Abstract
The role of the peptide hormone angiotensin (AngII) in promoting myocardial hypertrophy is well documented. Our studies demonstrate that AngII uses a signaling pathway in cardiac myocytes in which the promoter of the gene encoding its prohormone, angiotensinogen, serves as the target site for activated signal transduction and activator of transcription (STAT) proteins. Gel mobility-shift assay revealed that STAT3 and STAT6 are selectively activated by AngII treatment of cardiomyocytes in culture and bind to a sequence motif (St-domain) in the angiotensinogen promoter to activate its transcription in transient transfection assay. We have also observed a dramatic increase in the St-domain binding activity of STAT proteins in the hypertrophied heart of the genetically hypertensive rat relative to that of the aged-matched normotensive strain WKY, providing a compelling argument in favor of the linkage of STAT pathway to the heart tissue autocrine AngII loop. These studies thus uncover a mechanism by which the activation of a selective set of STATs underlies mobilization of the gene activation program intrinsic to cardiac hypertrophy.
Angiotensin II (AngII), an extensively characterized peptide produced by successive proteolytic cleavages of its prohormone angiotensinogen (ANG), plays a critically important role in the regulation of cardiovascular and renal homeostasis (1, 2). Recent evidence based on experimental animal models and transgenic mice has documented the involvement of AngII in promotion of hypertension and myocardial hypertrophy due to activation of the renin–angiotensin system (RAS) (2–4). In human genetic studies (5), a clear linkage was established between ANG gene and hypertension. At the cellular level, the AngII-mediated signaling is achieved through its binding to the cell surface receptors, members of the G protein-associated superfamily (6), which involves activation of multiple second messenger systems (7–9). Recently, it was shown that AngII, which causes the positive feedback of ANG transcription, activates the Janus kinase (Jak) and STAT pathway in rat aortic smooth muscle and cardiac fibroblast cell (10–12). This led to the speculation that the cellular processes that are triggered by the cardiac RAS might involve the Jak/STAT pathway, and genes containing the binding sequences (GAS and sis inducing elements) for activated STATs might serve as functional target.
STATs are latent transcription factors located in the cytoplasm that are activated via the tyrosine phosphorylation cascade after ligand binding and stimulation of the receptor–kinase complex (13). The activated STATs are subsequently translocated to the nucleus where they bind to specific DNA sites as homo- or heterodimers to stimulate transcription of the responsive genes. Among the STAT proteins known to date, STAT3 has been implicated in transduction of the cellular signals that are involved in development of cardiac hypertrophy (14). The signal-transducing receptor protein gp130 stimulates the Jak/STAT3 pathway and is associated with the regulation of cardiac growth and development. gp130 also transduces cardiotrophin 1, a cytokine that can induce cardiac hypertrophy in vitro (15). Thus, these observations suggest that there is a convergence of the AngII- and cytokine-mediated signaling mechanisms to the Jak/STAT pathway that then elicits a common physiological response, e.g., cardiac hypertrophy, by triggering a specific gene activation program.
In this report, we demonstrate that the ANG gene promoter serves as the target site for STAT proteins that are activated after treatment of neonatal rat cardiac myocytes in culture with AngII. The activated STATs bind to a motif, denoted St-domain, in the ANG promoter and stimulate its transcription in transient transfection assay. Gel mobility shift assay with nuclear extracts from AngII-treated cardiomyocytes, the St-domain DNA as probe, and anti-STAT antibodies indicated that STAT3 and STAT6, and STAT1 at a lower level, are selectively and functionally activated in response to AngII. In addition to these STATs, other components of the Jak/STAT pathway, Jak2 and STAT5A are also tyrosine-phosphorylated in response to AngII treatment in cardiomyocytes. In a parallel experiment, we observed that the St-domain binding activity of three STAT proteins (STAT3, STAT5A, and STAT6) is significantly increased in extracts isolated from the hypertrophied myocardium of the 22-week-old spontaneously hypertensive rat (SHR) relative to those obtained from the age-matched normotensive strain WKY. Thus, these observations demonstrate that the Jak/STAT pathway is an integral part of the signaling mechanism in hypertrophy of the cardiac muscle and implicate STAT proteins in activation and maintenance of the cardiac autocrine loop of AngII.
MATERIALS AND METHODS
Antibodies and Chemicals.
Polyclonal antibodies against Jak2, Stat1, Stat3, Stat5A, and Stat6 were obtained from Santa Cruz Biotechnology. Monoclonal antibodies against phosphotyrosine (4G10) and protein A-agarose were from Upstate Biotechnology. Human AngII was from Sigma.
In Vivo AngII Treatment.
Male Wistar rats (150–200 g) and spontaneously hypertensive rats (150–200 g) were obtained from Charles Rivers Breeding Laboratories. Rats with food withdrawn 12 hr before experiments were anesthetized by intraperitoneal injection of sodium pentobarbital [100 mg/kg (body weight)]. In vivo stimulation of the Jak/STAT pathway in the heart was achieved by injecting 100 μl of AngII (10−7 M) in to the superior vena cava. The heart was excised after 30 min of treatment, and nuclear extracts were prepared as described below.
Construction of Reporter Plasmids.
For construction of plasmid STANGCAT, the 170-bp restriction fragment (BglI–XbaI) of the rat ANG promoter (a gift from A. Kumar, New York Medical College, Valhalla, NY), was cloned in plasmid Basic CAT (Promega) at SalI–XbaI in the polylinker. To achieve directional cloning, XbaI end fragment was allowed to ligate and was subjected to end-filling between BglI and SalI. The plasmid containing 150 bp (HaeIII–XbaI) of the rat ANG promoter was cloned in the polylinker SalI–XbaI as above to create plasmid ANGCAT. A substitution mutation was introduced in the St-domain by a PCR-based site-directed mutagenesis method (16). Adjacent primers were designed on opposite DNA strands (primer I, 5′-gggctGActggaaggga-3′; primer II, 5′-ctagtggcagatgagc-3′). Both primers were phosphorylated as described (16) and PCR amplification was carried out with 20 ng of template. Amplification parameters were an initial 4 min at 94°C, followed by 29 cycles of 1 min at 94°C, annealing was for 3 min at 60°C, and extension for 8 min at 68°C. The PCR product was phenol/chloroform-extracted and ethanol-precipitated, followed by a 4-hr ligation reaction. The reaction products were then digested with 60 units of DpnI (New England Biolabs) for 90 min at 37°C. One microliter was used for transformation reaction and the positive mutants were isolated.
Cell Cultures.
The neonatal rat cardiomyocytes were cultured by using the ventricular tissues placed in ice-cold Ca2+-free medium DMEM/F-12, which were minced and kept in trypsin overnight at 4°C. Cell were dissociated with purified collagenase (Worthington) for 30 min at 37°C in the presence of trypsin inhibitor, freed of fibroblasts by preplating, and cultured for 24 hr in medium with 5% horse serum and 5% fetal calf serum in Leibovitz’s medium. Serum-free medium was added to cells 2 hr before addition of agonists.
Transient Transfection Assay.
Transfections were done by using calcium phosphate precipitation and chloramphenicol acetyltransferase (CAT) enzyme activity in the extracts was assayed as described (17). Plasmid simian virus 40 luciferase (Promega) was used for normalization of CAT expression. Cells were lysed in 1× lysis buffer (Promega) and the luciferase activity was measured by following instructions provided by the supplier (Promega) in a monolight luminometer.
Preparation of Nuclear Extracts.
Tissues from embryonic or adult heart muscle were finely minced in PBS and the disassociated cells were lysed in lysis buffer containing 20 mM Hepes (pH 7.6), 20% glycerol, 10 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride, and leupeptin (10 μg/ml) in a Wheaton Dounce homogenizer. Nuclei were collected by centrifugation at 532 × g for 5 min at 4°C, suspended in lysis buffer containing 500 mM NaCl, gently rocked for 1 hr at 4°C, and centrifuged at 6,660 × g for 10 min. The supernatant was separated into aliquots and frozen in liquid N2 for use in gel shift assay.
Electrophoretic Gel Mobility-Shift Assay (GMSA).
The St-domain DNA probe for protein binding was a double-stranded oligonucleotide containing the sequence 5′-GGGTtcCTGGAAGGG-3′ and complementary strand 5′-CCCTTCCAGgaACCC-3′, respectively. The oligonucleotide sequences containing a mutation substitution of the St-domain were 5′-GGGgaCCTGGAAGGG-3′ (top strand) and 5′-CCCTTCCAGGctCCC-3′ (complementary strand). These probes were end-labeled by polynucleotide kinase and [γ-32P]ATP. Binding reaction mixture containing 0.5 ng of labeled DNA (1,000 cpm), 2 μg of poly(dI-C), and 1–12 μg of protein in 20 mM Hepes, pH 7.5/3% glycerol/1.5 mM MgCl2/1 mM DTT/2 mM EDTA/50 mM KCl and the reaction was incubated at 4°C for 30 min. Competitor DNA was added in a 100-fold excess and preincubated with the extracts for 30 min before adding labeled probe. After a 30-min incubation on ice, the reactions were analyzed by electrophoresis on 8% polyacrylamide gel in 0.375× TBE (0.33 mM Tris borate, pH 8.7/1.0 mM EDTA). After electrophoresis, the gels were dried and subjected to autoradiography. Polyclonal antibodies (4 μg) against STAT p84/p91, STAT3, STAT5A, or STAT6 were added and incubated for 4 hr before addition of the DNA probe.
Tyrosine Phosphorylation of STAT Proteins After AngII Treatment.
Neonatal rat primary cardiac cells were kept for 2 hr in serum-free medium and then stimulated for 0, 15, 30, 60, and 120 min with AngII (10−7 M). At each time point, cells were washed in ice-cold 1× PBS and lysed. Equal amounts of proteins were incubated at 4°C with 5 μg of 4G10 antibody (Upstate Biotechnology). Fifty microliters of 50% protein A-agarose, prewashed in lysis buffer (Upstate Biotechnology), was then added and the mixture was incubated for 2 hr at 4°C. Each sample was washed with washing buffer containing 150 mM NaCl, 50 mM Tris⋅HCl (pH 7.4), 5 mM EDTA, 0.25% Triton X-100, 2 mM phenylmethylsulfonyl fluoride, aprotinin (0.2 unit/ml), 1 mM Na3VO4, and 1 mM NaF. Samples were eluted in 2× Laemmli’s sample buffer. Protein were separated on a 7.5% SDS/polyacrylamide gel and transferred to nitrocellulose membrane, Nitropure (Micron Separations, Westboro, MA) Blots were probed with polyclonal antibodies from Santa Cruz Biotechnology, diluted 1:1,000, and developed according to the Chemiluminiscence protocol (Amersham).
RESULTS
St-Domain in ANG Promoter Is Transcriptionally Active.
The regulatory regions of the ANG promoter have been characterized (3, 18). In this study, we identified the interferon γ-activated sequence (GAS domain) (19) CTTCCTGGAAG (denoted herein as St-domain) located at positions −160 to −175 in the promoter of the rat ANG gene that shares similarity with the consensus TTNCNNNAA sequence for binding of STAT proteins (13). The St-domain sequence is also present in human and mouse ANG promoters (18). The presence of St-domain in ANG gene prompted us to examine the potential involvement of STAT proteins in the AngII-mediated regulation of ANG promoter activity.
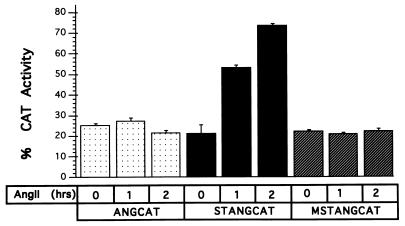
To examine whether the St-domain in ANG promoter is transcriptionally responsive to AngII treatment of the neonatal rat cardiac myocytes in culture, cells were transfected with plasmid STANGCAT containing the rat ANG promoter with St-domain (positions +47 to −175) or ANGCAT (positions +47 to −155) without St-domain. Transfected cells were maintained in serum-free medium for 2 hr and then treated with AngII as indicated, and the CAT activity was measured as described (17). As shown in Fig. 1, plasmid STANGCAT showed a time-dependent increase in CAT expression after AngII addition, whereas the activity of pANGCAT, measured under identical conditions, remained at the basal level. After 1 hr of treatment of cells with AngII, the CAT activity increased by approximately 2.5-fold and almost 4-fold in 2 hr. The AngII-mediated stimulation of the ANG promoter activity was dependent upon an intact St-domain, because substitution mutation in the St-domain (MSTANGCAT) caused a loss of the responsiveness to AngII. In absence of AngII, the level of expression of ANG promoter remained the same as that of a construct without St-domain or the substitution mutation.
Figure 1.
St-domain is active in primary cardiac cells. Plasmids ANGCAT, STANGCAT (carrying deletion of St-domain), and MSTANGCAT (carrying a substitution mutation of the St-domain) were constructed and used in a transient transfection experiment. The CAT reporter activity was evaluated before (0 hr) and after (1 and 2 hr) of treatment with AngII peptide (1 × 10−7 M) of rat primary cardiac cells. Each bar represents the mean of four experiments.
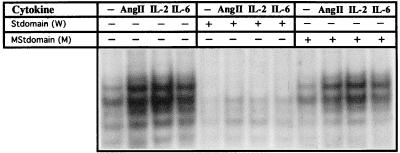
When nuclear extracts from 2-hr AngII-treated rat cardiac cells were examined by GMSA using St-domain DNA as probe, at least four complexes were observed in both treated and untreated extracts (Fig. 2). All four complexes showed a significant increase in intensity in the AngII-treated extract compared with untreated control. We also treated the cardiac cells with interleukin 2 (IL-2) and IL-6. The signal transduction mediated by IL-6, an activator of STAT proteins (20), is implicated in development of cardiac hypertrophy because overexpression of IL-6 in transgenic mice led to cardiac abnormalities, including thickening of the ventricular walls (14). Both agonists, AngII and interleukins, induced St-domain binding activity, which was competed by the addition of nonlabeled excess St-domain DNA but not by DNA carrying a mutation in the St-domain. These results thus demonstrate that the St-domain mediates the AngII-dependent up-regulation of ANG promoter via recognition of St-domain DNA binding proteins.
Figure 2.
Inducible DNA-binding activity of the St-domain. Rat primary cardiac cells were treated for 2 hr with AngII peptide (1 × 10−7 M), IL-2 (100 ng/ml), and IL-6 (30 ng/ml), after which nuclear extracts were obtained and analyzed by GMSA using an end-labeled wild-type St-domain DNA as probe. The specificity of the complexes was evaluated with unlabeled St-domain (W) and mutated St-domain (M) as competitors.
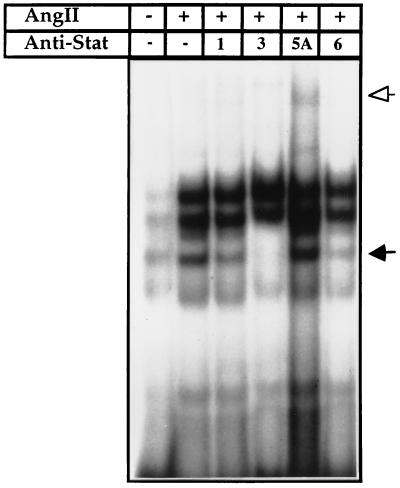
It has been suggested the STAT binding DNA core sequence with an adenosine or thymidine as the central nucleotide, as in ANG St-domain, serves as the preferential binding site for STAT5 and STAT6 (13). To identify the putative STAT proteins present in the St-domain/protein complexes, nuclear extracts from the AngII-treated myocytes in culture were incubated with polyclonal anti-STAT1, -STAT3, -STAT5A, and -STAT6 antibodies before binding the St-domain/DNA probe. As shown in Fig. 3, preincubation of extracts with anti-STAT3 and anti-STAT6 selectively disrupted the third complex. The same complex was reduced in intensity, but to a lower level, by anti-STAT1. The two major slow-moving complexes whose identity remain unknown were not affected. With anti-STAT5A, there was a partial supershift without significantly affecting any of the four complexes. The antibody against STAT5B, an isoform of STAT5, when examined in a parallel experiment, did not affect the STAT binding pattern (data not shown). These results suggest that AngII activates STAT6 and STAT3, which are involved in activation of the ANG promoter via recognition of St-domain sequence. The disruption of the same DNA–protein complex by anti-STAT3 and anti-STAT6 suggests that these proteins presumably bind the DNA as heterodimers.
Figure 3.
Analysis of STAT interaction with the St-domain by GMSA. Radiolabeled St-domain was incubated with nuclear extract from untreated and AngII-treated (1 × 10−7 M) primary cardiac cells. Nuclear extract were preincubated with polyclonal anti-STAT1, -STAT3, -STAT5, or -STAT6. Arrows indicate DNA-binding protein–St-domain complexes disrupted (solid) or shifted (open).
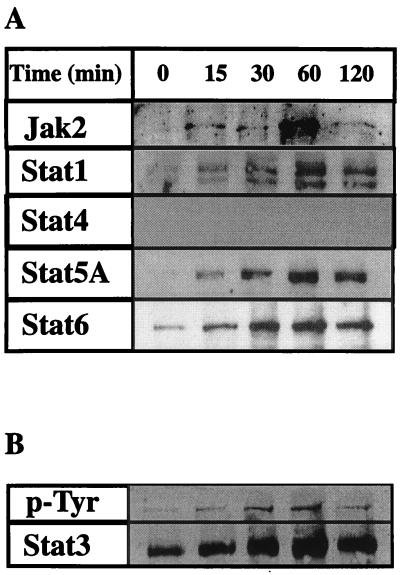
We then examined whether other proteins are activated via phosphotyrosine phosphorylation in AngII-treated cardiomyocyte. As shown in Fig. 4A, three STATs, namely, the two isoforms α and β of STAT1, which are recognized by the polyclonal anti-STAT1 antibody (11), STAT5A, and STAT6 were activated dramatically within 15 min of AngII treatment. Fig. 4B shows the activation pattern for STAT3 when measured by anti-phosphotyrosine (G418) and anti-STAT3 antibody, which serves as the loading control as well. Tyrosine phosphorylation of Jak2 was also induced within 15 min. Thus, STAT1, STAT3, STAT5, and STAT6 all were tyrosine-phosphorylated in response to AngII; only STAT3 and STAT6 participated in the complex formation with St-domain (see Fig. 3). STAT1 was previously shown to be activated by AngII and bind the sis inducing elements present in the c-fos promoter, a known immediate early responsive gene, which also responds positively to AngII treatment (21).
Figure 4.
Tyrosine phosphorylation of STATs after Ang II treatment of rat primary cardiac cells. (A) Extracts from control and AngII-treated cell at different time points were immunoprecipitated with anti-phosphotyrosine antibodies (4G10). After isolating the tyrosine phosphorylated protein by agarose-protein-A treatment, the proteins were separated in a 7% SDS/polyacrylamide gel and electroblotted on to nitrocellulose membrane. Each membrane was tested against polyclonal anti-Jak2, -STAT1, -STAT3, -STAT4, -STAT5A, and -STAT6 antibodies (Santa Cruz Biotechnology). (B) To evaluate that the increased phosphorylation activity of STAT proteins was due to AngII treatment, cell extracts from control and AngII-treated cardiomyocyte were immunoprecipitated with anti-STAT3, blotted against anti-phosphotyrosine (4G10), and then reblotted against STAT3. As observed equal amount of STAT3 is present at each time point, providing a loading control for the protein.
Activation of St-Domain Binding Activity in Hypertrophied SHR Cardiac Muscle Cells.
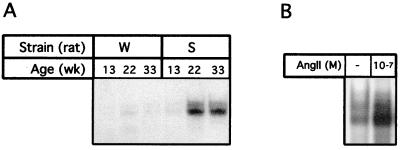
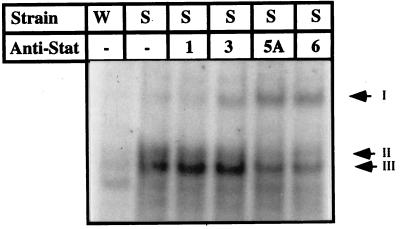
The SHR is a well established model for evaluating the effects of local and systemic RAS (22), which is implicated in development of hypertension and cardiac hypertrophy in SHRs (23). The intrinsic properties of these animals correlate well with the functional and physiological features of the diseased human heart. To examine whether the intracellular signaling observed in AngII-treated cardiomyocytes in culture is involved in SHR in activation of STAT proteins, we examined the nuclear extracts from cardiac muscle of hypertrophied SHR rats and the aged-matched normotensive rats strain WKY for St-domain DNA binding as above. As seen in Fig. 5, the DNA–protein profile in SHRs showed two closely migrating complexes that appeared pronounced in SHRs at 22 weeks of age when myocardial hypertrophy is well established (23). The St-domain binding activity was minimal at 13 weeks and comparable to that observed in age-matched normotensive rat strain WKY of all ages. To ascertain whether the stimulated STAT activity in SHRs at 22 wk age when myocardial hypertrophy is well established was indeed a response to AngII in vivo, we injected normotensive strain WKY rat with AngII. As shown in Fig. 5B, the constitutive level of STAT binding activity increased dramatically. The activation of the Jak/STAT pathway by AngII peptide in normotensive rats was also described by others (24, 25). The STAT–DNA complexes in SHR were disrupted significantly by anti-STAT6 and anti-STAT5A and to a lesser level by anti-STAT3 (Fig. 6). Although supershift signal observed for STAT3 relative to STAT5A in the SHR cardiac extracts is weaker than that observed in culture cardiomyocyte extracts, the results, nevertheless, demonstrate that a specific set of STATs is activated in the SHR myocardium and identify that the St-domain in ANG gene serves as the binding site of activated STATs linking the autocrine AngII loop to the Jak/STAT pathway.
Figure 5.
STAT–St-domain interaction on GMSA with extracts from SHR. (A) Cardiac muscle tissues obtained at 13, 23, and 33 weeks of age from both WKY and SHR were used to prepare nuclear extract for analysis by GMSA with St-domain as probe. W, normotensive strain WKY; S, hypertensive strain SHR. (B) Nuclear extracts from heart of WKY rat were prepared after AngII (10−7 M) treatment of WKY rat and compared for binding to St-domain with untreated WKY rat (−) on GMSA as in A.
Figure 6.
Identification of STAT proteins that interact with St-domain in vivo. Nuclear extract from a 23-week-old SHR rat was used in GMSA in the presence of polyclonal anti-Stat antibodies. W, extract from normotensive strain WKY; S, extract from SHR. Arrows: II and III, positions of two complexes; I, supershift.
DISCUSSION
The importance of the convergence of distinct mechanisms involved in transducing extracellular signals to activate transcription of the target genes using a common pathway has long been recognized. The Jak/STAT pathway is an important signaling mechanism used by a growing number of widely scattered extracellular ligands for transcriptional activation of genes (13). Although more than 30 extracellular proteins cause phosphorylation of STATs in various mammalian systems, only a few document the direct dependence of the physiological responses by activation of STATs (26–28).
Herein, we provide evidence that the ANG gene itself is the target for the activated STAT proteins in cardiac muscle, through a GAS regulatory region, the St-domain. The sequence-specific interaction of STAT proteins with the STAT binding domain (5spA) was reported earlier in an artificial promoter (29). The interaction of activated STATs with St-domain in the ANG promoter is specific as demonstrated in vitro by the lack of transcriptional activation of the ANG promoter containing a substitution mutation within the St-domain as compared with the wild-type promoter. In addition the competition gel shift analysis also identifies STAT3 and STAT6 as selective STAT proteins that recognize the St-domain. More evidence for activation of the STAT proteins was obtained by Western blot analysis. The profile of activation in cardiomyocyte partially resemble the pattern obtained in vascular smooth muscle (11), where Jak2, STAT1, and STAT3 were activated by AngII treatment. However, in cardiomyocytes AngII induced the activation of STAT5 and STAT6 as well. STAT4 was not induced and appears to be activated only by IL-12 (30). The long period of STAT activation (at least 2 hr) is consistent with the observation that exposure to AngII for an extended period is required for growth effects (31). STAT1, which was tyrosine-phosphorylated in response to AngII, did not participate effectively in binding to the St-domain in ANG promoter. It is likely that genes other than ANG also serve as target sites for activated STATs in cardiac myocytes treated with AngII. Indeed, STAT1 activated by AngII in cardiac fibroblast recognizes sis inducing elements present in several genes (21). Selective activation of STAT3, STAT5, and STAT6 by other cytokines have been reported in different cellular environments (32). Perhaps, the specificity in transcriptional activation of the target gene(s) may be afforded by the homo- and heterodimerization potential among STAT proteins STAT3, STAT5, and STAT6 homodimer, as well as STAT3/STAT5 heterodimer, as reported earlier (33–35).
The finding that in vivo injection of the AngII peptide mimics the increased St-domain binding activity of STAT proteins observed in cardiac extracts from the hypertrophied heart of the SHR relative to that of age-matched normotensive strain WKY represents a significant step in our understanding of the regulatory pathway(s) that controls the tissue autocrine AngII loop. Although STAT3 has been implicated in the development of cardiac hypertrophy (14, 15), our study has demonstrated that in addition to STAT3, STAT6 is also involved as a second messenger in the signal transduction triggered by AngII peptide and point to the underlying complexity in the regulation of the local and, perhaps, the systemic RAS. The pattern of activated STAT proteins in vitro after AngII treatment of cardiac cells in culture is not identical to that observed in SHR. It is likely that those involved in activation and maintenance of local RAS via binding to ANG promoter are common STATs (STAT3 and STAT6) and the remaining target other responsive genes. The results, nonetheless, provide a compelling argument in favor of the linkage of the Jak/STAT pathway with the AngII autocrine loop and uncover a mechanism by which the selective activation of a set of STAT proteins underlies mobilization of the gene activation program intrinsic to the pathophysiological state, such as the myocardial hypertrophy, which is known to be accompanied with specific modulations in gene-specific transcription factors (ref. 36 and unpublished results). The generation of STAT-deficient animals (26–28) should prove to be useful in testing the precise involvement of STATs in cardiac abnormalities.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: AngII, angiotensin; ANG, angiotensinogen; STAT, signal transduction and activator of transcription; RAS, renin–angiotensin system; Jak, Janus kinase; CAT, chloramphenicol acetyltransferase; GMSA, gel mobility-shift assay; IL, interleukin.
References
- 1.Pfeffer J M, Fisher J A, Pfeffer M A. Annu Rev Physiol. 1995;57:805–826. doi: 10.1146/annurev.ph.57.030195.004105. [DOI] [PubMed] [Google Scholar]
- 2.Raizada M, Phillips M, Summers C. Cellular and Molecular Biology of the Renin-Angiotensin System. Boca Raton, FL: CRC; 1993. [Google Scholar]
- 3.Kimura S, Mullins J J, Bunnemann B, Metzger R, Hilgenfeldt U, Zimmermann F, Jacob H, Fuxe K, Ganten D, Kaling M. EMBO J. 1992;11:821–827. doi: 10.1002/j.1460-2075.1992.tb05119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukamizu A, Sugimura K, Takimoto E, Sugiyama F, Seo M S, Takahashi S, Hatae T, Kajiwara N, Yagami K, Murakami K. J Biol Chem. 1993;268:11617–11621. [PubMed] [Google Scholar]
- 5.Jeunemaitre X, Soubrier F, Kotelevtsev Y V, Lifton R P, Williams C S, Charru A, Hunt S C, Hopkins P N, Williams R R, Lalouel J M, Corvol P. Cell. 1992;71:169–180. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- 6.Hunyady L, Balla T, Catt K J. Trends Pharmacol Sci. 1996;17:135–140. doi: 10.1016/0165-6147(96)81588-4. [DOI] [PubMed] [Google Scholar]
- 7.Sadoshima J, Izumo S. Circ Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 8.Booz G W, Baker K M. Cardiovasc Res. 1995;30:537–543. [PubMed] [Google Scholar]
- 9.Sadoshima J, Xu Y, Slayton H S, Izumo S. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 10.Marrero M B, Schieffer B, Paxton W G, Duff J L, Berk B C, Bernstein K E. Cardiovasc Res. 1995;30:530–536. [PubMed] [Google Scholar]
- 11.Marrero M B, Schieffer B, Paxton WG, Heerdt L, Berk BC, Delafontaine P, Bernstein K E. Nature (London) 1995;375:247–50. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- 12.Bhat G J, Thekkumkara T J, Thomas W G, Conrad K M, Baker K M. J Biol Chem. 1994;269:31443–31449. [PubMed] [Google Scholar]
- 13.Ihle J N. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 14.Hirota H, Yoshida K, Kishimoto T, Taga T. Proc Natl Acad Sci USA. 1995;92:4862–4866. doi: 10.1073/pnas.92.11.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pennica D, King K L, Shaw K J, Luis E, Rullamas J, Luoh S M, Darbonne W C, Knutzon D S, Yen R, Chien K R, et al. Proc Natl Acad Sci USA. 1995;92:1142–1146. doi: 10.1073/pnas.92.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher C L, Guo K P. Biotechniques. 1997;23:570–574. doi: 10.2144/97234bm01. [DOI] [PubMed] [Google Scholar]
- 17.Goswami S, Qasba P, Ghatpande S, Carleton S, Deshpande A K, Baig M, Siddiqui M A Q. Mol Cell Biol. 1994;14:5130–5138. doi: 10.1128/mcb.14.8.5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Congiu M, Clouston W M, Fernley R T, Richards R I. J Mol End. 1992;9:19–29. doi: 10.1677/jme.0.0090019. [DOI] [PubMed] [Google Scholar]
- 19.Shuai K C, Schindler V R, Prezioso J, Darnell J E. Science. 1992;258:1808–1812. doi: 10.1126/science.1281555. [DOI] [PubMed] [Google Scholar]
- 20.Ripperger J, Fritz S, Richter K, Dreier B, Schneider K, Lochner K. Ann NY Acad Sci. 1995;762:252–260. doi: 10.1111/j.1749-6632.1995.tb32330.x. [DOI] [PubMed] [Google Scholar]
- 21.Bradford C, Corson C, Marshall A. Circ Res. 1997;80:607–616. [Google Scholar]
- 22.Kagoshima T, Masuda J, Sutani T, Sakaguchi Y, Tsuchihashi M, Tsuruta S, Iwano M, Dohi K, Nakamura Y, Konishi N. Blood Pressure Suppl. 1994;5:89–93. [PubMed] [Google Scholar]
- 23.Ohta K, Kim S, Iwao H. Hypertension. 1996;28:627–634. doi: 10.1161/01.hyp.28.4.627. [DOI] [PubMed] [Google Scholar]
- 24.Velloso L A, Folli F, Sun X J, White M F, Saad M J, Kahn C R. Proc Natl Acad Sci USA. 1996;93:12490–12495. doi: 10.1073/pnas.93.22.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan J, Fukuda K, Kodama H, Makino S, Takahashi T, Sano M, Hori S, Ogawa S. Circ Res. 1997;81:611–617. doi: 10.1161/01.res.81.4.611. [DOI] [PubMed] [Google Scholar]
- 26.Meraz M A, White J M, Sheeban K C, Bach E A, Rodig S J, Dighe A S, Kaplan D H, Schreiber R D. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 27.Shimoda K, van Deursen J, Sangster M Y, Sarawar S R, Carson R T, Tripp R A, Chu C, Quelle F W, Nosaka T, Vignali D A, et al. Nature (London) 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Robinson G W, Wagner K U, Garret L, Wynshaw-Boris A, Hennighausen L. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- 29.Seidel H M, Milocco L H, Lamb P, Darnell J E, Stein R B, Rosen J. Proc Natl Acad Sci USA. 1995;92:3041–3045. doi: 10.1073/pnas.92.7.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson N G, Szabo S J, Weber-Nordt R M, Zhong Z, Scraiber R D, Darnell J E, Jr, Murphy K M. J Exp Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas W G, Thekkumkara T J, Baker K M. Adv Exp Med Biol. 1996;396:59–69. [PubMed] [Google Scholar]
- 32.Ghilardi N, Zeigler S, Wiestner A, Staffel R, Heim M H, Skoda R C. Proc Natl Acad Sci USA. 1996;93:6231–6235. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novak U, Mui A, Miyajima A, Paradiso L. J Biol Chem. 1996;271:18350–18354. doi: 10.1074/jbc.271.31.18350. [DOI] [PubMed] [Google Scholar]
- 34.Stancato L F, David M, Carter-Su C, Larner A C, Pratt W B. J Biol Chem. 1996;271:4134–4137. doi: 10.1074/jbc.271.8.4134. [DOI] [PubMed] [Google Scholar]
- 35.Kammer W, Lischke A, Moriggl R, Groner B, Ziemiecki A, Gurniak C B, Berg L J, Friedrich K. J Biol Chem. 1996;271:23634–23637. doi: 10.1074/jbc.271.39.23634. [DOI] [PubMed] [Google Scholar]
- 36.Doud S K, Pan L, Carleton S, Marmorstein S, Siddiqui M A Q. J Mol Cell Cardiol. 1995;27:2359–2372. doi: 10.1016/s0022-2828(95)92019-6. [DOI] [PubMed] [Google Scholar]