Abstract
Chagas disease remains a serious obstacle to health and economic development in Latin America, especially for the rural poor. We report the long-term effects of interventions in rural villages in northern Argentina during 1984–2006. Two community-wide campaigns of residual insecticide spraying immediately and strongly reduced domestic infestation and infection with Trypanosoma cruzi in Triatoma infestans bugs and dogs and more gradually reduced the seroprevalence of children <15 years of age. Because no effective surveillance and control actions followed the first campaign in 1985, transmission resurged in 2–3 years. Renewed interventions in 1992 followed by sustained, supervised, community-based vector control largely suppressed the reestablishment of domestic bug colonies and finally led to the interruption of local human T. cruzi transmission. Human incidence of infection was nearly an order of magnitude higher in peripheral rural areas under pulsed, unsupervised, community-based interventions, where human transmission became apparent in 2000. The sustained, supervised, community-based strategy nearly interrupted domestic transmission to dogs but did not eliminate T. infestans despite the absence of pyrethroid-insecticide resistance. T. infestans persisted in part because of the lack of major changes in housing construction and quality. Sustained community participation grew out of establishing a trusted relationship with the affected communities and the local schools. The process included health promotion and community mobilization, motivation, and supervision in close cooperation with locally nominated leaders.
Keywords: community participation, deltamethrin, pyrethroids, Triatoma infestans, Trypanosoma cruzi
In Latin America, the burden of Chagas disease was estimated as 2.7 times the joint burden of malaria, schistosomiasis, leishmaniasis, and leprosy in 2002 (1). Ten to 14 million people are infected by Trypanosoma cruzi (Kinetoplastida: Trypanosomatidae), and 40–120 million people are at risk of infection (2). More than 150 species of mammals are hosts. T. cruzi is spread in humans by nearly a dozen bloodsucking triatomine species that infest poor rural houses and their outbuildings, by blood transfusion, and from infected mothers to offspring. After a short, mostly subclinical, acute phase and a very long asymptomatic phase, 25–40% of infected individuals develop chronic disease with cardiac, digestive, or neurologic manifestations (2, 3).
Chagas disease transmission is controlled mainly by spraying residual insecticide on houses and their outbuildings and by screening blood donors. Housing improvement should largely reduce infestations, but it has had a marginal role in vector control programs except in Venezuela (4). No effective vaccine for T. cruzi is available. The effectiveness of two drugs, benznidazole and nifurtimox, currently recommended for etiologic treatment, is high during the acute phase and in young children ≤12 years of age but is not well defined in chronic adult patients (2).
The “Southern Cone Initiative” is a regional intergovernmental control program launched in 1991 with the original objectives of eliminating all domestic and peridomestic populations of the main vector Triatoma infestans and transmission of T. cruzi via blood transfusion by the year 2000 (3, 5). Insecticide spraying reduced the geographic range of T. infestans and led to the interruption of transmission in Uruguay, Chile, Brazil, and some other areas (3, 5). However, at the heart of its distribution in the Gran Chaco, T. infestans persists for reasons that remain poorly defined, and growing numbers of symptomatic, vector-mediated acute cases of Chagas disease have been reported in eight Argentine provinces since 2001 (www.direpi.vigia.org.ar/boletines/semana47N52.xls; accessed January 29, 2007). This persistence of infestation raises questions about the sustainable management of vector-mediated transmission. The 2005 target for interruption of transmission of Chagas disease set by the World Health Assembly in 1998 and others (ref. 2, p. 69) has not been met, the elimination of T. infestans is lagging, and the Gran Chaco has recently become one of the current priorities of the ongoing Southern Cone Initiative.
The Gran Chaco, an ecoregion covering 1.3 million square kilometers and mainly extending over Argentina, Paraguay, and Bolivia, has experienced accelerated ecosystem degradation leading to high levels of poverty (6). With typical mud-and-thatch rural houses, it is hyperendemic for Chagas and other neglected diseases. Insecticide resistance to pyrethroids in T. infestans recently emerged there (7). The main challenges are fast reinfestation after residual insecticide spraying and the lack of a sustainable surveillance system for sparse and remote rural populations.
Vector control programs traditionally had a centralized vertical structure with highly motivated professional spray workers and technicians, frequently with the time-limited goal of vector elimination, and massive funding (8). Failure to eliminate the vectors from defined regions led to more emphasis on control (suppression) and long-term sustainability through community-based broad social participation (9, 10). The present project, conducted in cooperation with the Argentine National Vector Control Program (NVCP) and other research institutions, addressed the long-term sustainability and effectiveness of a community-based control strategy in the Gran Chaco. The initial objectives of the project were to characterize transmission risk and establish the interrelations among environmental, entomological, parasitological, and serological variables of Chagas disease in a well defined rural area centered in Amamá village, typical of regional conditions in the Argentine Chaco; to assess the long-term effects on house reinfestation and infection with T. cruzi of residual spraying with pyrethroid insecticides as conducted routinely by vector control personnel (alone or in combination with plastering of walls) or villagers; and to construct and test an empirically based mathematical model of the transmission of T. cruzi (11). In this article, we integrate published evidence and present new data on the long-term effects of community practices on bug-related indices and human transmission during 1984–2006. By using a before–after study design, we assessed the outcomes of sustained, supervised, community-based control by comparing the preintervention, reinfestation, and sustained-intervention states of the study area centered in Amamá.
Results
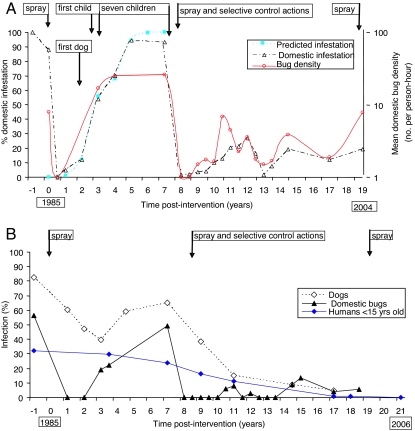
Two community-wide campaigns of insecticide spraying 7 years apart exerted immediate and very strong impacts on infestation and mean bug density in infested domestic sites (i.e., human sleeping quarters, as opposed to animal corrals and other outbuildings) and infection with T. cruzi in domestic bugs and dogs (Fig. 1). The seroprevalence of children also decreased, although more gradually and linearly (r2 = 0.96). Before any intervention, in Amamá the human prevalence of seropositivity to T. cruzi was 38.2% (number examined, n = 178), and Chagas disease myocardiopathy was frequent (12, 13). The second intervention era caused an ever- decreasing trend in dog infection, very low domestic bug infection, and no seropositive child by 21 years postintervention (YPI). Domestic bug density did not surpass one-third of previous peaks. Throughout the study, 1,645 diagnoses of infection in dogs and 2,316 in humans (from 870 identified residents) were obtained.
Fig. 1.
Prevalence of domestic infestation (observed and predicted by a logistic model), mean domestic density of T. infestans, and the timing of appearance of new cases of T. cruzi infection after two community-wide campaigns including residual insecticide spraying, Amamá and neighboring villages, 1984–2006. (A) Domestic infestation and bug density and timing of appearance of new cases and insecticide sprays. (B) Infection with T. cruzi in domestic T. infestans, dogs, and children <15 years of age.
During the first era of professional community-wide insecticide spraying followed by sporadic bug monitoring and no control action in Amamá village (1985–1992), the first insecticide campaign ever done (in 1985) reduced domestic infestation from 88% of houses at baseline to nil during the first 6 months after intervention (Fig. 1A). Domestic reinfestation returned to baseline values at 5–7 YPI, fitting closely to a logistic model (r2 = 0.997) (14). Mean domestic bug density also increased sigmoidally. The speed of domestic reinfestation was positively associated with the preintervention abundance of domestic bugs and the occurrence of unplastered or cracked walls. Infection with T. cruzi in domestic bugs fell from 56.5% to 19.1–22.4% at ≈3 YPI and thereafter increased to 49.1% at 7 YPI (Fig. 1B). Infection in dogs declined exponentially from 82.5% to 39.8% at 3 YPI (coefficient, −0.26/year, r2 = 0.98) and rose again to 65.1% at 7 YPI as domestic reinfestation increased, departing after 3 YPI from the predicted exponential decay in the absence of transmission (15) (Fig. 1B). In Trinidad and Marcedes villages before any intervention, dogs and cats experienced a similar force of infection even at very low infected-bug densities and contributed, respectively, 13.9 and 4.8 times as much as humans to infection of domestic bugs (16).
New human cases of T. cruzi occurred 2–3 years after the initial detection of domestic reinfestation at the child's house, and cases among dogs preceded the first child case (Fig. 1A). We report incidence as a number of new cases per 100 years (person-year or dog-year) of observation and use the abbreviation PHY (per hundred years). The average incidence in children (by seroconversion) between 4 and 7 YPI (4.3 PHY) was ≈17 times lower than the force of infection estimated from age-seroprevalence data in the local dog population (72.7 PHY, 95% confidence interval, 17.7–100 PHY) (17); the latter was similar to those recorded before any intervention [supporting information (SI) Fig. 4]. All seven child incident cases detected were reportedly asymptomatic and autochthonous; one occurred under a very light domestic infestation detected only at the endpoint, when most bugs had fed on humans.
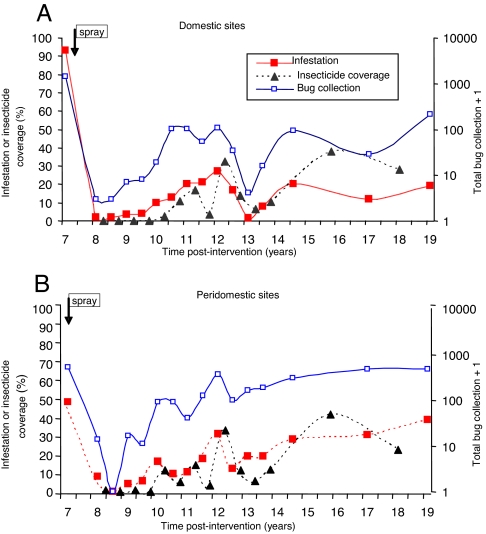
During the second era of professional community-wide insecticide spraying followed by intense bug monitoring and community-based selective control in five villages (1992–2006), the prevalence of T. infestans decreased in domiciles from ≈100% at 7 YPI (1992) to 4% at 9 YPI and thereafter peaked at 27% and 20%, whereas peridomestic infestation peaked three times (Fig. 2). The total catch of bugs in peridomestic structures was 3.1-fold higher than in domiciles, where bug densities were typically low and colonies transient. The earliest detected foci after interventions occurred in peridomestic sites (18). One of them remained persistently infested despite repeated insecticide sprays and was the putative source of new foci detected up to 450 m around over successive years (19). Domestic reinfestation was significantly and positively associated with the occurrence of an infested peridomestic site at the house compound and with high-density domestic infestations before interventions; a few cases of passive transportation of T. infestans in householders' belongings from infested peripheral villages were detected (18). Householders were very effective in gathering evidence of domestic bug infestation and house invasion. Domestic infestation prevalence (as determined by any of three methods) in well plastered houses was consistently higher than in houses with walls with some or many cracks; this pattern was determined by householders' collections of adult bugs invading from elsewhere and not by established colonizations (18) (SI Table 1). Domestic bug abundance in infested houses was equally depressed in Amamá vs. Trinidad-Mercedes villages regardless of the current state of wall plaster, whereas domestic bug infection only occurred in houses with wall plasters currently cracked or nonexistent.
Fig. 2.
Prevalence of infestation by T. infestans, total bug collection, and insecticide spray coverage carried out by professional spray workers (1993–1995) or householders (1996–2004) in Amamá and four neighboring villages during sustained surveillance with selective control actions. (A) Domestic sites. (B) Peridomestic sites.
The percentage of existing house compounds that were sprayed with insecticide at least once during a given year (crude insecticide coverage) closely followed peaks of infestation and strongly reduced infestation and bug abundance at the community level at 12 YPI but caused less-evident effects thereafter (Fig. 2). Adherence to insecticide spray fluctuated with infestation and bug abundance over time. T. infestans populations collected locally and in Santiago del Estero have not been found to be pyrethroid-resistant so far.
The impact of sustained surveillance and selective insecticide sprays on infection was larger than on infestation. The prevalence of T. cruzi in domestic T. infestans decreased from 49.1% (20) at 7 YPI to a mean of 5.6% over the subsequent 12 years, whereas peridomestic bug infection steadily decreased from 5.8% to a mean of 1.3% despite the steady rise in local bug abundance (SI Fig. 5). The two small peaks of infection in domestic bugs at ≈11 and 15 YPI coincided with upsurges of domestic infestations. All of the few infected bugs found after the 1992 interventions were fifth instars and adults, whereas infections were detected as early as second or third instars (28% infected) in 1992 (SI Fig. 6).
Based on early results (e.g., refs. 15–17), we used dogs as sentinels of domestic transmission of T. cruzi to minimize recurrent human blood sampling. The infection prevalence in dogs, mainly determined by three serologic tests at the same laboratory (21), decreased exponentially (coefficient, −0.27/year, r2 = 0.97) from 65.1% at 7 YPI to 4.7% at 17 YPI (Fig. 1B). Age-prevalence curves after 7 YPI progressively decayed to reach a very flat line at 17 YPI (Fig. 3A). Every survey after 7 YPI detected very young infected dogs, most of which were born to seropositive mothers (i.e., putative congenital cases) or were in-migrant dogs from nearby infested villages (22, 23). No seroconversion of dogs was detected between 9 and 11 YPI (n = 43) and between 14.5 and 17 YPI (n = 74), whereas only one incident case (with permanent residence in a house with T. cruzi-infected bugs) was detected between 11 and 14.5 YPI (n = 59), concurrently with the observed rise in domestic infestation. The observed incidence by seroconversion (0.26 PHY) from 11 to 17 YPI was very close to the average annual force of infection estimated from age-prevalence data at 17 YPI for all dogs (0.39 YPI) or for native dogs born after the 1992 interventions (0.28 PHY; 95% confidence interval, 0.00–0.72) (23).
Fig. 3.
Age-specific prevalence of T. cruzi infection in Amamá and four neighboring villages, 1984–2006, before initial interventions in 1985 and during sustained surveillance with selective control actions 1992–2006. (A) Prevalence of T. cruzi infection in dogs. (B) Seropositivity in humans <25 years of age. The prevalence of infection at birth (age 0) was assumed to be zero for both humans and dogs.
Control interventions exerted a sustained impact on human infection with T. cruzi (Fig. 3B). The age-seroprevalence curve recorded at 7 YPI did not differ significantly between Amamá and neighboring villages (24) and from that recorded at baseline (13) (SI Fig. 7). Age-prevalence curves were slightly less steep at 9 YPI (n = 255) and 11 YPI (n = 295), when infection in children <5 years of age declined to 7.5% and 0%, respectively. At 17–18 YPI (n = 153), we recorded one seropositive 12-year-old child (an in-migrant case who serorecovered after treatment) and a 4.8% prevalence in residents aged 15–24 years (i.e., two seropositive cases who had been treated for T. cruzi infection at 7 YPI). No seropositive child <15 years old was detected 20–21 YPI (n = 180).
Of 456 seronegative persons at first examination in 1992 or thereafter, 378 (83%) were reexamined serologically one to five times and contributed 2,246 person-years of observation (SI Table 2). After renewed interventions at 7 YPI, three individuals aged 2, 4, and 16 years seroconverted for T. cruzi, yielding an overall incidence of 0.134 PHY over 1992–2006. All of them seroconverted while living in houses not recolonized by triatomine bugs, and two of them reported spending several nights at highly infested houses in other rural villages; therefore, they were all considered regionally imported (not autochthonous) cases. Seven dubious seroconversions (with borderline serologic titers on one or two tests that subsequently either serorecovered or could not be confirmed) were considered false seroconversions; they were adult people living in houses not recolonized by bugs during sustained control. The incidence of infection fluctuated very little over time. Of 26 children seropositive for T. cruzi who were given standard parasiticidal treatment, 17 (65%) found to be seronegative at least once after treatment were considered cured.
All transmission indices observed at 17–18 YPI in the five study villages (core area, 137 houses) under sustained, supervised control were significantly exceeded by those recorded simultaneously in 35 peripheral rural villages (with 186 houses) under pulsed, nonsupervised, community-based control actions. These actions were promoted by NVCP mainly during 1993–1996 and executed by NVCP in 2000 in response to the local occurrence of a symptomatic Chagas acute case. The mean domestic abundance and infection prevalence of T. infestans in the pulsed control area were significantly higher (6.5-fold and 2.9-fold, respectively) than in the area under sustained control (23). Infection prevalence in dogs (11.8%, n = 221) and cats (6.6%, n = 61) in the pulsed control area doubled or tripled those in the sustained control area (4.7% and 2.1%, respectively). Unlike the latter, most of the infected dogs in the pulsed control area qualified as autochthonous, vector-mediated cases; evidence of recent transmission was indicated by the frequent occurrence of infected native dogs <3 years of age. The prevalence of T. cruzi in children <15 years of age varied little between 7 and 11 YPI (range = 17.9–25%, mean = 21.1%, n = 57), at approximately the onset of renewed province-wide control actions, and declined to 4.4% (n = 45) and 0% (n = 26) at 17–19 and 21 YPI, respectively. Of 49 residents of the pulsed control area seronegative at first examination in 1992 or thereafter and contributing 304 person-years of observation, three seroconverted for T. cruzi, yielding an overall incidence of 0.986 PHY over 1992–2006 (SI Table 2).
Discussion
Renewed interventions followed by sustained, supervised, community-based actions finally led to the interruption of local human T. cruzi transmission. Sustained vector control largely suppressed the reestablishment of domestic bug colonies and quickly reduced domestic bug and reservoir host infections. Seroprevalence in children decreased gradually through interruption of transmission, high local birth rates, case treatment, and emigration of infected individuals (no deaths were recorded). Different indices gave a consistent picture of current transmission levels. Age-prevalence curves (especially in dogs) reflected current and past levels of transmission at local and peripheral villages and can be used to infer the cumulative impact of control measures.
The first campaign in 1985 hardly modified transmission indices by 7 YPI compared with baseline levels. In the absence of modification of thatched roofs and peridomestic structures, plastering of walls did not detectably modify domestic reinfestation between 7 and 11 YPI. Domestic bug abundance and infections of bugs and mammalian hosts were equally depressed in the five study villages in sites with and without plastering. Had no selective vector control actions been conducted after insecticide spraying in 1992 and had the previous reinfestation process repeated, the cumulative number of new human infections that would have occurred but were averted by sustained interventions is 156 (calculations in SI Text).
Human infection usually is the crucial outcome for evaluating the impact of disease-control interventions. Human incidence with T. cruzi was greatly reduced, but exactly how much is uncertain because various serologic tests were used over >20 years; classical methods fail to detect a small fraction of human infections and to resolve <5% of serologically discordant sera (25, 26); a low incidence of infection greatly affects the positive predictive value of serologic tests, and fewer follow-ups were conducted on people in the peripheral villages. Repeated serodiagnosis in endemic rural areas over many years showed some departures from the expected pattern of lifelong seroreactivity to T. cruzi in humans (ref. 27 and this study) but hardly ever in dogs (16, 22). Although improved, highly specific serological tests are necessary to assess the impact of field interventions and treatment in humans, the use of dogs as disease sentinels and bug-related indices demonstrated large differences in domestic transmission between sustained and pulsed-control areas.
Sustained community participation in the core area grew out of establishing a trusted relationship with the affected communities and the local schools. The process included health promotion and community mobilization, motivation, and supervision, in close cooperation with locally nominated leaders; these leaders or their spouses held the very few stable jobs that were locally available, which allowed most of them to perform long-term unpaid volunteer work. Community participation was very likely enhanced by frustration at the previous failure to eliminate bugs during the first era; community awareness of the chronic lack of actions by the health sector; renewed, effective control interventions transferred to the local communities jointly with a continuous message to take action; increased community awareness of the occurrence of many infected children and of disease consequences, followed by etiologic treatment for T. cruzi leading to frequent cure; medical counseling of adult people; and regular guidance and provision of insecticides. When the study team visited the core villages less frequently, the population still adhered to insecticide spraying. Lack of continuity in the delivery of insecticides or replacement of compression sprayers in the peripheral area largely demobilized villagers and halted actions. Community participation is no spontaneous panacea. It plays a crucial role during long-term vector surveillance and control, whereas the suitability of community-based insecticide spraying during the attack phase is more controversial in terms of equity and cost-effectiveness. An important lesson learned was that case detection and treatment (unusual in the early 1990s and even now) combined with vector control enhances community acceptance and participation and contributes to sustainability of disease suppression.
The sustained community-based strategy succeeded in nearly interrupting domestic transmission of T. cruzi to dogs for over a decade but not in eliminating T. infestans despite the absence of any insecticide resistance. The reasons for such repeated failures in the Gran Chaco are multiple: (i) in the absence of major changes in housing construction or quality, many habitats suitable for T. infestans are available in mud-and-thatch houses and in the numerous peridomestic shelters for domestic animals; (ii) the effectiveness of pyrethroid insecticides is much lower at the prevailing high temperatures (when most insecticide sprays are typically conducted reactively), in peridomestic structures vs. indoors, and when infestations are heavy (14, 18, 28); (iii) insecticide coverage and vector surveillance are frequently incomplete in space and time; (iv) rural villagers' response to reinfestation lagged because they did not perceive peridomestic infestations as a hazard to their animals or themselves, which facilitated local bug propagation; and (v) political and economic support for Chagas control programs waned because it rarely exceeds the duration of a given governmental administration.
Disorganized decentralization of health services throughout Latin America in the early 1980s transferred responsibilities and few resources to the affected provinces or districts (29, 30). In the Argentine Chaco, residual provincial vector control programs progressively lost operational capacity (staff, vehicles), expertise, and coordination; when insecticide spraying rates fell, transmission resurged. Decentralization of vector control operations to the municipal level adds the unmet challenge of coordinating efforts among districts differing in control status, resources, and priorities and among national, provincial, and municipal public health levels. Districts that eliminate or control T. infestans in traditionally endemic settings are doomed to continuing expenditure on vector surveillance and eventual failure if neighboring districts fail to reduce transmission and infestation, as our study suggests. Such heterogeneities posed an insurmountable challenge to the sustainable elimination of T. infestans and transmission in the Argentine Chaco.
In the presence of persisting peridomestic infestations and a sizable domestic parasite reservoir, even very low levels of infestation allowed focal resurgence of transmission when vector control activities were relaxed. Flight dispersal of infected domestic bugs from a recently recolonized house to its peridomestic sites was recorded in the core area at 19 YPI (31). This dispersal event indicates that vector surveillance in high-risk areas must be continuous and open-ended, even after many years of vector control and declining prevalence. Targeting infected dogs to suppress infection is predicted to exert a large impact on domestic transmission (11). Etiologic treatment of infected children in the early chronic phase would prevent future disease and further reduce transmission but has been limited by inadequate treatment policies, the lack of access of the rural poor to diagnosis and treatment, and reduced availability of benznidazole or nifurtimox. Detection and treatment of infected children should be intensified widely for medical and epidemiological reasons.
Although insecticide spraying allows immediate and substantial reductions in transmission, rural housing improvement and other environmental management measures are highly cost-effective in the long run and will contribute to long-term sustainability by lessening the dependence on insecticides (32, 33). In the Chaco and nearby areas, plastering of walls and replacement of thatched roofs and of peridomestic enclosures for animals with appropriate designs and materials (e.g., wire fencing instead of piled branches of thorny shrubs) would render them less susceptible to triatomine colonization. Achieving such aims requires linking rural development agencies that promote improvements in agriculture and livestock production to housing and health ministries at national and provincial levels. Such housing improvements are justified on their own and require long-term economic, social, and political support.
This longitudinal study detected some persons who most likely acquired infection during short visits to other villages with higher levels of transmission and flux of infected dogs between houses, villages, and control areas. Transmission control, beyond its local impact in endemic regions, also protects others who travel to or from endemic regions or who receive a blood transfusion. Because sustained rural out-migration to cities causes socioeconomic problems that exceed the limits of the Gran Chaco, Chagas vector control and disease management must remain a regional effort within the frame of sustainable development rather than being viewed exclusively as a matter of health (34).
The tradeoff between the desire for complete elimination of infestation and transmission and the competing demands on limited resources in Chagas-endemic countries and districts is coupled with the limited political and economic power of the neglected populations affected by the disease. Sustainable social, political, and economic development; allocation and utilization of resources; and political leadership are essential for sustainable Chagas disease management. Without such transformations, Chagas disease will remain a health problem in large areas of rural and periurban poverty and a threat in much larger areas. In the Gran Chaco, health systems have to be strengthened and local personnel recruited, trained, and institutionalized. School-based health promotion and education may contribute significantly to sustainable vector surveillance and control but have not been exploited to their full potential. In such resource-limited settings, the welcome call for integrated, intersectoral, interprogrammatic action toward neglected disease control (34) finds very few potential local actors and many competing challenges (e.g., potable water delivery). Community-based efforts to control T. infestans are not sustainable unless the surveillance system is part of an established long-term national policy that mitigates the recurrent economic and political instability in the region. If successfully validated, the supervised community-based strategy after due adjustments may prove to be the most cost-effective approach for sustained control of transmission in the Andean and Central American regions where triatomine bugs thrive in sylvatic habitats and houses. The elimination of Chagas disease transmission and the sustained control of T. infestans in the Gran Chaco, closely related to several Millennium Development Goals, require more resources and a greater intensity of scientifically based coordinated control efforts at multiple scales from the village to the hemisphere.
Materials and Methods
Details of methods, study area and population, vector ecology, history of infestation, parasitology, and epidemiology appear in SI Materials and Methods and in refs. 11–24 and 31.
Study Area and Population.
Field studies were carried out in the neighboring rural villages of Amamá (latitude 27°1′S, longitude 63°0′W and latitude 27°8′S, longitude 63°8′W), Trinidad, Mercedes, Villa Matilde, and Pampa Pozo (core area), Moreno Department, Province of Santiago del Estero, Argentina. Moreno Department continues to have the highest number of Chagas acute cases ever reported in Argentina. In 1992–1993, 107 houses were marked with a numbered plaque and mapped, and 491 people were identified. Environmental, demographic, and lifestyle patterns were approximately similar between villages and areas (SI Table 3).
Study Design.
The first era (1985–1992, only in Amamá) included baseline surveys of triatomine infestation (by sensor boxes, timed manual and insecticide knockdown collections) and infection followed by residual insecticide spraying by NVCP in August-September 1985; annual surveys to interview householders and monitor domestic bug infestation and infection from 1986 to 1990 and in 1992; annual serological and xenodiagnosis (infection) surveys of dogs in 1984 (taken as baseline), 1986–1990, and 1992; and human serologic surveys in 1984 (all ages), 1989 (including only children <16 years old), and 1992–1993 (intended to achieve full coverage).
The second era (1992–2006, including five villages) included surveys of triatomine infestation and infection, dog and human infection carried out in February–October 1992 and May 1993; residual insecticide spraying (preceded by plastering of walls only in Amamá) in August–December 1992; annual or semiannual surveys to interview householders, monitor triatomine infestation and infection, and conduct or record insecticide sprays, since May 1993; periodic dog and human infection surveys starting in November 1994; clinical and electrocardiographic surveys of the human population (aimed at full coverage); and etiologic treatment of seropositive children <15 years of age during 1992–1996.
In March 1992, 71 houses were visited to search for triatomine bugs in all bedroom areas and peridomestic structures, to assess environmental risk factors, and to canvas householders for demographic data and habits, using similar procedures as in the first era. In August 1992, Amamá householders (but not those in the other four core villages) were invited to improve the plaster of indoor walls of their houses before the scheduled insecticide spraying of all domestic and peridomestic areas to take advantage of its residual effects (18). After house-to-house visits, an open meeting was held at the school to explain the program rationale and how this was expected to benefit householders. Householders were offered cement and assistance by two construction workers supported by the research project and were required to contribute at least by carrying water and soil. House improvements lasted 50 days. The state of wall plasters was assessed during spraying operations and every 6 months until late 1997. A total of 94 (98%) houses and their peridomestic structures were sprayed with deltamethrin by NVCP as before in October 1992. The effectiveness of spraying was assessed in November–December 1992, and the eight peridomestic foci of T. infestans detected were immediately resprayed with insecticides as before. All 13 houses from Villa Matilde and Pampa Pozo were sprayed with insecticides between October 1993 and May 1994.
The surveillance phase, from December 1992 to April 2004, included a strong community-participation component. During each visit, householders were asked about their bug collections and site of capture, the presence or sighting of triatomines in bedrooms or peridomestic areas, and other environmental and demographic data. Infestation was assessed by three methods as before. Health education related to Chagas disease was part of our interaction during home visits and with school teachers. Two construction workshops were held at the local schools, where a storeroom was built.
Control Actions.
From 1993 to 1995, only sites with at least one T. infestans adult and nymph were treated selectively with deltamethrin by NVCP as before. In line with the vector control strategy active in Argentina between 1993 and 1999 (“Plan Ramón Carrillo”) (29), vector surveillance and control activities were transferred to the communities through six 4-hour workshops conducted separately in Amamá and Mercedes schools between November 1995 and 1996 (20). Local residents were briefed on the main results of the research project and taught how to assemble and inspect sensor boxes, apply fumigant canisters, and spray their homes and outbuildings with insecticides. Members of 90 (87%) of the families with stable local residence participated in ≥1 of the workshops. Each community chose a member who would receive notifications of T. infestans captures, store the manual compression sprayers and insecticide, keep records of notifications and sprays, and help the affected families to treat their houses. Starting in 1996, the capture of one T. infestans bug of any stage was considered sufficient to justify treatment of all domestic and peridomestic areas of each house. Later, insecticide sprays were sometimes allowed when householders reported infestations without delivering bugs and when new houses were built. Insecticides were provided free of charge by NVCP and delivered during our periodic inspections for infestation; deliveries occurred less regularly between 1999 and 2001 because the federal government failed to make purchases and the nationwide community-based vector control plan was gradually weakening.
All 137 houses in the core area and 186 houses from the peripheral area were surveyed for demographic, entomologic, serological, and parasitological data in October 2002 to July 2003 as described in ref. 23. All houses and outbuildings were resprayed with deltamethrin by NVCP personnel in April 2004. The infested peridomestic sites were randomly sprayed with a standard or double insecticide dose. A blanket insecticide intervention trial launched in March 2005 included an additional 350 rural houses surrounding the peripheral area.
Supplementary Material
Acknowledgments
We thank María Moyano's family, Roberto Chuit, M. V. Cardinal, M. B. Castañera, L. A. Ceballos, J. M. Gurevitz, L. Lanati, P. L. Marcet, M. M. Orozco, R. M. Petersen, F. G. Petrocco, R. Piccinali, R. Rotondaro, J. Schachter-Broide, D. P. Vazquez, R. de Marco, G. M. Vazquez-Prokopec, E. Cura, M. A. Lauricella, C. Maidana, D. M. Canale, I. Castro, C. Spillmann, R. Stariolo, M. Zaidenberg, J. E. Zárate, and H. Coto. For assistance and data sharing, we thank personnel from Hospital Pirovano (Buenos Aires), who have developed a community health care project in Trinidad and Mercedes since 2002, and C. Piazza, L. Pons, and H. López Alcoba. J.E.C. thanks Mr. and Mrs. William T. Golden for hospitality and Priscilla K. Rogerson for assistance. R.E.G. thanks the European Community–Latin American Network for Research on the Biology and Control of Triatominae and Chagas Disease Intervention Activities networks for helpful comments. This work was supported by the National Institutes of Health (NIH)/U.S. National Science Foundation (NSF) Ecology of Disease program through NIH Research Grant R01 TW05836, the Fogarty International Center and the National Institute of Environmental Health Sciences (U.K., R.E.G., and J.E.C.), the University of Buenos Aires, the Rockefeller Foundation, Agencia Nacional de Promoción Científica y Técnica (Argentina), Fundación Mundo Sano, Fundación Bunge and Born (M.C.C.), and U.S. NSF Grant DMS-0443803 (to J.E.C.). E.L.S., R.E.G. and M.C.C. are members of Consejo Nacional de Investigaciones Científicas y Téchnicas de Argentina Researcher's Career, Argentina.
Abbreviations
- NVCP
National Vector Control Program
- PHY
per hundred years
- YPI
years post-initial intervention with year 0 in 1985.
Footnotes
This paper is part of a special series on Sustainable Health. See the related editorial on page 15969 and accompanying articles on pages 16038, 16044, and 16263.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700863104/DC1.
References
- 1.World Health Organization. World Health Report 2004: Changing History. Geneva: WHO; 2004. [Accessed May 30, 2007]. Available at www.who.int/whr/2004/en. [Google Scholar]
- 2.World Health Organization. Control of Chagas Disease. Geneva: WHO; 2002. p. 109. [Google Scholar]
- 3.Dias JCP, Silveira AC, Schofield CJ. Mem Inst Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- 4.Feliciangeli MD, Campbell-Lendrum D, Martinez C, Gonzalez D, Coleman P, Davies C. Trends Parasitol. 2003;19:44–49. doi: 10.1016/s1471-4922(02)00013-2. [DOI] [PubMed] [Google Scholar]
- 5.Silveira AC. In: El control de la enfermedad de Chagas en los países del Cono Sur de América. Historia de una iniciativa internacional. 1991-2001. Silveira AC, Rojas de Arias A, Guillén G, Russomando G, Schenone H, Dias JCP, Valdes Padilla J, Lorca M, Salvatella R, editors. Uberaba, Brazil: Facultad de Medicina do Triangulo Mineiro; 2002. pp. 15–43. [Google Scholar]
- 6.Bucher EH, Huszar PC. J Environ Manage. 1999;57:99–108. [Google Scholar]
- 7.Picollo MI, Vassena C, Santo Orihuela P, Barrios S, Zaidenberg M, Zerba E. J Med Entomol. 2005;42:637–642. doi: 10.1093/jmedent/42.4.637. [DOI] [PubMed] [Google Scholar]
- 8.Kitron U, Spielman A. Rev Infect Dis. 1989;11:391–406. doi: 10.1093/clinids/11.3.391. [DOI] [PubMed] [Google Scholar]
- 9.Rifkin S. Acta Trop. 1996;61:79–92. doi: 10.1016/0001-706x(95)00105-n. [DOI] [PubMed] [Google Scholar]
- 10.Forget G, Lebel J. Int J Occup Environ Health. 2001;7(Suppl 2):S3–S38. [PubMed] [Google Scholar]
- 11.Cohen JE, Gürtler RE. Science. 2001;293:694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- 12.Wisnivesky-Colli C, Ruiz AM, Ledesma O, Gürtler RE, Solarz ND, Lazzari J, Bujas MA, de Rissio AM, Barbieri G, Marteleur A, et al. Medicina (Buenos Aires) 1989;49:341–350. [PubMed] [Google Scholar]
- 13.Chuit R, Subias E, Perez AC, Paulone I, Wisnivesky-Colli C, Segura EL. Rev Soc Bras Med Trop. 1989;22:119–124. doi: 10.1590/s0037-86821989000300002. [DOI] [PubMed] [Google Scholar]
- 14.Gürtler RE, Petersen RM, Cecere MC, Schweigmann NJ, Chuit R, Gualtieri JM, Wisnivesky-Colli C. Trans R Soc Trop Med Hyg. 1994;88:27–30. doi: 10.1016/0035-9203(94)90483-9. [DOI] [PubMed] [Google Scholar]
- 15.Gürtler RE, Kravetz FO, Petersen RM, Lauricella MA, Wisnivesky-Colli C. Ann Trop Med Parasitol. 1990;84:313–323. doi: 10.1080/00034983.1990.11812475. [DOI] [PubMed] [Google Scholar]
- 16.Gürtler RE, Cecere MC, Lauricella MA, Cardinal MV, Kitron U, Cohen JE. Parasitology. 2007;134:1–14. doi: 10.1017/S0031182006001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gürtler RE, Cecere MC, Lauricella MA, Petersen RM, Canale D, Castañera MB, Chuit R, Segura EL, Cohen JE. Am J Trop Med Hyg. 2005;73:95–103. [PMC free article] [PubMed] [Google Scholar]
- 18.Cecere MC, Gürtler RE, Canale DM, Chuit R, Cohen JE. Acta Trop. 2002;84:101–116. doi: 10.1016/s0001-706x(02)00183-3. [DOI] [PubMed] [Google Scholar]
- 19.Cecere MC, Vazquez-Prokopec GM, Gürtler RE, Kitron U. Am J Trop Med Hyg. 2004;71:803–810. [PMC free article] [PubMed] [Google Scholar]
- 20.Cecere MC, Castañera MB, Canale DM, Chuit R, Gürtler RE. Rev Panam Salud Pública. 1999;5:392–399. doi: 10.1590/s1020-49891999000500003. [DOI] [PubMed] [Google Scholar]
- 21.Lauricella MA, Castañera MB, Gürtler RE, Segura EL. Mem Inst Oswaldo Cruz. 1998;93:501–507. doi: 10.1590/s0074-02761998000400016. [DOI] [PubMed] [Google Scholar]
- 22.Castañera MB, Lauricella MA, Chuit R, Gürtler RE. Ann Trop Med Parasitol. 1998;92:671–683. doi: 10.1080/00034983.1998.11813327. [DOI] [PubMed] [Google Scholar]
- 23.Cardinal MV, Castañera MB, Lauricella MA, Cecere MC, Ceballos LA, Vazquez-Prokopec GM, Kitron U, Gürtler RE. Am J Trop Med Hyg. 2006;75:753–761. [PMC free article] [PubMed] [Google Scholar]
- 24.Gürtler RE, Chuit R, Cecere MC, Castañera MB, Cohen JE, Segura EL. Am J Trop Med Hyg. 1998;59:741–749. doi: 10.4269/ajtmh.1998.59.741. [DOI] [PubMed] [Google Scholar]
- 25.Pirard M, Iihoshi M, Boelaert M, Basanta P, López F, Van der Stuyft P. Transfusion. 2005;45:554–561. doi: 10.1111/j.0041-1132.2005.04214.x. [DOI] [PubMed] [Google Scholar]
- 26.Luquetti AO, Rassi A. In: Trypanosoma cruzi e Doenca de Chagas. 2nd Ed. Brener Z, Andrade ZA, Barral-Netto M, editors. Rio de Janeiro: Guanabara Koogan; 1999. pp. 344–368. [Google Scholar]
- 27.Peñaranda-Carrillo R, Moreira EF, Silveira AC, Leite J, Vinhaes CM, Castro C, Macêdo V. Rev Soc Brasil Med Trop. 2002;35:331–338. [PubMed] [Google Scholar]
- 28.Gürtler RE, Canale DM, Spillmann C, Stariolo R, Salomón OD, Blanco S, Segura EL. Bull World Health Organ. 2004;82:196–205. [PMC free article] [PubMed] [Google Scholar]
- 29.Segura EL. In: El control de la enfermedad de Chagas en los países del Cono Sur de América. Historia de una iniciativa internacional. 1991–2001. Rojas de Arias A, Guillén G, Russomando G, Schenone H, Dias JCP, Valdes Padilla J, Lorca M, Salvatella R, editors. Uberaba: Facultad de Medicina do Triangulo Mineiro; 2002. pp. 45–108. [Google Scholar]
- 30.Tobar F. In: Decentralización y gestión del control de las enfermedades transmisibles en América Latina. Yadón ZE, Gürtler RE, Tobar F, Medici AM, editors. Buenos Aires: Organización Panamericana de la Salud; 2006. pp. 65–114. [Google Scholar]
- 31.Vazquez-Prokopec GM, Ceballos LA, Marcet PL, Cecere MC, Cardinal MV, Kitron U, Gürtler RE. Med Vet Entomol. 2006;20:1–6. doi: 10.1111/j.1365-2915.2006.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Utzinger J, Tozan Y, Singer BH. Trop Med Int Health. 2001;6:677–687. doi: 10.1046/j.1365-3156.2001.00769.x. [DOI] [PubMed] [Google Scholar]
- 33.Bryan RT, Balderrama F, Tonn RJ, Dias JCP. Am J Trop Med Hyg. 1994;50(Suppl):61–71. doi: 10.4269/ajtmh.1994.50.61. [DOI] [PubMed] [Google Scholar]
- 34.Holveck JC, Ehrenberg JP, Ault SK, Rojas R, Vasquez J, Cerqueira MT, Ippolito-Shepherd J, Genovese MA, Periago MR. BMC Public Health. 2007;7:1–21. doi: 10.1186/1471-2458-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.