Abstract
We used functional MRI and the anesthetic agent propofol to assess the relationship among neural responses to speech, successful comprehension, and conscious awareness. Volunteers were scanned while listening to sentences containing ambiguous words, matched sentences without ambiguous words, and signal-correlated noise (SCN). During three scanning sessions, participants were nonsedated (awake), lightly sedated (a slowed response to conversation), and deeply sedated (no conversational response, rousable by loud command). Bilateral temporal-lobe responses for sentences compared with signal-correlated noise were observed at all three levels of sedation, although prefrontal and premotor responses to speech were absent at the deepest level of sedation. Additional inferior frontal and posterior temporal responses to ambiguous sentences provide a neural correlate of semantic processes critical for comprehending sentences containing ambiguous words. However, this additional response was absent during light sedation, suggesting a marked impairment of sentence comprehension. A significant decline in postscan recognition memory for sentences also suggests that sedation impaired encoding of sentences into memory, with left inferior frontal and temporal lobe responses during light sedation predicting subsequent recognition memory. These findings suggest a graded degradation of cognitive function in response to sedation such that “higher-level” semantic and mnemonic processes can be impaired at relatively low levels of sedation, whereas perceptual processing of speech remains resilient even during deep sedation. These results have important implications for understanding the relationship between speech comprehension and awareness in the healthy brain in patients receiving sedation and in patients with disorders of consciousness.
Keywords: anesthesia, functional MRI, language, memory, sedation
As anyone who has tried to converse with a drowsy partner can testify, spoken language comprehension appears to be severely impaired at reduced levels of awareness. This impairment is difficult to assess behaviorally, because it is possible that speech comprehension remains intact, but the ability to produce spoken responses or to remember speech is impaired. Such a phenomenon may have parallels in two clinical situations: first, patients diagnosed as in a vegetative state (VS) or minimally conscious state may comprehend some or all speech but may be unable to report that fact because of impaired motor responses (1, 2). Indeed, near-normal neural responses in such patients have been reported in association with tests of speech comprehension (3). Second, some patients undergoing surgery under general anesthesia may comprehend some or all of what is going on around them. However, unless specific techniques are used, patients may be unable to signal that comprehension was intact or remember speech for later report (4). Such phenomena might suggest that speech can be both perceived and comprehended in the absence of conscious awareness or subsequent memory. However, functional imaging studies have so far failed to answer whether higher cognitive processing of speech is preserved in the absence of conscious awareness and have not provided neural correlates of the transition between conscious and nonconscious perception of spoken language.
In the present study, we tested the hypothesis that perceptual and semantic processes that contribute to speech comprehension and memory encoding can be differentially affected at reduced levels of awareness. We use functional imaging during graded sedation with an anesthetic agent to assess neural correlates of covert language comprehension at reduced levels of awareness. Previous functional MRI (fMRI) studies of volunteers under the influence of anesthetic agents have suggested that the additional activity normally observed in awake volunteers when speech is compared with matched nonspeech sounds is not observed after sedation or anesthesia (5, 6). What is unclear is whether this absence of speech-specific activity during anesthesia also implies a lack of comprehension. Neural responses observed in sedation-induced low-awareness states may also assist interpretation of responses to speech in VS and minimally conscious state patients. It is unclear whether neural activity that has been observed in some patients implies intact perception and comprehension of speech (2).
We used fMRI in conjunction with well controlled speech and nonspeech materials (7) to assess neural responses to speech sounds and sentence meaning during sedation with the anesthetic agent propofol. By comparing responses to sentences and acoustically matched nonspeech noises (signal-correlated noise, SCN), we can assess activity in regions of the superior and middle temporal gyri involved in perceiving spoken sentences while controlling for low-level auditory processes that are engaged for all sounds (8, 9). To assess neural responses to sentence meaning, we contrast responses to sentences that contain ambiguous words (such as bark or rain/reign) with matched sentences that lack equivalent ambiguities. Ambiguous words are ubiquitous in spoken language (10), and an additional process of contextually constrained meaning selection is necessary for successful comprehension of sentences that contain an ambiguous word (11, 12). Existing work has highlighted bilateral inferior frontal and left posterior inferior temporal regions, which show an elevated response to sentences containing ambiguities (7, 13). In the present study, we use this neural correlate of ambiguity resolution as a marker for intact sentence comprehension, which we can then assess at reduced levels of awareness.
In previous fMRI studies using propofol sedation, the drug was administered at a single infusion rate (6) or by a computer-controlled infusion system with a single “target” plasma concentration in all volunteers (5). In contrast, we individually tailored propofol infusion rates to generate specific levels of sedation in each of three scanning runs in each volunteer. Sedation levels were systematically assessed by using spoken response guided by the Ramsay sedation scale (14). Such ratings have previously shown a high degree of interrater reliability and therefore provide a systematic well validated measurement scale for the assessment of awareness levels (15). We assessed speech-specific perceptual processes and higher-level semantic processes at three qualitatively different levels of sedation: nonsedated (awake), lightly sedated (a slowed response to conversation), and deeply sedated (no conversational response but rousable by loud commands).
Results
Speech Perception and Comprehension.
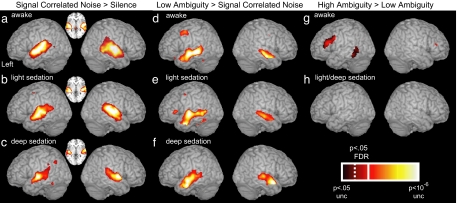
Contrasting blood-oxygen-level-dependent (BOLD) responses to SCN and silence (SCN vs. silence) highlighted activity in primary auditory regions on the superior temporal plane, centered on Heschl's gyrus. As shown previously (5, 6), auditory responses compared with a silent baseline are robust at all three levels of sedation tested, demonstrating that cortical auditory processes remain functional during sedation [see Fig. 1 a–c and supporting information (SI) Table 1]. To assess speech-specific neural responses, we contrast the BOLD response to low-ambiguity sentences and SCN (low vs. SCN). Consistent with previous fMRI findings in awake volunteers (7, 9), this contrast highlights anterior and posterior regions in the superior and middle temporal gyri (Fig. 1d; SI Table 2) that support perceptual processes critical for speech comprehension (16, 17). We also observe an additional response to speech in inferior frontal and premotor regions. A striking finding, however, is that the response of both temporal- and frontal-lobe regions remains robust in lightly sedated volunteers (Fig. 1e). Despite participants showing no response to conversational speech once deeply sedated, temporal (but not frontal) responses to speech remain largely intact (Fig. 1f).
Fig. 1.
fMRI response for simple contrasts between listening conditions at three levels of sedation. Contrast of SCN and silence thresholded at P < 0.05 FDR whole-brain corrected (solid line on scale) rendered onto a canonical brain image for nonsedated (i.e., awake) (a), lightly sedated (b), and deeply sedated (c) volunteers. Inset shows an axial slice at the level of Heschl's Gyrus (z = 0 mm). Activity for the contrast of low-ambiguity sentences and SCN at P < 0.05 FDR whole-brain corrected for awake (d), lightly sedated (e), and deeply sedated (f) volunteers. Brain regions showing significant activation for high- vs. low-ambiguity sentences for awake (g) and lightly/deeply sedated (h) volunteers. Results are thresholded at P < 0.05 FDR (dotted line on scale) within a search volume based on previous results (7). No voxels reach a corrected or uncorrected threshold (P < 0.01) during light or deep sedation.
Neural correlates of semantic processes involved in sentence comprehension were assessed by using the contrast between responses to high- and low-ambiguity sentences (high vs. low). The previous findings of inferior frontal and inferior temporal activity in this contrast are replicated for awake volunteers (Fig. 1g; SI Table 3). However, despite speech-specific activity in both lightly and deeply sedated volunteers, the contrast between high- and low-ambiguity sentences did not reveal any significant difference in activity even during light sedation (Fig. 1h).
The interaction between condition (SCN, low- and high-ambiguity sentences) and sedation (awake, light, and deep sedation) reveals a number of left-lateralized regions that show a condition-specific response to sedation, including the middle temporal, precentral, and inferior frontal gyri (see Fig. 2a; SI Table 4). As confirmed by paired comparisons, not all brain areas show the same condition by sedation interaction, and thus we observe dissociations among regions showing a progressive reduction of perceptual and semantic responses to speech at reduced levels of awareness (see Fig. 2 b–d; SI Table 5 and SI Fig. 4). For instance, the inferior frontal gyrus (IFG; Fig. 2b) shows a significant decline in the ambiguity effect (high vs. low) between awake and light sedation conditions but no reduction in perceptual responses to speech (low vs. SCN) during light sedation. In contrast, the middle temporal gyrus (MTG; Fig. 2c) and precentral gyrus (PCG; Fig. 2d) show a significant reduction in speech responses (low vs. SCN) during light sedation. Comparing the ambiguity response (high vs. low) during light and deep sedation fails to reveal any brain area that shows a significant decline in semantic processing. However, we do see a further decline in activity for speech (low vs. SCN) in all three regions mentioned previously (IFG, MTG, and PCG), although this fails to reach false discovery rate (FDR)-corrected significance in the MTG. Statistical comparison of the response profiles of the three peak voxels in the IFG, MTG, and PCG plotted in Fig. 2 b–d reveal a significant three-way brain region by condition by sedation interaction (P < 0.01) with paired comparisons of regions confirming that the response of the IFG is significantly different from both the MTG and PCG, which did not differ from each other. These three regions thus show differential condition-specific responses to sedation.
Fig. 2.
Condition-specific effects of propofol sedation on fMRI responses. (a) BOLD signal interaction between condition and sedation level rendered onto a canonical brain image. Results are thresholded at P < 0.05 FDR corrected (solid line on scale) within a search volume of areas responding to all sentences compared with SCN in awake participants. (b) Plot of BOLD signal changes compared with rest for high-ambiguity sentences, low-ambiguity sentences, and SCN at three levels of sedation from a peak voxel in the left IFG [solid white arrow in a, Montreal Neurological Institute (MNI) coordinates: x = −44, y = +22, z = +22]. Error bars show standard error of the mean excluding between-subject variability, appropriate for repeated-measures comparisons between conditions. Significance of two conditions by two sedation level interaction shown by braces (∗∗ reaches P < 0.05 FDR corrected, equivalent to P < 0.01 uncorrected; see SI Table 5 and SI Fig. 4). (c) BOLD signal in the left MTG (broken white arrow in a: x = −62, y = −34, z = −4), plotted as in b. ∗ interaction reaches P < 0.05 uncorrected. (d) BOLD signal in the left PCG (solid black arrow in a: x = −50, y = 0, z = +50).
Effects of Sedation on Subsequent Memory.
Recognition-memory data collected for 11 of the 12 volunteers revealed that memory performance was near ceiling for sentences presented while subjects were completely awake, at chance levels for sentences presented during deep sedation, and at intermediate levels for sentences presented during light sedation (Fig. 3a). Recognition-memory performance did not correlate with predicted propofol concentrations during either light or deep sedation (both P > 0.05). The effect of propofol sedation on memory for spoken sentences appears to be similar to that for words and pictures (18, 19). Of 11 volunteers, 9 remembered <50% of sentences presented during light sedation at mean effect-site concentration of 1.0 μg/ml, similar to previous estimates of effect site concentration for 50% correct memory (1.26 and 0.43 μg/ml; refs. 18 and 19).
Fig. 3.
Effects of sedation on sentence memory. (a) d′ recognition-memory performance at three levels of sedation (awake, light sedation, and deep sedation). Error bars show the standard error of the mean over subjects. Memory was above chance for sentences presented to awake and lightly sedated participants (∗∗∗, P < 0.001), although not for deeply sedated participants. Sentence recognition was impaired by light sedation [awake vs. light: t (10) = 6.16, P < 0.001] and further impaired during deep sedation [light vs. deep: t(10) = 4.13, P < 0.005]. (b) Brain regions showing a signification correlation between activity for sentences compared with SCN and recognition-memory performance during light sedation, thresholded at P < 0.05 FDR corrected (solid line on scale) within the same search volume as a. (c) BOLD response for sentences vs. SCN against d′ recognition-memory score for the left IFG [arrow in b, Montreal Neurological Institute (MNI) coordinates: x = −50, y = +28, z = −16]. The dashed line shows the best-fitting linear regression line (y = 0.023x − 0.013).
Memory for sentences presented during light sedation varied among volunteers and correlated with the magnitude of BOLD response to sentences in the left inferior frontal and middle temporal gyri (Fig. 3 b and c; SI Table 6). Comparison of neural responses to subsequently recognized and subsequently forgotten sentences presented during light sedation showed an additional response for remembered sentences in the posterior MTG (x = −52, y = −34, z = −2, Z = 4.10, cluster size 28 voxels, P < 0.05 FDR). A further analysis to assess the relationship between neural activity and recognition memory focused on responses in an anterior portion of the left hippocampus that has previously been associated with encoding verbal materials (y > −20, 20) Although neither analysis reached a corrected level of significance, a cluster of 21 voxels showed an additional response for subsequently remembered sentences during light sedation at P < 0.01 uncorrected (peak voxel, x = −10, y = −4, z = −16, Z = 3.02, P = 0.001 uncorrected, P = 0.145 FDR; see SI Fig. 5). We tested for an association between activity for high- vs. low-ambiguity sentences and recognition-memory scores (d′), but these did not correlate significantly, nor did ambiguity effects during light sedation become significant when responses were assessed only for sentences that were subsequently recognized.
Discussion
We demonstrate a graded and hierarchical reduction in the recruitment of processes involved in speech perception and comprehension with increased sedation. In awake and fully conscious participants, fMRI activation provides evidence for processes engaged in perceiving speech sounds, computing the meaning of ambiguous spoken sentences and encoding these sentences for subsequent memory. During light sedation, activity associated with speech–sound perception remained robust, yet recognition memory for sentences was variable and activity related to semantic ambiguity processing was absent. At the deepest level of sedation tested, participants were often difficult to rouse, showed substantially impaired responses to conversational speech, and were unable to encode sentences for subsequent memory. These observations are consistent with substantially impaired awareness of sentences presented during deep sedation. However, cortical activation was still observed for speech sounds in regions of the MTG that have previously been ascribed a critical role in speech perception (8, 9, 16, 17). Although there have been reports of temporal-lobe responses for participants' names compared with beeps during sleep (21) and for nonattended sentences compared with nonattended background noise (22), our work goes beyond these reports by demonstrating significant changes in neural responses associated with changes in awareness of speech sounds and by assessing neural correlates of semantic and mnemonic processing of sentences at declining levels of awareness.
Neural Correlates of Perceptual Awareness of Speech.
We believe the changes we observe in speech-specific responses between light and deep sedation provide a neural correlate of the transition between conscious and nonconscious perception of speech. We observed a striking change in the magnitude of inferior frontal and premotor responses to speech; these are both areas that have been previously associated with speech perception (9, 23–25). Studies that have demonstrated neural responses to speech in the absence of awareness, during sleep (21), or for nonattended presentations (22) report activity that is confined to the temporal lobe. This is consistent with the temporal-lobe responses that we observed during deep sedation. Our data go beyond these previous reports, however, in suggesting a direct association between reduced activity in prefrontal and premotor regions and reduced awareness of speech.
The association between conscious awareness for spoken materials and activity in frontal and premotor regions suggested by the current results is consistent with fMRI responses to brief masked presentations of written words, which can similarly evoke posterior cortical activity in conjunction with reduced prefrontal and premotor activation (26). However, whereas masked visual presentation also produces a marked reduction in activity in posterior regions involved in perceptual processing (such as the fusiform gyrus), temporal-lobe responses for speech compared with SCN during deep sedation remained robust and showed only marginal reductions relative to light sedation. Thus, in our work, as in another recent study of the neural basis of visual consciousness (27), frontal activity is most clearly associated with conscious awareness, and posterior regions show smaller changes in activity despite significant changes in the degree of conscious awareness. Such findings are consistent with a global-workspace account (28) in which conscious awareness for speech is supported both by activity in specific frontal systems and through coherent or synchronous activity in distributed frontotemporal regions.
Semantic and Mnemonic Processing of Speech at Reduced Levels of Awareness.
What is the role of conscious awareness in comprehension and memory for speech stimuli? This issue has previously been explored by examining behavioral and neurophysiological responses to speech that is outside the focus of attention. Listeners attending to one channel of dichotically presented speech can detect whether the nonattended channel contains speech or nonspeech sounds but fail to notice whether nonattended speech changes to a foreign language (29). Nonetheless, unattended words attract our attention, such as when one's name is called, a “cocktail party effect” that suggests some words are recognized in unattended speech (30), and recent behavioral work has demonstrated repetition priming effects from nonconsciously perceived words (31, 32). Mismatch negativity studies have shown evoked electrophysiological responses that vary with the lexical, semantic, and syntactic properties of isolated words in the absence of directed attention (33). However, in our work, neural correlates of ambiguity resolution, a process that is critical for successful comprehension, were significantly reduced and abolished even by light sedation with propofol. This finding suggests that propofol sedation produces an impairment in speech comprehension consistent with both reduced recognition-memory performance and slower and more hesitant verbal interactions during light sedation.
Our results provide evidence for an association between speech comprehension (as indexed by ambiguity-related activity) and awareness level. This may arise because the rapid efficient disentangling of semantic ambiguity requires coordinated activity in anatomically distant frontal and temporal regions, and these long-range functional interactions are highly susceptible to sedation. Previous observations of frontal and temporal cortical responses for the contrast of high- vs. low-ambiguity sentences in VS patients (2, 3) are therefore likely to be of great clinical significance. This conclusion is supported by the recent observation that one patient who fulfilled clinical criteria for VS demonstrated both sensitivity to semantic ambiguity and showed changes in neural activation in response to spoken instructions (3). Whether semantic ambiguity resolution can proceed in the absence of conscious awareness in healthy nonsedated volunteers remains unclear. Further research to assess neural correlates of ambiguity resolution in other low-awareness states, such as during dichotic speech presentation or using mismatch negativity paradigms, would be valuable.
Recognition-memory performance remained above chance for sentences presented during light sedation, even while activity related to semantic ambiguity processing was absent. This may indicate that participants can remember sentences they initially failed to comprehend. Indeed, we think that above-chance performance on the recognition-memory test could arise solely from familiarity with the perceptual form of heard words. In future studies, foil sentences that include a previously unused meaning of an ambiguous word could be used in the recognition-memory test to assess this possibility. However, given the complete failure of recognition memory for sentences presented during deep sedation, despite speech-specific responses in the temporal lobe, the present results confirm that neural correlates of perceptual processing of speech can remain intact even in the absence of subsequent memory for speech content.
Implications for Anesthesia.
Our results provide direct evidence of differential dose-dependent effects of i.v. anesthetics on the hierarchy of cortical processing required for speech comprehension. The dissociations we have observed among perceptual, semantic, and mnemonic responses to speech during sedation demonstrate that anesthetic agents affect cognitive function in a graded fashion. Higher-level processes involved in computing the meaning of sentences or encoding them into memory can be affected at relatively low levels of sedation, whereas lower-level perceptual processing of speech remains intact. These results support the view that anesthesia is a behavioral and neural continuum rather than a discrete event. This conclusion has potentially important implications for how patients with low residual blood concentrations of anesthetic drugs receive postoperative instructions. For instance, we saw a significant decline in left IFG activation in response to high-ambiguity sentences at sedation levels that produce only a moderate impairment in conversational speech. Impaired sentence comprehension may be particularly relevant (both clinically and medicolegally) in the context of ambulatory surgery, where patients are discharged home without formal clinical supervision after short surgical procedures under general anesthesia.
Our findings are also highly relevant to the phenomenon of awareness under anesthesia (34, 35), typically diagnosed when patients report postoperative recollection of events occurring during general anesthesia. Anesthetic awareness is a distressing complication with an overall incidence of 0.1–0.2% after surgery and a significantly greater incidence in patients undergoing cardiac surgery, cesarean section, and trauma surgery. Our results show that postsedation memory is correlated with activity in left frontal and temporal regions that have been associated with successful memory encoding of verbal materials in awake participants (36, 37). A previous positron-emission tomography study assessing the effect of propofol on resting cerebral blood flow has shown increased hippocampal activity during sedation (38). However, the present data are equivocal as to whether activity in hippocampal regions serves to support residual sentence memory during light sedation. Our observations may provide a neuranatomical substrate for successful memory encoding of speech overheard during anesthesia. The implied connections between the neuroimaging literature on memory encoding and postanesthetic recall provide a basis for further research on this clinically important topic.
Current electroencephalographic monitoring of anesthetic depth may significantly reduce awareness by providing neurophysiological targets for titration of anesthesia (34). However, the neurophysiological targets used for such titration have so far been validated against a definition of awareness, which includes explicit postoperative recollection of events. Such a definition may underestimate the true incidence of complex cortical processing of speech, because our data show that speech-specific neural activity in lateral temporal regions may occur without subsequent memory. The paradigms we have used may provide one way of calibrating anesthetic monitors, so that drug administration can be titrated to also prevent comprehension and both conscious and nonconscious perception of speech, rather than just subsequent recollection, thus satisfying both doctors' and patients' expectations concerning general anesthesia.
Methods
Participants and Paradigm.
Twelve right-handed English-speaking volunteers participated in the experiment (nine male); mean (range) age was 34 (29–42) years, and mean (range) mass was 70 (52–85) kg. All participants were medically trained anesthetists who gave written informed consent under the guidance of the Cambridge Local Research Ethics Committee in accordance with the Helsinki declaration.
We used the stimulus materials and experimental design from a recent fMRI study (7). There were two experimental conditions in which spoken sentences were presented: 59 high-ambiguity sentences containing two or more ambiguous words (e.g., “there were ‘dates’ and ‘pears’ in the fruit bowl”) and 59 well matched low-ambiguity sentences without “ambiguous” words (e.g., “there was beer and cider on the kitchen shelf”) (for further information, see ref. 7). An additional 59 sentences matched for duration and number of syllables and words were converted to speech-spectrum SCN using Praat software (www.praat.org). These stimuli have the same spectral profile and amplitude envelope as the original speech but are entirely unintelligible. An additional 60 silent trials were also included to allow comparisons between responses to nonspeech SCN and silent rest. These trials were divided into three runs of 79 trials with different conditions presented in a pseudorandom order. The order of the three runs was counterbalanced, so that different stimulus items were presented at each of three levels of sedation (nonsedated, lightly sedated, and deeply sedated) in different volunteers.
After participants were removed from the scanner and the sedative effects of propofol had worn off, a sentence-recognition-memory test was administered to 11 of the participants. This consisted of a written list of 236 sentences, including the 59 high- and 59 low-ambiguity sentences. Participants were asked to indicate which sentences they recognized having heard during scanning. Hit and false-alarm responses were analyzed to derive a signal-detection measure of sentence recognition-memory performance (d′), which corrects for biases induced by participants that respond consistently “yes” or “no” when unsure.
Sedation Procedure.
The 12 volunteers were scanned in three 12-min fMRI sessions while nonsedated (awake), lightly sedated (Ramsay score 2; ref. 14), and deeply sedated (Ramsay score 3), using propofol. Propofol is a GABAA potentiating compound commonly used by i.v. infusion for sedation during surgical procedures and intensive care. In higher doses, it is used for induction and maintenance of general anesthesia. In the present study, we used a manually implemented “effect-site steering” algorithm in conjunction with a computer-controlled infusion pump to achieve step-wise increments in the sedative effect of propofol. This method allowed us to individually tailor propofol infusion rates to generate specific levels of sedation for scanning runs in each volunteer (for further details, see SI Text).
Before each scanning run, we assessed participants' level of sedation by talking to them through the headphone and microphone system in the scanner and judged their level of sedation according to their verbal responses. Before administration of propofol, volunteers were fully awake, alert, and responsive, and thus could not be scored on the Ramsay sedation scale, which is intended for patients in an intensive care setting. During administration of propofol, participants become more relaxed and slowed in their responses to conversations, although without obvious slurring or impairment in conversation, consistent with level 2 on the Ramsay scale. Once participants no longer spontaneously engaged in conversation and responded in a slurred and impaired voice to loud commands or to their name being shouted, we deemed them to be at Ramsay 3. The mean (range) estimated effect-site propofol concentration was 1.0 (0.5–1.3) μg/ml during light sedation (Ramsay 2) and 1.5 (0.8–2.5) μg/ml during deep sedation (Ramsay 3). Mean (range) total mass of propofol administered was 231 (131–342) mg, equivalent to 3.4 (1.9–5.4) mg/kg. The variability of these concentrations and doses is typical for studies of the pharmacokinetics and pharmacodynamics of propofol.
Because of the time taken to recover from propofol sedation, it was not practical to fully counterbalance the order of the three sedation levels in different participants. Data were acquired in the following order: nonsedated, Ramsay 2, Ramsay 3 in 9 of 12 volunteers; and nonsedated, Ramsay 3, Ramsay 2 in the remaining three participants. Statistical comparisons of experimental and control data demonstrate that scan order effects are insufficient to account for observed effects of sedation (see SI Text).
fMRI Acquisition and Analysis.
fMRI data were acquired by using a Bruker Medspec 3 Tesla MR system (Ettlingen, Germany) and a sparse imaging procedure (40). For each volunteer, 243 echo-planar image volumes were acquired [repetition time = 9 s; acquisition time (TA) = 1.6 s; 21 × 4-mm-thick slices; interslice gap 1 mm; field of view, 24 × 24 cm; in-plane resolution of 3.75 × 3.75 mm], in three scanning runs of 83 scans with four dummy scans discarded before analysis. Preprocessing was performed by using standard procedures with SPM2 software (www.fil.ion.ucl.ac.uk/spm/software/spm2), and analysis was conducted by using a general linear model and random effects analysis (for details, see SI Text and ref. 7). Results are reported that exceed FDR-corrected threshold of P < 0.05 (41) for both whole-brain analysis and analyses using search volumes from previous results.
The interaction between stimulus condition and sedation level was assessed with an ANOVA implemented in SPM (42). i.v. anesthetics such as propofol have no major direct effects on cerebrovascular tone and preserve flow-metabolism coupling (43). Condition-specific effects of propofol sedation detected by this interaction analysis are unlikely to arise without differences in neural activity and cannot be explained by effects of scan order (see SI Text). To characterize these interactions, we performed a series of two-by-two interaction analyses comparing condition-specific responses at different sedation levels. Parameter estimates from peak voxels in the condition by sedation interaction were entered into repeated-measures ANOVAs. Significant three-way brain region by condition by sedation-level interactions demonstrate neural dissociations between perceptual and semantic responses to speech at different levels of sedation (44). These interactions cannot arise from vascular effects of an anesthetic drug in the absence of concurrent local effects on neuronal activity.
To relate recognition-memory performance and neural activity, we correlated the magnitude of activity differences between sentences vs. SCN with d′ recognition-memory scores (see SI Text for details). We also contrasted sentences that were subsequently recognized or forgotten in the postscan memory test. For both the sedation-by-condition interaction and subsequent memory analyses, a search volume (the brain region active for all sentences compared with SCN during the awake scanning session) was used to increase sensitivity.
Supplementary Material
Acknowledgments
We thank our volunteers and the radiographers at the Wolfson Brain Imaging Centre for their assistance. We thank Rik Henson, William Marslen-Wilson, and anonymous reviewers for comments and suggestions. We acknowledge the financial support of the U.K. Medical Research Council (including U.1055.04.013, to M.H.D.; U.1055.01.002, to A.M.O.; and Grant G9439390, ID 56833, to M.R.C. and D.K.M.), the British Oxygen Professorship of the Royal College of Anesthetists (A.R.A. and D.K.M.), the Leverhulme Trust (J.M.R.), and the Canadian Institute for Health Research and Canada Research Chairs Program (I.S.J.).
Abbreviations
- VS
vegetative state
- fMRI
functional MRI
- SCN
signal-correlated noise
- IFG
inferior frontal gyrus
- MTG
middle temporal gyrus
- PCG
precentral gyrus
- FDR
false discovery rate
- BOLD
blood-oxygen-level dependent.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701309104/DC1.
References
- 1.Schiff ND, Rodriguez-Moreno D, Kamal A, Kim KH, Giacino JT, Plum F, Hirsch J. Neurology. 2005;64:514–523. doi: 10.1212/01.WNL.0000150883.10285.44. [DOI] [PubMed] [Google Scholar]
- 2.Coleman MR, Rodd JM, Davis MHJ, IS , Menon DK, Owen AM. Brain. 2007 in press. [Google Scholar]
- 3.Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- 4.Russell IF. Br J Anaesth. 2006;96:346–352. doi: 10.1093/bja/ael017. [DOI] [PubMed] [Google Scholar]
- 5.Plourde G, Belin P, Chartrand D, Fiset P, Backman SB, Xie G, Zatorre RJ. Anesthesiology. 2006;104:448–457. doi: 10.1097/00000542-200603000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Heinke W, Fiebach CJ, Schwarzbauer C, Meyer M, Olthoff D, Alter K. Br J Anaes. 2004;92:641–650. doi: 10.1093/bja/aeh133. [DOI] [PubMed] [Google Scholar]
- 7.Rodd J, Davis MH, Johnsrude IS. Cerebral Cortex. 2005;15:1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- 8.Scott SK, Blank CC, Rosen S, Wise RJS. Brain. 2000;123:2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis MH, Johnsrude IS. J Neurosci. 2003;23:3423–3431. doi: 10.1523/JNEUROSCI.23-08-03423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodd JM, Gaskell MG, Marslen-Wilson WD. J Mem Lang. 2002;46:245–266. [Google Scholar]
- 11.Rayner K, Duffy SA. Mem Cognit. 1986;14:191–201. doi: 10.3758/bf03197692. [DOI] [PubMed] [Google Scholar]
- 12.Gernsbacher MA, Faust ME. J Exp Psychol Learn Mem Cognit. 1991;17:245–262. doi: 10.1037//0278-7393.17.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zempleni MZ, Renken R, Hoeks JC, Hoogduin JM, Stowe LA. NeuroImage. 2007;34:1270–1279. doi: 10.1016/j.neuroimage.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 14.Ramsay M, Savage T, Simpson B, Goodwin R. Br Med J. 1974;2:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Jonghe B, Cook D, Appere-De-Vecchi C, Guyatt G, Meade M, Outin H. Intensive Care Med. 2000;26:275–285. doi: 10.1007/s001340051150. [DOI] [PubMed] [Google Scholar]
- 16.Scott SK, Johnsrude IS. Trends Neurosci. 2003;26:100–107. doi: 10.1016/S0166-2236(02)00037-1. [DOI] [PubMed] [Google Scholar]
- 17.Hickok G, Poeppel D. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Henderson F, Absalom A, Kenny G. Anaesthesia. 2002;57:387–390. doi: 10.1046/j.1365-2044.2002.2410_1.x. [DOI] [PubMed] [Google Scholar]
- 19.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Anesthesiology. 1997;86:836–847. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Henson R. Q J Exp Psychol B. 2005;58:340–360. doi: 10.1080/02724990444000113. [DOI] [PubMed] [Google Scholar]
- 21.Portas CM, Krakow K, Allen P, Josephs O, Armony JL, Frith CD. Neuron. 2000;28:991–999. doi: 10.1016/s0896-6273(00)00169-0. [DOI] [PubMed] [Google Scholar]
- 22.Scott SK, Rosen S, Wickham L, Wise RJS. J Acoust Soc Am. 2004;115:813–821. doi: 10.1121/1.1639336. [DOI] [PubMed] [Google Scholar]
- 23.Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Nat Neurosci. 2004;7:701–702. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- 24.Burton MW, Small SL, Blumstein SE. J Cognit Neurosci. 2000;12:679–690. doi: 10.1162/089892900562309. [DOI] [PubMed] [Google Scholar]
- 25.Watkins K, Paus T. J Cognit Neurosci. 2004;16:978–987. doi: 10.1162/0898929041502616. [DOI] [PubMed] [Google Scholar]
- 26.Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, Riviere D. Nat Neurosci. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- 27.Lau HC, Passingham RE. Proc Natl Acad Sci USA. 2006;103:18763–18768. doi: 10.1073/pnas.0607716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Trends Cognit Sci. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Cherry EC. J Acoust Soc Am. 1953;25:975–979. [Google Scholar]
- 30.Moray N. Q J Exp Psychol. 1959;11:56–60. [Google Scholar]
- 31.Kouider S, Dupoux E. Psychol Sci. 2005;16:617–625. doi: 10.1111/j.1467-9280.2005.01584.x. [DOI] [PubMed] [Google Scholar]
- 32.Rivenez M, Darwin CJ, Guillaume A. J Acoust Soc Am. 2006;119:4027–4040. doi: 10.1121/1.2190162. [DOI] [PubMed] [Google Scholar]
- 33.Pulvermuller F, Shtyrov Y. Prog Neurobiol. 2006:49–71. doi: 10.1016/j.pneurobio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Myles PS, Leslie K, McNeil J, Forbes A, Chan MT. Lancet. 2004;363:1757–1763. doi: 10.1016/S0140-6736(04)16300-9. [DOI] [PubMed] [Google Scholar]
- 35.Sandin RH, Enlund G, Samuelsson P, Lennmarken C. Lancet. 2000;355:707–711. doi: 10.1016/S0140-6736(99)11010-9. [DOI] [PubMed] [Google Scholar]
- 36.Wagner A, Schacter D, Rotte M, Koutstaal W, Maril A, Dale A, Rosen B, Buckner R. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 37.Otten LJ, Henson RN, Rugg MD. Nat Neurosci. 2002;5:1339–1344. doi: 10.1038/nn967. [DOI] [PubMed] [Google Scholar]
- 38.Byas-Smith M, Frolich MA, Votaw JR, Faber TL, Hoffman JM. Mol Imag Biol. 2002;4:139–146. doi: 10.1016/s1536-1632(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 39.Schroeder M. J Acoust Soc Am. 1968;44:1735–1736. [Google Scholar]
- 40.Hall DA, Haggard MP, Akeroyd MA, Palmer AR, Summerfeld AQ, Elliott MR, Gurney EM, Bowtell RW. Hum Brain Mapp. 1999;7:213–223. doi: 10.1002/(SICI)1097-0193(1999)7:3<213::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Genovese CR, Lazar NA, Nichols T. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 42.Henson RNA, Penny W. ANOVAs in SPM, Technical Report. London: Wellcome Department of Imaging Neuroscience; 2003. [Google Scholar]
- 43.Menon DK. In: Fundamentals of Anaesthesia and Acute Medicine. Priebe H-J, Skarvan K, editors. London: BMJ Publications; 2000. [Google Scholar]
- 44.Henson R. Trends Cognit Sci. 2006;10:64–69. doi: 10.1016/j.tics.2005.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.