Abstract
Reversible encapsulation complexes create spaces where two or more molecules can be temporarily isolated. When the mobility of encapsulated molecules is restricted, different arrangements in space are possible, and new forms of isomerism (“social isomerism”) are created: the orientation of one encapsulated molecule influences that of the other in the confined space. Expansion of a capsule's length is possible through addition of small-molecule spacer elements. The expanded capsules have dimensions that permit the observation of social isomerism of two identical guests, and they adopt arrangements that properly fill the host's space. The host also can adapt to longer guests by incorporating additional spacers, much as protein modules are added to a viral capsid in response to larger genomes. Arachidonic and related fatty acid derivatives act in this way to induce the assembly of further extended capsules having sufficient length to accommodate them.
Keywords: encapsulation, self-assembly, social isomers
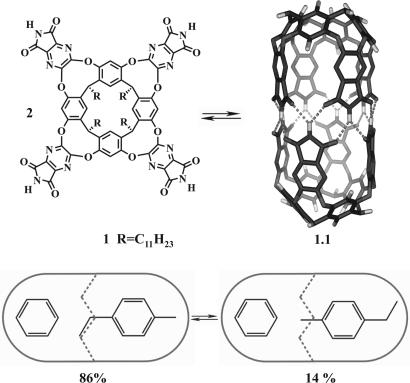
Encapsulation complexes are synthetic, self-assembled hosts that more or less completely surround their guest molecules. They are dynamic and form reversibly in solution with lifetimes ranging from milliseconds to days. The capsules isolate molecules from the bulk solution, and they reveal behaviors that cannot be seen otherwise. They have become a tool of modern physical organic chemistry (1). For example, cylindrical host 1.1 (Fig. 1) self-assembles only in the presence of suitable guests to give encapsulation complexes (2–4). Combinations of different guests (5) inside this capsule have allowed its use as a reaction chamber (6), a chiral receptor (7), and a space where single-molecule solvation can be observed (8). Elsewhere, reversible encapsulation complexes have been used to stabilize reactive intermediates (9, 10) and even transition states (11, 12) as well as to alter the course of reactions in the limited quarters (13). New forms of stereochemistry also have emerged from coencapsulation studies (14). For example, with benzene and p-ethyl toluene, two complexes coexist (Fig. 1). The two molecules are too large to slip past each other, and the p-ethyl toluene is too long to tumble freely while inside the capsule. Either of these motions could interconvert the complexes, but they are slow on the NMR time scale and separate spectra are seen for the two isomers. These “social isomers” are different arrangements in space that arise from the interactions of at least two encapsulated molecules. They also are constitutional isomers, but mechanical barriers hold these arrays in place rather than covalent bond “connectedness.” Accordingly, the term supramolecular diastereomers better describes the relationships. This stereoisomerism is related to the carceroisomerism (15) observed in covalently bound capsules with one guest.
Fig. 1.
The self-assembled cylindrical capule. (Upper) Chemical structure of the cavitand component and calculated structure of the capsule 1.1 with peripheral alkyl groups removed. (Lower) Cartoons of the capsule demonstrating the social isomers of benzene with p-ethyl toluene.
The self-assembly of the benzene/p-ethyl toluene pair owes its stability to the optimal filling of the capsule's space. Two benzene molecules can assemble the capsule as guests in that solvent and fill 41% of the space. With p-ethyl toluene, one molecule fills only 33% of the space, but two molecules are too long to be accommodated in the cavity. Accordingly, p-ethyl toluene can be combined with a number of coencapsulation partners, of which the benzene is at near optimum at filling 53% of the space (16). This matching of host space and guest size drives much encapsulation and is especially good for arranging bimolecular reactions inside (17, 18). It lies at the heart of the studies reported here.
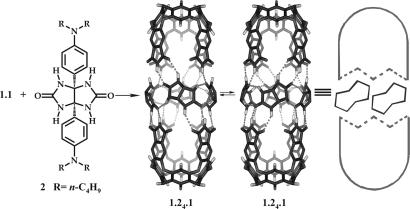
Capsule 1.1 reversibly incorporates glycoluril spacers 2 (Fig. 2) to give an expanded assembly 1.24.1 with suitable guests (19). Four glycoluril spacers were added and increased the cavity's volume by ≈200 Å3 and its length by ≈7 Å. A number of longer alkanes were encapsulated in this host assembly, but the expanded capsule also offered broadened possibilities for isomerism and accommodated other guests that we elaborate on here. The results indicate that highly complex molecular assemblies can emerge from only a few modules acting as hosts and guests (20, 21).
Fig. 2.
Formula of the glycoluril 2 spacer and semiempirical energy-minimized structure of the expanded capsule 1.24.1. Both enantiomers and hydrogen bonds are shown, but peripheral alkyl and aryl groups have been removed for viewing clarity. The cartoon schematic used elsewhere in the article is shown on the right.
Results
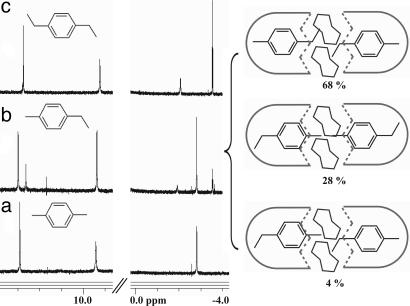
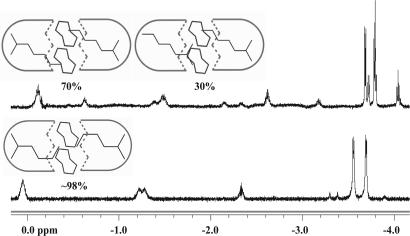
Simple p-disubstituted benzene derivatives are bound in 1.24.1, and the NMR spectra of p-xylene and p-diethyl benzene show complexes of high symmetry with two guests inside. The spectrum of encapsulated p-ethyl toluene, however, shows that three species are present (Fig. 3bLeft). These are social isomers; they reveal that there are several ways to fill the space, but what rules govern the arrangements?
Fig. 3.
Encapsulation of p-dialkylated benzenes. (Left) Downfield and upfield regions of the 1H-NMR spectra (600 MHz, mesitylene-d12) of p-xylene (a), p-ethyl toluene (b), and p-diethyl benzene (c) encapsulated in 1.24.1. (Right) The social isomers of p-ethyl toluene related to the spectra in b Left.
In the most favorable isomer, ethyl groups appear in the narrower center of the capsule, whereas the centrally positioned methyl of the tolyl group penetrates deeper into the tapered the resorcinarene ends. This arrangement apparently creates stronger C H/π interactions (22, 23) at the ends of the capsule than does a (rotating) ethyl group. Surprisingly, the unsymmetrical isomer, although favored statistically, is the highest-energy form of the three arrangements. The energetic differences between social isomers are small (ΔG° varies from ≈0.3 to 1.5 kcal/mol) and reflect not only the better “fit” in the space but also the interactions of the coencapsulated molecules.
H/π interactions (22, 23) at the ends of the capsule than does a (rotating) ethyl group. Surprisingly, the unsymmetrical isomer, although favored statistically, is the highest-energy form of the three arrangements. The energetic differences between social isomers are small (ΔG° varies from ≈0.3 to 1.5 kcal/mol) and reflect not only the better “fit” in the space but also the interactions of the coencapsulated molecules.
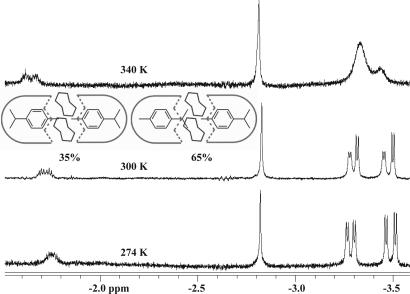
The social isomerism of two p-cymenes provided a surprise: a higher stability for the isomer with the methyl toward tapered ends of the cavitand (as for p-ethyl toluene) was expected, but this isomer was not detectable. Instead, the unsymmetrical isomer was favored 2 to 1 over the isomer with both isopropyl groups near the tapered ends (Fig. 4). Apparently, two isopropyls at the tapered ends or at the constricted middle of the capsule create steric clashes and do not fit the space comfortably. The chiral nature of the assembly is reflected in the doubling of signals of the isopropyl group: its methyls are diastereotopic. At first glance, the asymmetric elements are far from the cavity's end, but the chiral arrangement of the glycolurils at the center of the assembly can shift the eight imide walls in a manner that is transmitted effectively by the very rigidity of those walls (24). At higher temperatures, the chiral arrangement of the glycolurils begin to interconvert, and the signals of the diastereotopic methyls of the cymene guest coalesce.
Fig. 4.
Upfield region of the 1H-NMR spectra (600 MHz, mesitylene-d12) of p-cymene encapsulated in 1.24.1 at various temperatures. The broadening at higher temperatures is caused by interconversion of the two enantiomeric arrays of the glycoluril spacers in the capsules; the social isomers do not interconvert on the NMR time scale.
Simple alkanes that are too long to tumble freely in the capsule also show that multiple arrangements are accommodated. Fig. 5 shows the spectra of 2-methyl heptane and 2-methyl hexane. The former shows two of the three possible isomers, whereas the latter shows only one in the capsule at room temperature. Unlike alkyl aryls, alkanes with increased rotational degrees of freedom prefer to keep their bulky side in the tapered end of capsule to minimize steric clashes in the middle.
Fig. 5.
Upfield region of the 1H-NMR spectra (600 MHz, mesitylene-d12) of 2-methyl heptane and 2-methyl hexane in 1.24.1.
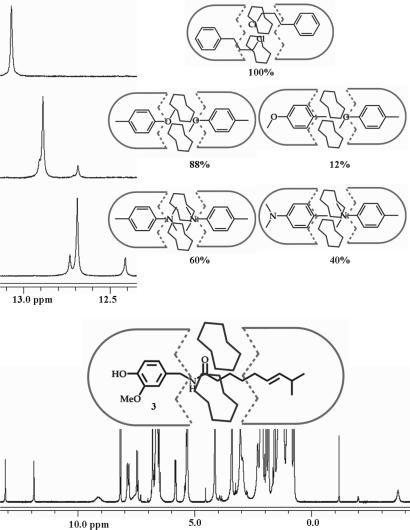
The seams of hydrogen bonds at the center of the capsular assembly favor polar guests, and this preference was evident in the spectra of N,N-dimethyl-p-toluidine, alkyl anisoles, and aryl alkyl halides. In each case, the major (if not the exclusive) social isomer showed the heteroatoms near the glycolurils [Fig. 6 and supporting information (SI)]. This preference is reinforced by the constricted center of the assembly, which is too narrow to comfortably accommodate substituted aryl groups. The irritant capsaicin 3 isolated from paprika also bears a terminal isopropyl group and is well encapsulated by 1.24.1. The broadened signal for this function suggests an intermediate rate of racemization of the glycoluril array.
Fig. 6.
Encapsulation of polar guests. (Upper) Downfield regions of the 1H-NMR spectra (600 MHz, mesitylene-d12) of N,N-dimethyl-p-toluidine, 4-methyl anisole, and 1-chloro-3-phenyl propane encapsulated in 1.24.1 and their social isomers in the extended capsule. (Lower) The NMR spectrum and cartoon of encapsulated capsaicin 3 also is shown.
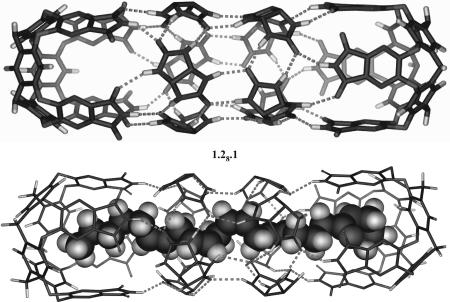
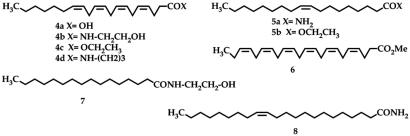
Preliminary results with normal hydrocarbons longer than C20 showed that an additional belt of glycolurils is incorporated to accommodate these guests when an excess amount of 2 is available. The formulation is shown in Fig. 7 as 1.28.1. We probed the adaptability of the larger assembly with a number of natural products having long and narrow shapes and found that they are readily encapsulated by the cavitand/glycoluril system (Fig. 8). These include arachidonic acid, 4a, the substrate for the biosynthesis of the prostaglandins, and several of its derivatives, including anandamide, 4b, the endogenous ligand for the cannabinoid receptor (25). Oleamide, 5a, one of the fatty acid amides involved in sleep induction (26), and its ethyl ester, 5b, also were encapsulated. The lengths of these guests in their extended conformations reached 26 Å, and they filled between 40% and 50% of the doubly extended capsule's space. The omega-3 acid methyl ester, 6, showed that at least six cis double bonds can be accommodated, and the ethanolamide of palmitic acid, 7, a saturated fat derivative, also was a good guest. The longest guest, at 27 Å, was 8. In the solvent mesitylene-d12, slightly more than one equivalent of these molecules is needed for complete encapsulation. Fig. 9(and the SI) shows their NMR spectra; no evidence of coiling in the alkyl regions took place in the capsule 1.28.1.
Fig. 7.
Hyperextension of the cylindrical capsule. (Upper) The energy-minimized structure of the doubly expanded capsule 1.28.1. Only one enantiomer is shown, and peripheral alkyl and aryl groups have been removed for viewing clarity. (Lower) Calculated structure of anandamide 4b in 1.28.1.
Fig. 8.
Structures of natural products and derivatives, including arachidonic acid, 4a; anandamide, 4b; oleamide, 5a; an omega-3 ester, 6; and palmitic ethanolamide, 7. All are encapsulated in 1.28.1.
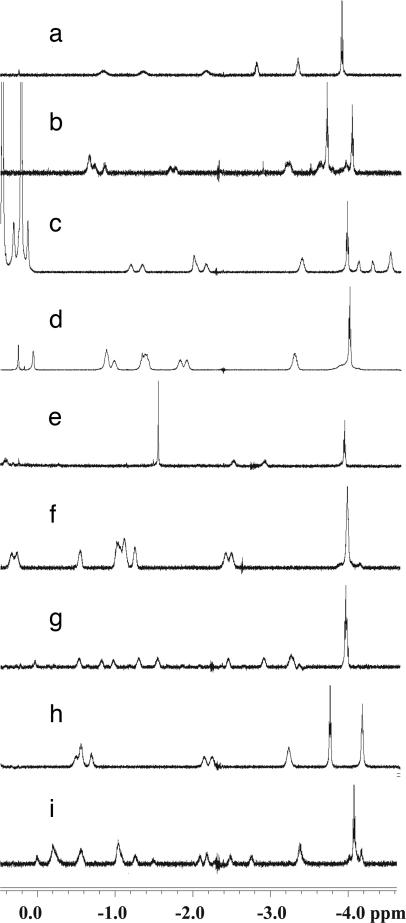
Fig. 9.
Upfield regions of the NMR spectra of encapsulated arachidonic acid, 4a (a); ethyl arachidonate, 4c (b); arachidonyl cyclopropylamide, 4d (c); anandamide, 4b (d); methyl docosohexaenoate, 6 (e); palmitoyl ethanolamide, 7 (f); 9-octadecenamide, 5a (g); ethyloleate, 5b (h);and 13-docosenamide, 8 (i).
The release of these natural products from the capsules can be accomplished by the addition of hydroxylic solvents. A few percent of methanol added to the mesitylene solvent disrupts the hydrogen bonds and destroys the assembly. The weakly basic sites of the glycolurils offer a site for protonation, and when acids are added to the mesitylene solvent, precipitation of the protonated glycolurils occurs with release of the guests. The assembly can be restored by the subsequent addition of tertiary amines (27). Accordingly, the uptake and release of these guests can be controlled through the polarity of the medium or acid/base chemistry.
Discussion
The multiple host/guest arrays shown by these assemblies differ from a “lock and key” recognition paradigm; there are several energetically comparable ways to fill the space given a specified “filling.” Rather, the term “induced fit” is more apt if it applies to the behavior of both partners; they mutually adapt for the optimal filling of space. The polar guests position themselves to best interact with the hydrogen bonding seams, and the flexible guests arrange themselves for a comfortable fit. The hosts do likewise by incorporating additional spacers to provide the capacity. There are examples in biology that resemble this behavior in those viral capsids that add protein units to expand the volume inside for longer genomes (28). For applications in the future, we note that two different molecules, each capable of social isomerism in 1.24.1, could result in four different arrangements. All combinations and the chiral nature of the assembly then expand the discrete possibilities to 20, and even many more are possible if different glycolurils are used simultaneously. If these arrangements can be assembled at will, maintained for longer time periods, and read out rapidly, then nanoscale information storage in these capsules would be possible. The encapsulation complexes of the natural products may be useful in situations where very slow release of biologically active agents, one molecule at a time, is desirable.
Materials and Methods
Reagents.
All reagents were obtained from commercial suppliers and used without further purification. NMR spectra were recorded on a Bruker DRX-600 spectrometer. The NMR samples were prepared as follows. For capsule 1.1, a 2 mM concentration of 1 and 1–20 mM concentrations of the appropriate guest were mixed with 0.6 ml of mesitylene-d12 in a NMR tube. The tube was placed in an ultrasonic bath (230 W) and sonicated for 5–10 min. Capsules 1.24.1 and 1.28.1 were prepared similarly to the above procedure, except for the addition of glycoluril 2 (4 mM for 1.24.1 and 8 mM for 1.28.1) to the mixture.
1H-NMR Spectra.
Complete 1H-NMR spectra of encapsulated N,N-dimethyl-p-toluidine, aryl alkyl halides, aryl alkyls, and natural products 3-8 are available in the SI.
Supplementary Material
Acknowledgments
We thank Prof. B. Cravatt (The Scripps Research Institute) for samples of the fatty acid derivatives. We are grateful to The Skaggs Institute and the National Institutes of Health (Grant GM 50174) for financial support. D.A. is a Skaggs Postdoctoral Fellow.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707759104/DC1.
References
- 1.Schmuck C. Angew Chem Int Ed. 2007;46:5830–5833. doi: 10.1002/anie.200702125. [DOI] [PubMed] [Google Scholar]
- 2.Heinz T, Rudkevich DM, Rebek J., Jr Nature. 1998;394:764–766. [Google Scholar]
- 3.Heinz T, Rudkevich DM, Rebek J., Jr Angew Chem Int Ed. 1999;38:1136–1139. doi: 10.1002/(SICI)1521-3773(19990419)38:8<1136::AID-ANIE1136>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 4.Körner SK, Tucci FC, Rudkevich DM, Heinz T, Rebek J., Jr Chem Eur J. 2000;6:187–195. doi: 10.1002/(sici)1521-3765(20000103)6:1<187::aid-chem187>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Rebek J., Jr Angew Chem Int Ed. 2005;44:2068–2078. doi: 10.1002/anie.200462839. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Rebek J., Jr Org Lett. 2002;4:327–329. doi: 10.1021/ol0168115. [DOI] [PubMed] [Google Scholar]
- 7.Scarso A, Shivanyuk A, Hayashida O, Rebek J., Jr J Am Chem Soc. 2003;125:6239–6243. doi: 10.1021/ja029574p. [DOI] [PubMed] [Google Scholar]
- 8.Scarso A, Shivanyuk A, Rebek J., Jr J Am Chem Soc. 2003;125:13981–13983. doi: 10.1021/ja037808e. [DOI] [PubMed] [Google Scholar]
- 9.Dong VM, Fiedler D, Carl B, Bergman RG, Raymond KN. J Am Chem Soc. 2006;128:14464–14465. doi: 10.1021/ja0657915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshizawa M, Kuskawa T, Fujita M, Yamaguchi K. J Am Chem Soc. 2000;122:6311–6312. [Google Scholar]
- 11.Yoshizawa M, Tamura M, Fujita M. Science. 2006;312:251–254. doi: 10.1126/science.1124985. [DOI] [PubMed] [Google Scholar]
- 12.Fiedler D, Bergman RG, Raymond KN. Angew Chem Int Ed. 2004;43:6748–6751. doi: 10.1002/anie.200461776. [DOI] [PubMed] [Google Scholar]
- 13.Kaanumalle LS, Gibb CLD, Gibb BC, Ramamurthy V. J Am Chem Soc. 2004;126:14366–14367. doi: 10.1021/ja0450197. [DOI] [PubMed] [Google Scholar]
- 14.Shivanyuk A, Rebek J., Jr J Am Chem Soc. 2002;124:12074–12075. doi: 10.1021/ja020607a. [DOI] [PubMed] [Google Scholar]
- 15.Timmerman P, Verboom W, van Veggel FCJM, van Duynhoven JPM, Reinhoudt DN. Angew Chem Int Ed. 1994;33:2345–2348. [Google Scholar]
- 16.Mecozzi S, Rebek J., Jr Chem Eur J. 1998;4:1016–1022. [Google Scholar]
- 17.Yoshizawa M, Takeyama Y, Okano T, Fujita M. J Am Chem Soc. 2003;125:3243–3247. doi: 10.1021/ja020718+. [DOI] [PubMed] [Google Scholar]
- 18.Yoshizawa M, Takeyama Y, Kusukawa T, Fujita M. Angew Chem Int Ed. 2002;41:1347–1349. doi: 10.1002/1521-3773(20020415)41:8<1347::aid-anie1347>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Ajami D, Rebek J., Jr J Am Chem Soc. 2006;128:5314–5315. doi: 10.1021/ja060095q. [DOI] [PubMed] [Google Scholar]
- 20.Ajami D, Schramm MP, Volonterio A, Rebek J., Jr Angew Chem Intl Ed. 2007;46:242–244. doi: 10.1002/anie.200603421. [DOI] [PubMed] [Google Scholar]
- 21.Valdés C, Spitz UP, Toledo L, Kubik S, Rebek J., Jr J Am Chem Soc. 1995;117:12733–12745. [Google Scholar]
- 22.Nishio M, Hirota M, Umezawa Y. The C—H/π Interaction: Evidence, Nature, and Consequences. New York: Wiley; 1998. [Google Scholar]
- 23.Grotzfeld RM, Branda N, Rebek J., Jr Science. 1996;271:487–489. doi: 10.1126/science.271.5248.487. [DOI] [PubMed] [Google Scholar]
- 24.Saito S, Nuckolls C, Rebek J., Jr J Am Chem Soc. 2000;122:9628–9630. [Google Scholar]
- 25.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 26.Cravatt BF, Lerner RA, Boger DL. J Am Chem Soc. 1996;118:580–590. [Google Scholar]
- 27.Ajami D, Rebek J., Jr J Am Chem Soc. 2006;128:15038–15039. doi: 10.1021/ja064233n. [DOI] [PubMed] [Google Scholar]
- 28.Verduin BJM, Bancroft JB. Virology. 1965;37:501–506. doi: 10.1016/0042-6822(69)90240-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.