Wound healing is essential to the survival of an organism. A breach in tissue composed of a continuous layer of cells provokes a very complex response intended to close, and eventually heal, the wound. In epithelial tissue, the opening of a gap induces the proliferation and movement of the surrounding intact cells, which eventually closes the gap. A multitude of chemical signals are produced when a wound occurs, and thereafter, which leads to proliferation and motility in the surrounding intact layer (1). In this issue of PNAS, Poujade et al. (2) demonstrate that cell motility that closes an open gap in a cell culture (a “wound”) can be triggered even in the absence of cell damage. A unique experimental setup allows the authors to follow the behavior of a continuous and undisturbed monolayer of cells that suddenly finds itself with a free boundary as a model of an open wound. The authors observe that the subsequent cell motility is highly nonuniform and develops distinct morphological patterns and collective motion.
The response of a cell culture to damage that mimics wounding has been analyzed previously. In previous studies (3, 4), a uniform layer of cells was grown on a substrate and then scratched (wounded) with a sharp object. Although these experiments have advanced our knowledge of the wound-healing process, they are complicated to interpret because they involve several effects. First, the sharp object breaks up the cells and releases their constituents into the solution as cellular debris (1). The surrounding cells are therefore affected by the chemical triggers released from the neighboring damaged cells. In addition, the cells at the edges of the open gap are further affected by the sudden loss of neighbors, which opens a free boundary on one of their sides. The cells at this free boundary undergo morphological changes that lead to their motility through the induction of membrane extensions (lamellipodia and filopodia) (5, 6). Observations of cell motility and morphology in these experiments are further complicated by the fact that the edges of the scratch are rough and nonuniform. Finally, the substrate in the open gap is covered with the remains of the cells that were removed and also has a rough and nonuniform topography. In view of these complications, it seems natural to seek a cleaner method for studying the response of a cell culture to a sudden change that triggers motility. This is exactly what Poujade et al. (2) have done.
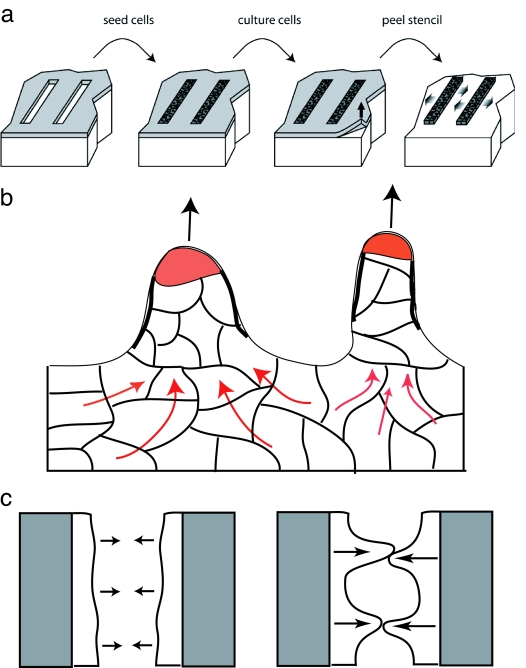
Their basic scheme is very straightforward in principle (Fig. 1a). A monolayer of Madin–Darby canine kidney (MDCK) cells (prototypical epithelial cells) is grown on areas of the substrate that are left uncovered by an adsorbed mask (stencil). The mask, made of a soft cross-linked polydimethylsiloxane (PDMS), is then carefully peeled away, resulting in the sudden appearance of free boundaries, which triggers cell motion. With this method, the initial free boundary of the culture is extremely smooth and uniform, even on the subcellular level. Furthermore, the open substrate that the cells now move onto is clean, smooth, and uniform. The authors then investigate the effects of these free boundaries on cell motion. It was found previously (4) that the cell culture invades the free substrate in a highly nonuniform manner, with some cells moving faster than others, leading to finger-like columns of motile cells. The cells at the tips of these columns were aptly named “leader cells.” Does such a nonuniform morphology arise in the controlled experiment as well? Surprisingly, Poujade et al. (2) answer, “Yes” (Fig. 1b).
Fig. 1.
Released cell culture motility patterns. (a) Scheme of the Poujade et al. (2) experiment. (b) After removal of confinement, the initial cell culture expands onto the surrounding substrate in the form of cell columns, with leader cells (red tips) moving fastest forward, dragging their neighbors by means of cytoskeletal scaffolds (heavy black lines) and strong cell–cell cadherin contacts. The continuity of the moving front is always maintained so that the leader cells do not dissociate from their neighbors. Behind the moving front, a collective cell motion (red arrows) feeds the column with cells. (c) Forming a pattern of cell columns may allow the cell culture to bridge the open wound faster (Right) than would a uniformly moving smooth front (Left).
The leader cells have a distinct morphology with a highly polarized shape and well developed lamellipodia (2, 6). These cells therefore have the marked characteristics of highly motile cells and, indeed, move with a higher velocity than their less polarized neighbors. The location of these cells along the free boundary of the culture seems to be random. What induces cells in the culture boundary to become motile and even to become leaders? The confinement of the cells by the boundaries produces a pressure that acts on the cells and results in signaling events that limit cell proliferation (7) and motility; these signals are presumably in response to the fact that the cells are part of a continuous tissue. When confinement is removed, pressure is released and the cells at the free boundary are able to expand and move outward. Although the pressure in the culture serves as a biochemical signal to the cells to become motile, it is too small to mechanically induce the actual outward movement of the cells. The existence of a free boundary triggers a shape transformation in a subset of cells that become highly motile “leaders.”
Once leader cells form, they begin moving outward, normal to the free boundary, at a generally constant velocity, “dragging” a column of cells behind them. The strong cadherin cell–cell contacts (8) maintain the continuity of the outer contour of the cell culture so that no breaks appear. At the same time, the proliferation of cells behind the moving front maintains a constant cell density and prevents holes from forming in the continuous culture. The leaders are highly efficient, motile cells, and their followers in the moving cell columns are now observed (2) to form cytoskeletal scaffolds (Fig. 1b) that maintain their motion in the direction of the leader cell. Behind the moving front, the cells respond to the formation of the cell columns by moving collectively in complex patterns (2) that resemble the dynamics of a sheared viscous fluid, including the formation of vortices with a diameter of ≈100 μm. These collective motions (5) seem to maintain the forward motion of the cell columns and feed them with a
A multitude of chemical signals are produced when a wound occurs.
flow of cells (Fig. 1b). It is still unclear what determines the length scale over which the velocities of the cells are correlated.
As was found in a previous study (9), the present work shows that the average width of the cell culture increases quadratically with time. The mechanism that drives this acceleration in the average velocity of the culture edge is not clear and may be related to the formation of leader cells and cell columns (Fig. 1c). Eventually the system reaches a terminal velocity, and the width grows linearly with time (9). Poujade et al. (2) observed that such linear behavior occurs very early for a culture whose initial width is small compared with the length scale of velocity–velocity correlations. This linear behavior appears for cultures that are thinner than the typical size of the internal cellular flows (≈100 μm). Below this size, the ability of the cell columns to induce large-scale cellular flows behind them may be hindered, and the average width velocity is constant. The roles of the collective cellular motions and the overall geometry of the cell culture need to be studied in greater detail.
The authors have examined the role of the cell–cell contacts by adding growth factors that are known to increase cell motility (10, 11) and reduce cell–cell adhesion. In the presence of these growth factors, the cell culture front moves rapidly, and almost all of the leading cells develop polarized shapes with lamellipodia. No large-scale patterns or cell columns are found in this case because all of the cells in the moving front behave as leaders. Note that when cells move in a more dispersed manner and do not maintain a strictly continuous front, there also seems to be no pattern-forming or cell column development as a result of the wound-healing motility (10–12).
We can speculate that the functional role of the leader cells and cell columns is to speed up the bridging of an open wound, given that the leaders and their follower columns move faster than a uniformly moving smooth front (Fig. 1c). It is quite remarkable that within an initially uniform layer of identical cells, some can show such extreme structural changes and transform into highly motile leaders. This transition involves dramatic changes in cell shape and behavior that are reminiscent of the epithelial–mesenchymal transition (13, 14), although in our case, there is no loss of cadherin cell–cell contacts. When the wound is sealed, these leader cells return to their normal epithelial form and behavior and disappear into the crowd; the leader has done its job and can return to “civilian life.” Thanks to the novel approach of Poujade et al. (2), the nature of these important cellular transformations can now be studied in a controlled manner. This will likely lead to a multitude of additional experiments to test the roles of different geometries and environments on cellular motility. The quantitative observations, and the ability to isolate each component, that are now possible will also permit more accurate theoretical and computational studies of this complex phenomenon (7, 12).
Footnotes
The author declares no conflict of interest.
See companion article on page 15988.
References
- 1.Yin J, Xu K, Zhang J, Kumar A, Yu F-SX. J Cell Sci. 2007;120:815–825. doi: 10.1242/jcs.03389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poujade M, Grasland-Mongrain E, Hertzog A, Jouanneau J, Chavrier P, Ladoux B, Buguin A, Silberzan P. Proc Natl Acad Sci USA. 2007;104:15988–15993. doi: 10.1073/pnas.0705062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang CC, Park AY, Guan JL. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 4.Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Proc Natl Acad Sci USA. 2003;100:10788–10793. doi: 10.1073/pnas.1834401100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farooqui R, Fenteany G. J Cell Sci. 2005;118:51–63. doi: 10.1242/jcs.01577. [DOI] [PubMed] [Google Scholar]
- 6.Fenteany G, Janmey PA, Stossel TP. Curr Biol. 2000;10:831–838. doi: 10.1016/s0960-9822(00)00579-0. [DOI] [PubMed] [Google Scholar]
- 7.Shraiman BI. Proc Natl Acad Sci USA. 2005;102:3318–3323. doi: 10.1073/pnas.0404782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y. Mol Cell Biol. 2004;24:6690–6700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen P, Misfeldt DS. Proc Natl Acad Sci USA. 1980;77:4760–4763. doi: 10.1073/pnas.77.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang S, Dang Y, Schnellmann RG. Am J Physiol. 2004;28:F365–F372. doi: 10.1152/ajprenal.00035.2004. [DOI] [PubMed] [Google Scholar]
- 11.Kurten RC, Chowdhury P, Sanders RC, Jr, Pittman LM, Sessions LW, Chambers TC, Lyle CS, Schnackenberg BJ, Jones SM. Am J Physiol. 2005;288:C109–C121. doi: 10.1152/ajpcell.00024.2003. [DOI] [PubMed] [Google Scholar]
- 12.Bindschadler M, McGrath JL. J Cell Sci. 2006;120:876–884. doi: 10.1242/jcs.03395. [DOI] [PubMed] [Google Scholar]
- 13.Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, Thompson EW. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 14.Thiery JP. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]