Abstract
Intracellular signaling pathways that share common components often elicit distinct physiological responses. In most cases, the biochemical mechanisms responsible for this signal specificity remain poorly understood. Protein scaffolds and cross-inhibition have been proposed as strategies to prevent unwanted cross-talk. Here, we report a mechanism for signal specificity termed “kinetic insulation.” In this approach signals are selectively transmitted through the appropriate pathway based on their temporal profile. In particular, we demonstrate how pathway architectures downstream of a common component can be designed to efficiently separate transient signals from signals that increase slowly over time. Furthermore, we demonstrate that upstream signaling proteins can generate the appropriate input to the common pathway component regardless of the temporal profile of the external stimulus. Our results suggest that multilevel signaling cascades may have evolved to modulate the temporal profile of pathway activity so that stimulus information can be efficiently encoded and transmitted while ensuring signal specificity.
Keywords: computational modeling, cross-talk, signal transduction, adaptive systems, filters
Intracellular signaling pathways that share components are common in nature. For example, in yeast, the mating, high-osmolarity, and starvation response pathways involve a common kinase (Ste11) (1, 2), yet the pathways respond to the appropriate environmental cues in very distinct and highly precise ways. In higher organisms, the situation is even more complex and undesired cross-talk underlies many pathological conditions (3–5). Therefore, understanding the mechanisms responsible for pathway specificity is a fundamental problem in signal transduction. Several solutions to this cross-talk problem have been proposed. Scaffold proteins are thought to limit cross-talk by sequestering and enabling activation of signaling molecules unique to a given response pathway (6–9). A classic example is the yeast scaffold protein Ste5, which is required for mating but not for the high-osmolarity or starvation responses. Another mechanism to achieve specificity is cross-inhibition, in which a signaling component unique to the stimulated pathway suppresses activation of the inappropriate response. Cross-inhibition is also used in the yeast mating response. In this case, stimulation with pheromone produces an increased degradation of a transcription factor (Tec1) involved in the starvation genetic program (10). Finally, in higher eukaryotes spatial localization of signaling molecules plays a role in pathway specificity as observed in the widely studied response of the ERK family of MAP kinases to different growth factors (reviewed in ref. 11) or in cAMP signaling in cardiac myocytes (12).
The mechanisms described above play important roles in achieving pathway specificity and have been analyzed systematically in recent studies (1, 13–16). However, one aspect of signaling networks that has not been considered in the context of signal specificity is the distinct temporal behavior exhibited by these systems. For example, the high-osmolarity and pheromone-response pathways display MAP kinase activity profiles that are markedly different. Highly osmotic conditions lead to a rapid and transient activation of the MAP kinase Hog1 with pathway deactivation occurring within 30 min (17). In contrast, saturating levels of pheromone produce a slow activation of the MAP kinase Fus3 with the maximum amplitude occurring at ≈60 min and significant pathway activity still present after 90 min. This striking difference in the dynamic response of these two networks led us to investigate the possibility that pathway specificity results from the temporal profile of the propagated signal.
We present a mechanism for signal specificity termed “kinetic insulation” that prevents cross-talk by virtue of the distinct chemical kinetics and network architectures of pathways that share common components. Our analysis reveals that temporal dynamics can be exploited by cellular systems to route information through a common component. We show that this approach is sufficient to maintain specificity and prevent cross-talk in a variety of scenarios. In addition, we discuss different strategies for encoding information that allows quantitative properties of the input stimulus (strength, rate of change, and duration) to be transmitted by the pathway and subsequently decoded by the cell.
Results
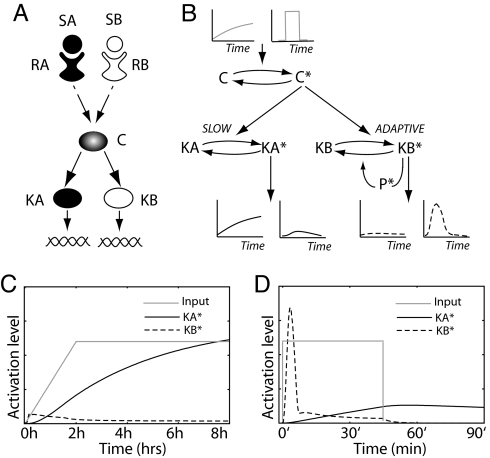
Fig. 1A shows a schematic diagram of the signaling system we consider. The system consists of two response pathways, A and B, that share the intermediate component C. Signaling through each pathway is initiated when the ligand, SA or SB, binds to the appropriate receptor, RA or RB, respectively. The correct response to either stimulus requires activation of the appropriate terminal kinase, KA or KB, without significant activation of the other. We demonstrate that, when the upstream components of pathway A cause C activity to increase slowly and the upstream components of pathway B cause activity to increase transiently, simple downstream pathway architectures can be constructed such that the kinases KA and KB discriminate between these two inputs (see Fig. 1B). We then show how the appropriate input signal to C can occur regardless of the temporal profile of the external stimulus.
Fig. 1.
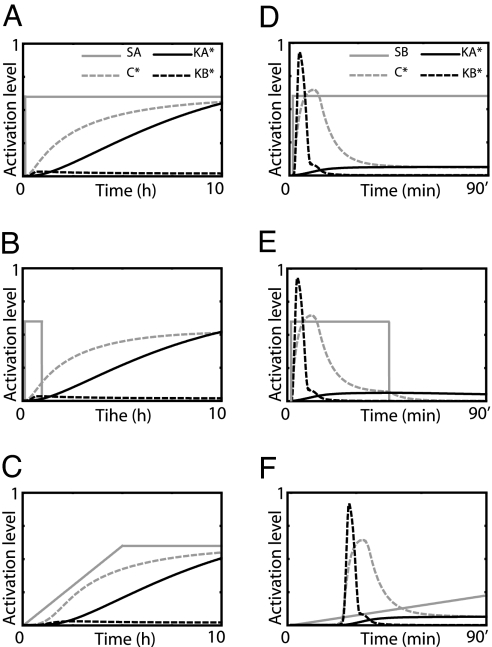
Kinetic insulation. (A) Pathways A and B share the component C. The terminal kinases, KA and KB, must respond to external cues received by receptors, RA and RB, respectively. (B) Slow kinetics prevents KA from being activated by a short transient signal, whereas the adaptive nature of KB prevents its activation by a slowly varying signal. (C and D) The temporal profiles of KA (black solid lines) and KB (black dashed lines) activity when component C is exposed to a slowly increasing signals (C, gray line) and square pulse lasting 45 min (D, gray line).
Discriminating Transient and Slowly Varying Signals.
In Fig. 1B, kinase KA responds to a slowly increasing signal while remaining virtually inactive if the signal is transient. The simplest way to achieve this behavior is if KA has slow activation kinetics relative to the time scale of the transient signal. Thus, KA acts as a “low-pass” filter ignoring the fast transient signal from pathway B. To achieve pathway specificity, KB has to be significantly activated only when C receives a transient input signal, but remains inactive if the input signal varies slowly in time. This is achieved by using an adaptive system. Adaptive systems generate a transient response, which eventually returns to prestimulus levels, even in the presence of a sustained input signal. This behavior can occur through the action of feed-forward (18) or negative-feedback loops (19). We recently demonstrated that to generate a large-amplitude signal an adaptive system must be excited by an input that increases rapidly with respect to the adaptation time scale (19). Otherwise, as the input signal increases, the system continuously adapts and the activity of the terminal kinase remains near its basal level. If the downstream components of pathway B form an adaptive system, then the pathway functions as a “high-pass” filter. That is, pathway B is unable to respond to the slowly increasing input signals resulting from the activation of pathway A, but produces a strong response to the fast transient signals from pathway B. Fig. 1B shows a schematic diagram of a pathway architecture involving a negative-feedback loop that produces such a system. In this case the kinase KB phosphorylates and activates a phosphatase (P*) that, in turn, dephosphorylates and deactivates KB (19). A consequence of this design is that KB activation is also transient. To provide a simple criterion for measuring the efficiency of kinetic insulation, we consider the maximum activation amplitude of KA and KB to be the most important characteristic of the propagated signal for determining whether a response is generated. Therefore, for the results presented below, the model parameters were chosen so that the duration of transient KB activation is roughly independent of the input signal strength, but the amplitude is signal strength-dependent (see Methods). We stress that this is not a requirement for kinetic insulation but rather a matter of convenience. Furthermore, the transient nature of the KB response is not a serious limitation because, as we show in the next section, a transient input signal can be transformed into a sustained output signal in a straightforward manner.
In general, the common pathway component C can play an active role in modulating the temporal response of the two branches. However, for simplicity, we assume that C undergoes stimulus-dependent phosphorylation and constitutive dephosphorylation. The rates of these two processes must be sufficiently fast so that C is able to respond to the transient input signal from pathway B without significant distortion of its temporal profile. The model equations for the system are given in Methods.
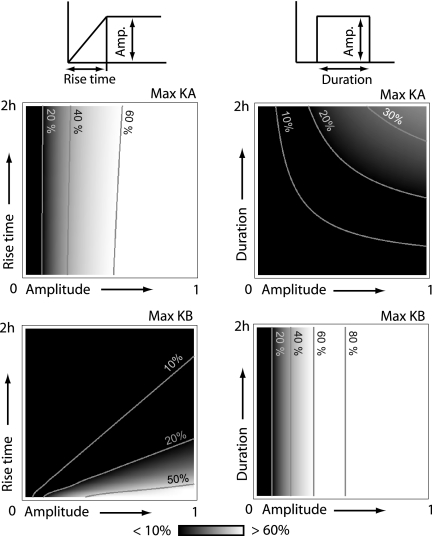
To test the ability of the system to discriminate between input signals with different temporal profiles, we performed a series of computational experiments in which C was activated by using either a slowly ramped input or square pulse. Typical input signals and temporal responses of KA and KB are shown in Fig. 1 C and D. As can be seen, KA and KB only respond when C is activated with an input signal of the appropriate temporal profile. To test the range of input signals that the system can discriminate, we repeated the simulations varying the final amplitude and rise time of the ramped input (Fig. 2Left) and the signal duration and amplitude for a square pulse (Fig. 2 Right). For a given input signal, we compared the maximum activation levels of KA (Fig. 2 Upper) and KB (Fig. 2 Lower) achieved during the 8-h period of the simulation. Fig. 2 Left clearly illustrates that, except for very small input amplitudes, KA is strongly activated by a ramped input whereas significant KB activation only occurs for very rapidly increasing input signals (Fig. 2 lower right in Lower Left). Fig. 2 Right shows the results when the input signals are square pulses of varying amplitude and duration. As can be seen, KB is strongly activated over a wide range of amplitudes and durations, whereas KA shows modest activity only for input signals of sufficient duration (>1 h).
Fig. 2.
System response to various input profiles. Species C is exposed to ramped inputs (Left) of various rise times and final amplitudes and square pulses (Right) of various amplitudes and durations. The gray scale indicates maximum activity level of KA (Upper) and KB (Lower) reached during the 8-h period. Black corresponds to activity levels of <10% of the total kinase abundance, and white corresponds to activity levels of >60% of the total kinase abundance.
The results presented above demonstrate that for pathways in which the signal must pass through a common element, simple downstream architectures can be used to selectively transmit a response based on the temporal profile of the input received by the shared component. We term this filtering mechanism “kinetic insulation.” We next considered what upstream architectures are required to convert receptor activity into appropriate input signals for the common pathway component. In the following section we describe mechanisms for encoding stimulus dose information into an appropriate temporal response.
Processing Stimulus Profiles into Appropriate Input Signals.
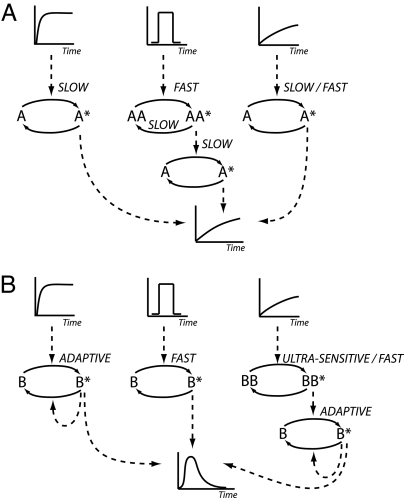
Kinetic insulation requires that the common signaling component receives distinct temporal inputs from the upstream branches of pathways A and B. However, depending on environmental conditions the cell can be presented with a variety of stimulus profiles. Therefore, a strategy must be in place to ensure that the appropriate signal is transmitted to the common component regardless of the temporal profile of the incoming stimulus. In our example, activation of pathway A requires that C receive a slowly increasing signal. However, the concentration of stimulus SA can vary slowly or rapidly in time. In the latter case, pathway A must be able to convert the rapidly changing stimulus level into a transmitted signal that slowly increases in time. In the same way, pathway B must be capable of transmitting a transient input signal to C in response to various SB profiles. Therefore, we consider strategies for generating transient and slowly increasing signals from three distinct stimulus concentration profiles: (i) sustained (a fast rise followed by a sustained stimulus), (ii) square pulse, and (iii) ramped. Fig. 3 depicts mechanisms that can be used to modulate pathway activity to generate the desired temporal profile for the six possible scenarios.
Fig. 3.
Simple architectures designed to modulate the temporal profile of the input stimulus. (A) Architectures that transform sustained, transient, and slowly increasing inputs into a slowly increasing output signal. (B) Architectures that transform the same set of inputs into a transient signal.
Transforming a sustained stimulus into a ramped response is easily achieved by a signaling species with slow activation kinetics (Fig. 3A Left). The simplest way to produce a ramped response from a square pulse stimulus involves two steps, designated here as AA and A (Fig. 3A Center). Initially, an upstream pathway component AA is rapidly activated by the transient stimulus to form the active and relatively stable AA*. The sustained level of AA* then slowly activates the species A generating the desired ramped response. Finally, a ramped stimulus can be transmitted directly as a ramped response (Fig. 3A Right). Or, if needed, an intermediate pathway component with slow activation kinetics can be used to slow down the response rate.
A sustained stimulus is converted into a transient signal by using an adaptive system (Fig. 3B Left). The production of a transient response from a square pulse stimulus is straightforward and achieved by using a signaling species with fast activation/deactivation kinetics (Fig. 3B Center). Additionally, an adaptive system can be used to guarantee a specific response duration or make other output properties independent of the stimulus profile. Converting a ramped stimulus profile into a fast transient signal requires multiple steps because adaptive systems tend to be insensitive to slowly increasing stimulus levels. The simplest architecture able to perform this conversion contains a signaling species BB with fast activation and slow deactivation kinetics directly upstream of an adaptive system consisting of signaling molecule B (Fig. 3B Right). Species BB rapidly amplifies the slowly increasing stimulus level and passes a relatively sustained signal to B, which in turn is converted into a transient output by the adaptive system. The high sensitivity of the upstream component BB makes this architecture very sensitive to noise. Use of an ultrasensitive switch (20) for BB helps to avoid a spurious response because the system only responds once the stimulus level crosses a threshold value. This has the additional effect of introducing a delay between the time the stimulus level starts increasing and when the downstream transient signal is generated. This delay depends on the rate at which the stimulus is increasing and provides a mechanism for modulating the response, depending on how fast the environmental conditions are changing. This analysis is not meant to be comprehensive, but rather provides the basic building blocks necessary to kinetically insulate pathways with common components.
Pathway Specificity Through Kinetic Insulation.
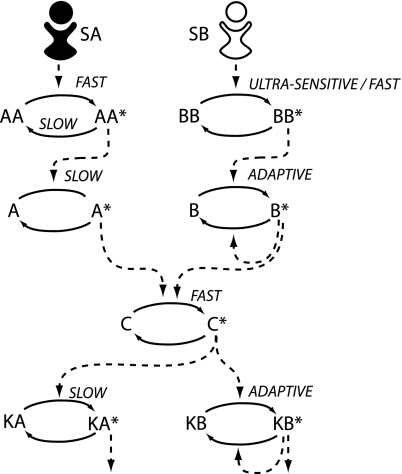
As a proof of principle, we combined the architectures shown in Fig. 3 with the kinetic insulation mechanism described in Fig. 1. The resulting model is shown in Fig. 4. For illustrative purposes, we designed the model so that both branches produce the correct signal profile for each of three stimulus profiles discussed above: sustained, square pulse, and ramped. To make pathway A responsive to a transient stimulus in addition to sustained and ramped stimuli, it is built from an upstream component AA possessing fast activation and slow deactivation kinetics, followed by a slow-reacting species A (Fig. 3A Center). Pathway B was designed with an upstream fast-reacting ultrasensitive species BB followed by an adapting species B (Fig. 3B Right). This endows pathway B with the ability to respond to ramped stimulus concentrations and to sustained and square pulse profiles. The model details and equations are given in Methods.
Fig. 4.
A model for kinetic insulation. The upstream components of pathway A transform diverse SA input signals into a slowly varying output signal that activates kinase KA but not KB. The upstream components of pathway B transform diverse SB input signals into a transient output signal, causing the activation of kinase KB but not KA.
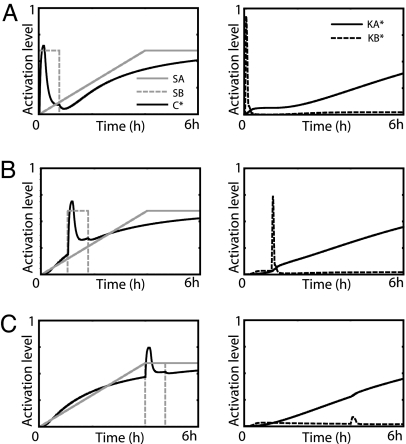
The three types of stimulus profiles discussed above were used for the simulations shown in Fig. 5. First, these profiles were taken as the concentration of SA and used to activate the receptor RA (Fig. 5 A–C). For all three stimuli (gray lines), AA is quickly activated, generating a long-lived signal that in turn causes the slow activation of A and the shared component C (dashed gray lines) as required for effective kinetic insulation. As a result, KA is significantly activated (black lines), whereas KB is activated only very weakly (dashed black lines) even when the stimulus is very transient.
Fig. 5.
Response to various stimulus profiles. A–C illustrate the system's response to sustained, transient (45 min), and ramped stimulus profiles, respectively, applied to pathway A. Times series for the stimulus SA (gray lines) and activity levels of C (dashed gray lines), KA (black lines), and KB (dashed black lines) are shown. D–F illustrate the system's response to the same stimulus profiles applied to pathway B. The gray lines are times series for SB. Note that, for this case, both KA and KB reach steady state within 90 min.
Fig. 5 D–F shows the response when pathway B is stimulated with the same profiles as used above (gray lines). As expected, the fast kinetics of the intermediate species BB and the adaptive nature of B work together to produce a transient signal that is propagated downstream by C (dashed gray line). This causes significant activation of KB (dashed black lines) with minimal activation of the slowly responding KA (black lines). The switch-like nature of the upstream component BB is evident by the delay seen in the activation of KB (Fig. 5F).
To test the model further, we studied the response when both branches are stimulated. If both stimuli appear simultaneously, the result is a superposition of the individual responses with KB activation occurring transiently and KA activation levels rising slowly (Fig. 6A). When the stimuli are not synchronized, the response depends on the order in which the branches are excited. When pathway B is stimulated first, pathway A remains competent for signaling and specificity is not affected. Conversely, when pathway A is stimulated first, signaling through pathway B is possible only if C activation is not saturated. Even then, depending on the mechanism of adaptation used in pathway B, KB activation may be diminished or not responsive to stimulation (19). In the example shown in Fig. 6, saturation of the common component C does not occur until long after pathway A is activated (Fig. 6 B and C). Interestingly, this feature provides an effective mechanism for cross-inhibition to prevent KB activation during the later stages of response A (Fig. 6C). However, it is possible to engineer pathway A in such a way that it does not saturate C, allowing pathway B always to remain competent for signaling.
Fig. 6.
Simultaneous stimulation of both pathways. (A) Application of a slowly increasing stimulus (Left, gray line) to pathway A and a square pulse lasting 45 min (Left, dashed gray line) to pathway B. Temporal profiles of the shared component C (Left, black line) and the terminal kinases KA (Right, solid line) and KB (Right, dashed line) are shown. (B) The same as in A, except the square pulse is applied to pathway B after a 1-h delay. (C) The same as in A, except the square pulse is applied after a 4-h delay. In this case, saturation of the common component C prevents signaling through pathway B.
Discussion
Many environmental stimuli elicit distinct cellular responses. However, most signaling systems contain components that are not unique to one pathway (1). Component sharing provides advantages for the cell, because it reduces the biological cost of assembling multiple signal transduction systems. However, it raises the fundamental question of how cellular pathways avoid inappropriate cross-activation. Signal specificity previously has been attributed to (i) scaffold proteins that recruit and assemble the appropriate signaling molecules and (ii) cross-inhibition in which the activity of competing pathways is suppressed (7, 8, 10, 13, 16, 21). We describe a third mechanism termed kinetic insulation that provides a means for achieving pathway specificity. In this scenario, signaling elements downstream of the common component are designed to respond only to specific temporal profiles of upstream pathway activity.
Our theory is founded on the observation that signaling cascades exhibit distinct dynamical behaviors, including differences in the persistence of activation (transient versus sustained signaling) as well as variations in activation and deactivation time scales. Indeed the dose and duration of an external stimulus can profoundly influence the magnitude or nature of the response. For example, yeast undergo two developmental fates in response to pheromone. Low levels of pheromone lead to filamentous growth, whereas high concentrations produce a mating response (22). Such observations led us to consider the possibility that cells use information encoded in the temporal profile of pathway activity to maintain signal identity. We reasoned that similar to modern communication devices that transmit multiple signals through a single channel, a cell might use biochemical networks to encode external cues into temporal patterns that can be received only by the intended target. Extending the analogy further, we designed simple architectures that can function as “filters” and combined them into a system capable of maintaining specificity under a wide range of conditions. Our computational experiments demonstrate how signal specificity can be achieved without the need for scaffold proteins or cross-inhibition. Furthermore, we showed that kinetic insulation allows the activation of one pathway without neutralizing the other, provided that the shared component is not saturated. This situation is easily achievable because the upstream portions of the pathways can be designed in such a way that saturation of the common component does not occur. Alternatively, saturation of the shared component by the slow pathway (pathway A in our example) provides an extra layer of control that can be used to prevent an undesired response to stimulus B once response A has been initiated.
Component sharing is particularly prevalent among MAP kinase signaling cascades. These pathways typically comprise three or more protein kinases acting in sequence. In the past, this level of organization was ascribed to a need for signal amplification or ultrasensitive responses (23, 24). In the context of kinetic insulation, the need to modulate the temporal profile of pathway activity necessitates the existence of multicomponent pathways. The yeast high-osmolarity and pheromone-response pathways could eventually be used to test the existence of kinetic insulation. Both pathways require the same MAP kinase kinase kinase (Ste11), yet the activation profiles of the respective MAP kinases, Hog1 and Fus3, are distinct. Pheromone stimulation produces a slow increase in Fus3 activity that persists for >90 min, whereas high osmotic stress leads to a rapid and transient increase in Hog1 activation (17). Furthermore, recent evidence (25) suggests that Hog1-dependent desensitization of the osmosensor Sho1 forms a feedback loop contributing to the adaptive nature of this pathway. Therefore, these two pathways display the required ingredients for kinetic insulation. In support of our model, a catalytically inactive form of Hog1 remains phosphorylated over a prolonged period, possibly due to loss of a negative-feedback loop responsible for adaptation; under these slowed activation conditions, stimuli that normally activate Hog1 result in inappropriate activation of Fus3 (26). Conversely, if a mutant can be found that accelerates Fus3 activation, the kinetic insulation hypothesis predicts that in this mutant Fus3 will be activated by high-osmolarity conditions.
The different modulation schemes studied above (Fig. 3) have important implications for how information about the stimulus profile is encoded. For example, information about a stimulus can be encoded as signal duration, amplitude, or rate of activation, depending on the kinetic requirements of the system. In the case of pathway A, which requires slowly increasing signals to produce a response, the activation rate is the only option. In pathway B, a transient signal is generated and therefore information about the stimulus can be encoded as signal duration or amplitude. However, if the pathway contains components that become saturated, information can only be encoded in the signal duration. The nature of the stimulus does not dictate the encoding strategy, as exemplified by the case of a sustained input generating a transient signal in pathway B. These considerations suggest that information processing and signal specificity are closely related issues.
As mentioned above, the addition of upstream components (AA and BB in Fig. 3) allows the system to respond appropriately to various stimulus profiles. However, it also tends to hinder the ability of the system to encode information about the quantitative characteristics of the stimulus. For example, in pathway B the conversion of slowly increasing signal into a transient output requires the saturation of component BB. This has the negative effect of rendering the system incapable of distinguishing transient versus sustained stimulus profiles as well as erasing quantitative information about the stimulus dose or duration. This feature is obviously undesirable when the detailed nature of the stimulus is important, and suggests that signaling pathways likely are tuned for a specific subset of stimulation profiles. Therefore, multibranched signaling pathways might result from the need to respond to different temporal profiles of the same stimulus. For example, one environmental cue that can occur over a wide range of time scales is osmotic stress. It is intriguing that the osmotic response of many simple eukaryotes consists of multiple branched pathways (27–29). This pathway architecture has been interpreted as providing either a backup system or a mechanism for sensing a wide range of osmotic conditions. The recent discovery that the Sho1 branch of the osmotic response of yeast is a rapidly adapting system (25) opens the possibility that the other pathway branches are necessary to allow the cell to respond to slow changes in osmolarity. Thus, multicomponent signaling architectures can provide enormous flexibility in transmitting detailed information about an incoming stimulus. In contrast, pathways leading to all-or-none responses would need only to relay qualitative information about the presence or absence of a stimulus, in which case a much simpler architecture would suffice.
Finally, a kinetic approach to specificity has some interesting advantages. First, it is robust. Cross-talk is avoided not just by virtue of a specific protein–protein interaction, but also by the inherent kinetics of the signaling species as well as network-level features of the upstream segments of the cascade. Additionally, kinetic insulation might reduce the biological cost and complexity of the system as compared with pathways that rely on protein scaffolds or cross-inhibition. Given the prevalence of multicomponent signaling cascades and the use of shared signaling components, it seems reasonable to speculate that the kinetic insulation mechanism described here applies to a broad array on intracellular signaling systems.
Methods
Activation and deactivation reactions were modeled using Michaelis–Menten kinetics. For simplicity, protein synthesis and degradation were not considered. Therefore, concentrations were normalized by the total amount of each species and the kinetic rate and Michaelis constants were modified accordingly. This resulted in the following set of equations:
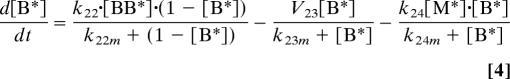 |
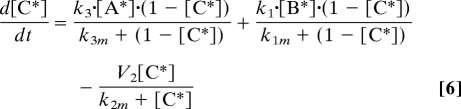 |
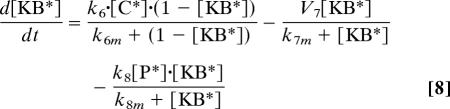 |
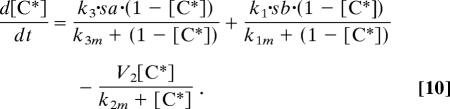 |
Eqs. 1–9 describe the full model of Fig. 4. Eqs. 7–10 correspond to the reduced model in Fig. 1 in which the input signal is fed directly to the common component C. The ordinary differential equations were solved in Mathematica (Wolfram Research, Champaign, IL).
The following set of or parameter values were manually selected to achieve efficient kinetic insulation: k1 = 5 × 10−2, k1m = 10, V2 = 2.5 × 10−2, k2m = 10, k3 = 5 × 10−2, k3m = 10, k4 = 7.5 × 10−4, k4m = 1, V5 = 6 × 10−5, k5m = 1, k6 = 5 × 10−2, k6m = 5 × 10−2, V7 = 1 × 10−3, k7m = 10−2, k8 = 4 × 10−1, k8m = 2 × 10−2, k9 = 1.5 × 10−4, k9m = 5 × 10−1, V10 = 5 × 10−5, k10m = 5 × 10−1, k20 = 2 × 10−1, k20m = 10−2, V21 = 10−2, k21m = 10−2, k22 = 10−1, k22m = 1 × 10−2, V23 = 3 × 10−2, k23m = 1 × 10−2, k24 = 5 × 10−1, k24m = 2 × 10−3, k25 = 5 × 10−4, k25m = 1, V26 = 5 × 10−5, k26m = 1, k30 = 5 × 10−3, k30m = 1, V31 = 2 × 10−5, k31m = 2.5 × 10−1, k32 = 10−4, k32m = 1, V33 = 5 × 10−5, k33m = 1.
The parameters were selected in a systematic fashion so that each pathway module has the appropriate input/output relationship and the system responds on biologically relevant time scales. The adaptive system used for KB can operate in three regimes (see ref. 19): (i) transient output signal with dose-dependent maximum amplitude and roughly constant width at half-maximum (low stimulus levels), (ii) transient output signal with constant amplitude and dose-dependent duration (medium stimulus levels), and (iii) sustained output signal (high stimulus levels). The parameters were chosen so the system operates in regime 1. Therefore, the degree of pathway activity is determined by the activation amplitude of KB.
The most important requirement for kinetic insulation is that the two pathways respond on distinct time scales. If this criterion is met, kinetic insulation is relatively insensitive to the choice of parameter values. Note that the Vmax values used in Michaelis–Menten expressions vary greatly. There are two potential explanations for this broad range. First, the pathways are built from very simple architectures. For example, to allow pathway B to adapt, we assume that a negative-feedback loop acts on the terminal kinase KB. Therefore, to produce significant pathway activity, the activation rate, k4, of the phosphatase that terminates signaling must be slow as compared with the activation rate, k6, of KB. This implies that the constitutive deactivation rate of the phosphatase, V5, must be even slower to allow the accumulation of significant phosphatase activity. By including more steps in the feedback loop or having the phosphatase target an upstream pathway component, the range of required rate constants can be made smaller and the sensitivity of the pathway improved (19). Second, the Vmax values are normalized by the total protein concentration, and therefore depend on the relative abundance of each species.
Acknowledgments
We thank Dr. Nan Hao for helpful discussions. This work was supported by National Institutes of Health Grants R01-GM079271 and R01-GM073180.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. B.K. is a guest editor invited by the Editorial Board.
References
- 1.Schwartz MA, Madhani HD. Annu Rev Genet. 2004;38:725–748. doi: 10.1146/annurev.genet.39.073003.112634. [DOI] [PubMed] [Google Scholar]
- 2.Dohlman HG. Annu Rev Physiol. 2002;64:129–152. doi: 10.1146/annurev.physiol.64.081701.133448. [DOI] [PubMed] [Google Scholar]
- 3.Muller R. J Cancer Res Clin Oncol. 2004;130:429–444. doi: 10.1007/s00432-004-0570-y. [DOI] [PubMed] [Google Scholar]
- 4.Shi W, Harris AL. J Mammary Gland Biol Neoplasia. 2006;11:41–52. doi: 10.1007/s10911-006-9011-7. [DOI] [PubMed] [Google Scholar]
- 5.Kalaitzidis D, Gilmore TD. Trends Endocrinol Metab. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Bardwell L. Biochem Soc Trans. 2006;34:837–841. doi: 10.1042/BST0340837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolch W. Nat Rev Mol Cell Biol. 2005;6:827–837. doi: 10.1038/nrm1743. [DOI] [PubMed] [Google Scholar]
- 8.Flatauer LJ, Zadeh SF, Bardwell L. Mol Cell Biol. 2005;25:1793–1803. doi: 10.1128/MCB.25.5.1793-1803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dard N, Peter M. BioEssays. 2006;28:146–156. doi: 10.1002/bies.20351. [DOI] [PubMed] [Google Scholar]
- 10.Chou S, Huang L, Liu H. Cell. 2004;119:981–990. doi: 10.1016/j.cell.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 11.Sacks DB. Biochem Soc Trans. 2006;34:833–836. doi: 10.1042/BST0340833. [DOI] [PubMed] [Google Scholar]
- 12.Vandecasteele G, Rochais F, Abi-Gerges A, Fischmeister R. Biochem Soc Trans. 2006;34:484–488. doi: 10.1042/BST0340484. [DOI] [PubMed] [Google Scholar]
- 13.Komarova NL, Zou X, Nie Q, Bardwell L. Mol Syst Biol. 2005;1:2005. doi: 10.1038/msb4100031. 0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardwell L, Zou X, Nie Q, Komarova NL. Biophys J. 2007;92:3425–3441. doi: 10.1529/biophysj.106.090084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaber J, Kofahl B, Kowald A, Klipp E. FEBS J. 2006;273:3520–3533. doi: 10.1111/j.1742-4658.2006.05359.x. [DOI] [PubMed] [Google Scholar]
- 16.Somsen OJ, Siderius M, Bauer FF, Snoep JL, Westerhoff HV. J Theor Biol. 2002;218:343–354. doi: 10.1006/jtbi.2002.3082. [DOI] [PubMed] [Google Scholar]
- 17.Klipp E, Nordlander B, Kruger R, Gennemark P, Hohmann S. Nat Biotechnol. 2005;23:975–982. doi: 10.1038/nbt1114. [DOI] [PubMed] [Google Scholar]
- 18.Alon U. An Introduction to Systems Biology: Design Principles of Biological Circuits. London: Chapman & Hall/CRC; 2007. [Google Scholar]
- 19.Behar M, Hao N, Dohlman HG, Elston TC. Biophys J. 2007;93:806–821. doi: 10.1529/biophysj.107.107516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldbeter A, Koshland DE., Jr Proc Natl Acad Sci USA. 1981;78:6840–6844. doi: 10.1073/pnas.78.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClean MN, Mody A, Broach JR, Ramanathan S. Nat Genet. 2007;39:409–414. doi: 10.1038/ng1957. [DOI] [PubMed] [Google Scholar]
- 22.Erdman S, Snyder M. Genetics. 2001;159:919–928. doi: 10.1093/genetics/159.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang CY, Ferrell JE., Jr Proc Natl Acad Sci USA. 1996;93:10078–10083. doi: 10.1073/pnas.93.19.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinrich R, Neel BG, Rapoport TA. Mol Cell. 2002;9:957–970. doi: 10.1016/s1097-2765(02)00528-2. [DOI] [PubMed] [Google Scholar]
- 25.Hao N, Behar M, Parnell SC, Torres MP, Borchers CH, Elston TC, Dohlman HG. Curr Biol. 2007;17:659–667. doi: 10.1016/j.cub.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 26.O'Rourke SM, Herskowitz I. Genes Dev. 1998;12:2874–2886. doi: 10.1101/gad.12.18.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Rourke SM, Herskowitz I. Mol Biol Cell. 2004;15:532–542. doi: 10.1091/mbc.E03-07-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gacto M, Soto T, Vicente-Soler J, Villa TG, Cansado J. Int Microbiol. 2003;6:211–219. doi: 10.1007/s10123-003-0136-x. [DOI] [PubMed] [Google Scholar]
- 29.Hohmann S. Microbiol Mol Biol Rev. 2002;66:300–372. doi: 10.1128/MMBR.66.2.300-372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]