted access to the diets. It is possible that the effects of type of diet on reversal learning and BDNF levels might be based on body weight differences and not the type of diet per se. One feature of the results reduces the plausibility of this hypothesis: both the unrestricted HFD and HFS groups weighed approximately the same throughout the experiment, yet only the unrestricted HFD group showed impaired reversal learning and reduced BDNF levels relative to the chow group. This indicates that the observed deficits were not simply based on increased body weight, but depended on some other property of the diet. For example, caloric restriction has been reported to increase BDNF levels in the hippocampal formation [38]. It may be that caloric restriction during the training period produced faster recovery of BDNF levels and hippocampal function for rats on the HFS compared to the HFD diet.
The findings that the HFS diet had little or no effects on learning performance or on BDNF levels was unexpected given previous reports that consumption of HFS diets produced impaired spatial learning and reduced hippocampal BDNF [11]. Unlike the present experiment, these prior studies did not include an extended period of caloric restriction (approximately 10 weeks over the course discrimination and reversal training) that immediately preceded analysis of BDNF levels. Rather, in the previous study, changes BDNF levels induced by consumption of a HFS diet were recorded following testing with a spatial memory tasks that did not involve food restriction. As previously mentioned, caloric restriction can increase BDNF levels in the hippocampus [38]. Thus, it may be that reduced BDNF levels for rats given the HFS diets would have been found in our study had we measured BDNF prior to the beginning of caloric restriction.
Regardless of this procedural difference, our findings indicate that the effects of chronic intake of diets high saturated fat and complex sugars on learning and hippocampal BDNF levels may depend not only on the conditions of access to the diet but also on the source of carbohydrate. The HFD diet has a higher glycemic index than the HFS diet based on the fact that dextrose is a more potent stimulator of insulin secretion than sucrose [39]. Previous research shows that diets with a high glycemic index promote the development of insulin resistance and hyperglycemia [40]. Insulin resistance has been linked to memory impairments in both human [41] and nonhuman animals [33]. Although, it is not clear whether or not insulin resistance involves reduced levels of BDNF, increased levels of BDNF have been associated with improved glucoregulation [42] and amelioration of insulin resistance [43] in rodents. Recent findings showing that manipulations of dietary carbohydrate that produce insulin resistance in peripheral tissues also give rise to insulin resistance in the brain [44] lend support to the hypothesis that changes in brain insulin-signaling may play a role in hippocampal- and prefrontocortical-dependent learning and in the regulation of BDNF in these brain areas. Additional research is needed to determine how dietary dextrose might be involved with changes in cognitive performance concomitant with reduced BDNF levels in the ventral hippocampus and prefrontal cortex.
In addition to a deficit in reversal learning, the present results revealed a modest impairment in extinction of responding to the former CS+ for rats that had received the HFD diet compared to chow controls. This deficit did not vary significantly as a function dietary restriction condition. Moreover, because BDNF levels were reduced in the ventral hippocampus and prefrontal cortex only for rats that were in the unrestricted HFD group, the finding that the deficit in extinction performance did not depend on restriction condition indicates that impaired extinction of responding to the former conditioned stimulus may not have been an effect of the HFD diet on BDNF. However, as noted above, this interpretation must be viewed cautiously because BDNF levels were not recorded at the time when impaired extinction performance was observed, and these levels may have increased subsequently as result of extended exposure to caloric restriction. It will be important to conduct additional studies that assess the effects of HFD and HFS diets on BDNF levels and on the ability of animals to suppress responding to cues associated with food, under conditions that do not involve restriction of caloric intake.
A recent model from our laboratory [45] hypothesized that if excessive intake of energy rich, high-fat, diets interfere with hippocampal functioning, this interference might then reduce the ability to suppress or inhibit the evocation of appetitive and eating behaviors by environmental cues that are associated with food. This impairment would presumably increase the tendency to engage in appetitive and consummatory behaviors, including those directed at obtaining the same high-fat foods that give rise to impaired hippocampal functioning. The present results agree with this model in that BDNF levels in the ventral hippocampus were reduced for rats that were given unrestricted access to a HFD diet prior to the onset of appetitive training.
However, we also found that intake of the same diet was accompanied by reduced BDNF in the prefrontal cortex, a brain area that has also been described as an important substrate for behavioral inhibition, including that related to food rewards and satiety [46, 47]. Anatomical evidence [48] shows that ventral hippocampal CA1 field axons project to the rostroventral region of the medial prefrontal cortex. In addition, projections have been documented from the ventral tip of the hippocampal CA1 cell field [49], and from the prefrontal cortex [50] to hypothalamic cell groups thought to be important for the control of ingestive behavior. The present results, combined with these anatomical findings suggest that the effects of high-fat diets on BDNF levels in what can be described as a hippocampal-prefrontocortical circuit [e.g., 51-53] may have special significance with respect to the performance of reward and/or appetitive motivational functions, including those involved with the inhibitory controls of energy intake regulation.
Acknowledgments
This work was done in partial fulfillment of the requirements for completion of a Master of Science degree by Scott E. Kanoski. The authors thank Jennie Mak, David Eagan, Andrea Tracy, Melissa McCurley, and Mamta Behl for their contributions. Support for this research was provided by National Institutes of Health in the form of grants R01 HD29792 (to TLD) and R01 DA13680 (to RLM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cordain L, Eaton SB, Sebastian A, et al. Origins and evolution of the Western diet: health implications for the 21st century. American Journal of Clinical Nutrition. 2005;81:341–54. doi: 10.1093/ajcn.81.2.341. see comment. [DOI] [PubMed] [Google Scholar]
- 2.Grant WB. Diet and risk of dementia: does fat matter? The Rotterdam Study. Neurology. 2003;60:2020–1. comment. [PubMed] [Google Scholar]
- 3.Grant WB, Campbell A, Itzhaki RF, Savory J. The significance of environmental factors in the etiology of Alzheimer's disease. Journal of Alzheimer's Disease. 2002;4:179–89. doi: 10.3233/jad-2002-4308. [DOI] [PubMed] [Google Scholar]
- 4.Kalmijn S. Fatty acid intake and the risk of dementia and cognitive decline: a review of clinical and epidemiological studies. Journal of Nutrition, Health & Aging. 2000;4:202–7. see comment. [PubMed] [Google Scholar]
- 5.Morris MC, Evans DA, Bienias JL, et al. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology. 2004;62:1573–9. doi: 10.1212/01.wnl.0000123250.82849.b6. [DOI] [PubMed] [Google Scholar]
- 6.Greenwood CE, Kaplan RJ, Hebblethwaite S, Jenkins DJ. Carbohydrate-induced memory impairment in adults with type 2 diabetes. Diabetes Care. 2003;26:1961–6. doi: 10.2337/diacare.26.7.1961. see comment. [DOI] [PubMed] [Google Scholar]
- 7.Papanikolaou Y, Palmer H, Binns MA, et al. Better cognitive performance following a low-glycaemic-index compared with a high-glycaemic-index carbohydrate meal in adults with type 2 diabetes. Diabetologia. 2006;49:855–62. doi: 10.1007/s00125-006-0183-x. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood CE, Winocur G. Learning and memory impairment in rats fed a high saturated fat diet. Behavioral & Neural Biology. 1990;53:74–87. doi: 10.1016/0163-1047(90)90831-p. [DOI] [PubMed] [Google Scholar]
- 9.Greenwood CE, Winocur G. Cognitive impairment in rats fed high-fat diets: a specific effect of saturated fatty-acid intake. Behavioral Neuroscience. 1996;110:451–9. doi: 10.1037//0735-7044.110.3.451. [DOI] [PubMed] [Google Scholar]
- 10.Winocur G, Greenwood CE. The effects of high fat diets and environmental influences on cognitive performance in rats. Behavioural Brain Research. 1999;101:153–61. doi: 10.1016/s0166-4328(98)00147-8. [DOI] [PubMed] [Google Scholar]
- 11.Molteni R, Barnard RJ, Ying Z, et al. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–14. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 12.Molteni R, Wu A, Vaynman S, et al. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–40. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. European Journal of Neuroscience. 2004;19:1699–707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- 14.Jarrard LE. What does the hippocampus really do? Behavioural Brain Research. 1995;71:1–10. doi: 10.1016/0166-4328(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 15.Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO Journal. 1982;1:549–53. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leibrock J, Lottspeich F, Hohn A, et al. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–52. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. Journal of Neurochemistry. 2002;82:1367–75. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 18.Monteggia LM, Barrot M, Powell CM, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10827–32. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rossi C, Angelucci A, Costantin L, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. European Journal of Neuroscience. 2006;24:1850–6. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- 20.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–7. doi: 10.1126/science.1128134. see comment. [DOI] [PubMed] [Google Scholar]
- 21.Winocur G, Wojtowicz JM, Sekeres M, et al. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- 22.Berger TW, Orr WB. Hippocampectomy selectively disrupts discrimination reversal conditioning of the rabbit nictitating membrane response. Behavioural Brain Research. 1983;8:49–68. doi: 10.1016/0166-4328(83)90171-7. [DOI] [PubMed] [Google Scholar]
- 23.Churchill JD, Green JT, Voss SE, et al. Discrimination reversal conditioning of an eyeblink response is impaired by NMDA receptor blockade. Integrative Physiological & Behavioral Science. 2001;36:62–74. doi: 10.1007/BF02733947. [DOI] [PubMed] [Google Scholar]
- 24.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience & Biobehavioral Reviews. 2004;28:771–84. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Davidson TL, Jarrard LE. The hippocampus and inhibitory learning: a ‘Gray’ area? Neuroscience & Biobehavioral Reviews. 2004;28:261–71. doi: 10.1016/j.neubiorev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiology of Learning & Memory. 2005;83:125–33. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salazar RF, White W, Lacroix L, et al. NMDA lesions in the medial prefrontal cortex impair the ability to inhibit responses during reversal of a simple spatial discrimination. Behavioural Brain Research. 2004;152:413–24. doi: 10.1016/j.bbr.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 28.Bannerman DM, Grubb M, Deacon RM, et al. Ventral hippocampal lesions affect anxiety but not spatial learning. Behavioural Brain Research. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- 29.Pothuizen HH, Zhang WN, Jongen-Relo AL, et al. Dissociation of function between the dorsal and the ventral hippocampus in spatial learning abilities of the rat: a within-subject, within-task comparison of reference and working spatial memory. European Journal of Neuroscience. 2004;19:705–12. doi: 10.1111/j.0953-816x.2004.03170.x. [DOI] [PubMed] [Google Scholar]
- 30.Trivedi MA, Coover GD. Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiology of Learning & Memory. 2004;81:172–84. doi: 10.1016/j.nlm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Gray JA, McNaughton N. The neuropsychology of anxiety. Oxford: Oxford University Press; 2000. [Google Scholar]
- 32.Wolever TM, Miller JB. Sugars and blood glucose control. American Journal of Clinical Nutrition. 1995;62:212S–221S. doi: 10.1093/ajcn/62.1.212S. discussion 221S-227S. [DOI] [PubMed] [Google Scholar]
- 33.Winocur G, Greenwood CE, Piroli GG, et al. Memory impairment in obese Zucker rats: an investigation of cognitive function in an animal model of insulin resistance and obesity. Behavioral Neuroscience. 2005;119:1389–95. doi: 10.1037/0735-7044.119.5.1389. [DOI] [PubMed] [Google Scholar]
- 34.Razay G, Wilcock GK. Hyperinsulinaemia and Alzheimer's disease. Age & Ageing. 1994;23:396–9. doi: 10.1093/ageing/23.5.396. [DOI] [PubMed] [Google Scholar]
- 35.Davidson TL, Flynn FW, Jarrard LE. Potency of food deprivation intensity cues as discriminative stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 1992;18:174–81. doi: 10.1037//0097-7403.18.2.174. [DOI] [PubMed] [Google Scholar]
- 36.Hearst E. Extinction reveals stimulus control: latent learning of feature-negative discriminations in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1987;13:52–64. [PubMed] [Google Scholar]
- 37.Chan KH, Morell JR, Jarrard LE, Davidson TL. Reconsideration of the role of the hippocampus in learned inhibition. Behavioural Brain Research. 2001;119:111–30. doi: 10.1016/s0166-4328(00)00363-6. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Duan W, Long JM, et al. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. Journal of Molecular Neuroscience. 2000;15:99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- 39.Trout DL, Hallfrisch J, Behall KM. Atypically high insulin responses to some foods relate to sugars and satiety. International Journal of Food Sciences & Nutrition. 2004;55:577–88. doi: 10.1080/09637480400029308. [DOI] [PubMed] [Google Scholar]
- 40.Brynes AE, Mark Edwards C, Ghatei MA, et al. A randomised four-intervention crossover study investigating the effect of carbohydrates on daytime profiles of insulin, glucose, non-esterified fatty acids and triacylglycerols in middle-aged men. British Journal of Nutrition. 2003;89:207–18. doi: 10.1079/BJN2002769. [DOI] [PubMed] [Google Scholar]
- 41.Craft S. Insulin resistance syndrome and Alzheimer's disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiology of Aging. 2005;26 1:65–9. doi: 10.1016/j.neurobiolaging.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 42.Ono M, Ichihara J, Nonomura T, et al. Brain-derived neurotrophic factor reduces blood glucose level in obese diabetic mice but not in normal mice. Biochemical & Biophysical Research Communications. 1997;238:633–7. doi: 10.1006/bbrc.1997.7220. [DOI] [PubMed] [Google Scholar]
- 43.Kuroda A, Yamasaki Y, Matsuhisa M, et al. Brain-derived neurotrophic factor ameliorates hepatic insulin resistance in Zucker fatty rats. Metabolism: Clinical & Experimental. 2003;52:203–8. doi: 10.1053/meta.2003.50026. [DOI] [PubMed] [Google Scholar]
- 44.Mielke JG, Taghibiglou C, Liu L, et al. A biochemical and functional characterization of diet-induced brain insulin resistance. Journal of Neurochemistry. 2005;93:1568–78. doi: 10.1111/j.1471-4159.2005.03155.x. [DOI] [PubMed] [Google Scholar]
- 45.Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiology & Behavior. 2005;86:731–46. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Ahn S, Phillips AG. Modulation by central and basolateral amygdalar nuclei of dopaminergic correlates of feeding to satiety in the rat nucleus accumbens and medial prefrontal cortex. Journal of Neuroscience. 2002;22:10958–65. doi: 10.1523/JNEUROSCI.22-24-10958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schneider M, Koch M. Behavioral and morphological alterations following neonatal excitotoxic lesions of the medial prefrontal cortex in rats. Experimental Neurology. 2005;195:185–98. doi: 10.1016/j.expneurol.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Swanson LW. A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Research. 1981;217:150–4. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- 49.Cenquizca LA, Swanson LW. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. Journal of Comparative Neurology. 2006;497:101–14. doi: 10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrovich GD, Holland PC, Gallagher M. Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. Journal of Neuroscience. 2005;25:8295–302. doi: 10.1523/JNEUROSCI.2480-05.2005. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalisch R, Korenfeld E, Stephan KE, et al. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. Journal of Neuroscience. 2006;26:9503–11. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 53.Wang GW, Cai JX. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behavioural Brain Research. 2006;175:329–36. doi: 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]


