Abstract
Although the functions of α-Ca2+/calmodulin-dependent kinase II (CaMKII) have been studied extensively, the role of βCaMKII, a coconstituent of the CaMKII holoenzyme in synaptic plasticity, learning, and memory has not been examined in vivo. Here we produce a transgenic mouse line in which the inducible and reversible manipulation of βCaMKII activity is restricted to the hippocampal dentate gyrus, the region where long-term potentiation was originally discovered. We demonstrate that βCaMKII activity in the dentate gyrus selectively impaired long-term potentiation in the dentate perforant path, but not in the CA1 Schaffer collateral pathway. Although the transgenic mice showed normal 1-day memories, they were severely impaired in 10-day contextual fear memory. Systematic manipulations of dentate βCaMKII activity during various distinct memory stages further reveal the initial day within the postlearning consolidation period as a critical time window that is highly sensitive to changes in βCaMKII activity. This study provides evidence not only for the functional role of βCaMKII in the dentate gyrus plasticity and hippocampal memory, but also for the notion that the mismatch between the actual learning pattern and reactivation patterns in the dentate gyrus circuit can underlie long-term memory consolidation.
Keywords: long-term memory, pharmacogenetic, long-term potentiation, protein knockout
Because long-term potentiation (LTP) was originally discovered in the dentate gyrus of the hippocampus (1), a research effort into the molecular mechanisms and function of LTP in the dentate gyrus has been at the heart of the neuroscience community. Thus, it would be useful to selectively manipulate signaling molecules in the dentate gyrus and to see whether such action would alter dentate LTP and memory processes, which include acquisition, consolidation, storage, and retrieval.
In the present work we set out to study the role of β-Ca2+/calmodulin-dependent kinase II (βCaMKII) in the dentate gyrus. β-CaMKII is a component of CaMKII holoenzyme that constitutes ≈1% of protein in the cortex and 2% of proteins in the hippocampus (2). In contrast to numerous in vivo studies on αCaMKII (3–5), the analysis of βCaMKII has been carried out in limited experiments using cell cultures (6–9).
Based on the sequence and biochemical analyses, the αCaMKII and βCaMKII seem to have distinctive properties while sharing a certain redundancy. For example, α- and β-subunits possess different enzymatic kinetics such that the binding affinities for Ca2+/calmodulin differ significantly (10, 11). The subcellular localization of CaMKII holoenzyme has also been attributed to the functional difference between αCaMKII and βCaMKII where the F-actin binding domain present only in β-subunit (but not in α-subunit) confers anchoring and subsequent postsynaptic translocation of CaMKII holoenzyme (6, 9). It would be of great interest to ask whether increased βCaMKII would alter synaptic plasticity and, if so, whether such a change would be correlated with any specific alteration in one or multiple temporal stages of memory processes.
Here we used an inducible and reversible chemical genetic method that is based on a creation of a hidden cavity inside the ATP-binding pocket in a given kinase such that it can then accept a highly specific and rationally designed bulky inhibitor that is otherwise too large to fit and therefore inert to all other WT endogenous kinases (12). We report the production of a transgenic (Tg) mouse line in which we can manipulate βCaMKII selectively in the dentate gyrus of the hippocampus during long-term memory process.
Results
Construction of Tg βCaMKII-F90G and Its Expression in Vivo.
Because αCaMKII and βCaMKII are highly similar [84% sequence identity when βCaMKII without its F-actin binding cassette (amino acids 315–387) is compared with α isoform], it has prevented a functional analysis using conventional CaMKII inhibitors alone (3). To investigate the functional difference between αCaMKII and βCaMKII in vivo, we have used a targeted chemical–genetic engineering technique to achieve specific inhibition of βCaMKII (5). By mutating a key residue(s) within the ATP-binding pocket to an amino acid with a smaller side chain, the silently mutant kinase is then made to accept a larger inhibitor. Through a primary sequence alignment and molecular modeling of the structures of kinases' catalytic domain, we have deduced that Phe-90 of βCaMKII may confer the role of size exclusion within the ATP-binding pocket (Fig. 1 A–C). GST fusion proteins of WT and mutated βCaMKII-F90G were then created and purified, and they show comparable activities when tested against a synthetic peptide substrate, AutoCamtide II, in vitro (Kcat/Km of 9.53E3 min−1·M−1 versus 1.12E4 min−1·M−1 for WT and F90G βCaMKII, respectively).
Fig. 1.
Project strategy and selection of custom inhibitor for βCaMKII-F90G. (A) Crystal structure modeling of C. elegans αCaMKII homolog (UNC43) and Mus musculus PKAα ATP-binding pockets. PKAα residues 88–176 (in green) and CaMKII residues 57–145 (in blue) are reconstructed and structurally superimposed by using SwissProt Viewer from published coordinates from the Protein Data Bank (ID codes 1ATP and 2BDW, respectively) (24, 25). Met-120 of PKAα (in green) and Phe-89 of CaMKII (in blue) show the key residues within the ATP-binding pocket that spatially complement N6 of adenine base. (B) The above structures with Met-120 and Phe-89 mutated to Gly (in orange and yellow, respectively). The ATP-binding pocket is enlarged with M120G and F89G mutations, effectively removing spatial constraints on bulkier competitive inhibitors. (C) Sequence alignment of M. musculus PKAα, αCaMKII, and βCaMKII. Red underlined residues show the key amino acid for a targeted enlargement mutation, and blue underlined residues show the catalytic regions of kinases. (D) Screening of most selective PP1 analog against βCaMKII-F90G. Derivatives of PP1 are tested for inhibition of recombinant βCaMKII activity. Purified GST-βCaMKII-F90G and GST-βCaMKII WT were used in the presence of 3.3 μM inhibitor series. (E) Structures of PP1, the progenitor inhibitor, and its derivatives with variable side groups “R” are shown. NM-PP1 has the R group corresponding to side group 1.
We next screened a combinatorial inhibitor library to identify the most specific inhibitor for the modified kinase (12, 13). We tested 4-amino-1-tert-butyl-3-(p-methylphenyl)pyrazolo[3,4-d]pyrimidine (PP1) and its derivatives with bulkier variable groups for the inhibition on recombinant βCaMKII activity. PP1's C-3-derivatized analog, 1-napthylmethyl-PP1 (NM-PP1), was identified as the most efficient inhibitor of βCaMKII-F90G while having little effect on WT βCaMKII (Fig. 1D). NM-PP1 was previously shown not to have any significant inhibition of other WT serine/threonine kinases in vitro (13).
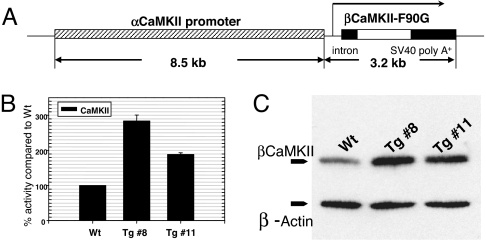
We then constructed αCaMKII promoter-driven βCaMKII-F90G expression vector to generate forebrain-specific Tg mouse lines (Fig. 2A). We produced two independent Tg founder lines (Tg 8 and Tg 11, respectively) using the pronuclear injection method (14). Upon gross inspections of WT and Tg animals, there were no differences in body weight, feeding and mating behaviors, and open-field activity between the genotypes. We used synaptosomal enriched fractions from the forebrain region and measured enzymatic activity of CaMKII from both adult Tg mice and WT littermates (Fig. 2B). Using AutoCamtide II as a substrate in an in vitro kinase assay, Tg line 8 and 11 show increased CaMKII activity compared with the WT counterparts [283.89 ± 17.18% and 187.83 ± 5.22% for Tg lines 8 and 11, respectively (mean ± SEM)] (Fig. 2B). Moreover, Western blot of the same fractions against βCaMKII immunoreactivity shows an elevated βCaMKII protein level in both Tg lines in comparison to that of the WT littermates (Fig. 2C). Theses suggest that the transgenically expressed βCaMKII-F90G has led to an increased synaptic CaMKII activity, and this resulted from the increased expression of βCaMKII-F90G. Because Tg line 8 has the highest Tg protein expression level and enzymatic activity, we focused on subsequent analyses on this line.
Fig. 2.
Overexpression of βCaMKII-F90G. (A) The Tg construct for expression of βCaMKII-F90G in the forebrain. (B) In vitro enzymatic activity of CaMKII in Tg and WT forebrain synaptosomal fraction is measured by using AutoCamtide II as a substrate. n = 7 animals in each group (6–8 months old). Data are mean percentages (± SEM) compared with WT. (C) Western blot of βCaMKII shows an elevated level of βCaMKII protein from the synaptosomal fraction of Tg lines 8 and 11 compared with the WT littermates. Immunoreactivity against β-actin is used as internal loading control.
Spatial Expression Pattern of βCaMKII-F90G and the Restricted Expression in Dentate Gyrus Lead to Impaired LTP.
To further characterize the expression pattern of βCaMKII-F90G in Tg line 8, we performed an in situ hybridization using a probe specific for the SV40 poly(A)+ tail of the βCaMKII-F90G mRNA. The specificity of the probe was confirmed because it did not yield any detectable signal in WT brains (Fig. 3A). Because it has been known that the CaMKII promoter sometimes exhibits an expression of transgene within restricted subregion(s) of the hippocampus at the young adult age (15), we decided to examine the transgene expression first in young adult Tg line 8 mice [postnatal (PN) days 60–85]. Interestingly, we found that the expression of βCaMKII-F90G at this young adult stage was spatially restricted to the dentate gyrus layer of the hippocampus (Fig. 3B; the brain of PN day 80 is shown).
Fig. 3.
Overexpression of spatially restricted βCaMKII-F90G in dentate gyrus layer in younger Tg animals (age 60–85 days PN). (A) In situ hybridization using PA probe EJ shows no discernible signal from brain slices from WT littermates of βCaMKII-F90G Tg (at the age of PN 60–80 days). (B) In situ hybridization using PA probe EJ shows dominant expression of βCaMKII-F90G mRNA in the Tg dentate gyrus layer over CA1 at PN 80 days. (C) Normal input–output curve of Tg mice in perforant pathway in the hippocampal slices (WT, n = 6 slices per three mice; Tg, n = 11 slices per five mice). (D) Normalized paired-pulse responses in dentate gyrus of the hippocampal slices from Tg mice (WT, n = 14 slices per seven mice; Tg, n = 14 slices per five mice). (E) βCaMKII-F90G overexpression does not alter the plasticity responses in the hippocampal Schaffer collateral pathway in younger animals aged 60–85 days PN. LTP induced by a tetanic stimulation (one train, 100 Hz, 1 s per train) in Tg slices (154 ± 8.8% at minutes 41–45; n = 6 slices per four mice) was no different from that of WT slices (152 ± 5.6% at minutes 41–45; n = 7 slices per five mice; P = 0.856 compared with Tg mice). (F) βCaMKII overexpression alters LTP in the hippocampal perforant pathway. LTP induced by a tetanic stimulation (six trains, 100 Hz, 1 s per train, interval 60 s, duration 0.1 ms) in Tg slices (131 ± 1.35% at minutes 81–90; n = 8 slices per four mice) was significantly smaller than that of WT slices (171 ± 12.3% at minutes 81–90; n = 13 slices per seven mice; P = 0.006 compared with Tg mice). (G) NM-PP1 (0.5 μM in artificial cerebrospinal fluid) restored LTP of the βCaMKII-F90G Tg mice in the hippocampal medial perforant pathway. LTP induced by a tetanic stimulation (same as in F) in Tg slices with NM-PP1 (179 ± 15.5% at minutes 81–90; n = 10 slices per seven mice) were restored to the level of WT slices with NM-PP1 (177 ± 25.2% at minutes 81–90; n = 6 slices per five mice; P = 0.49 compared with Tg mice with NM-PP1). All values are mean percentage compared with baseline response ± SEM. Statistical differences were evaluated with a t test. All mice used were 75–85 days old.
The dentate-specific expression of βCaMKII-F90G in this Tg line prompted us to take this unique opportunity to examine the correlation among βCaMKII, dentate synaptic plasticity, and hippocampal memory formation. We first measured input–output function in the medial perforant pathway of the dentate gyrus by comparing the ratio between initial slope of field excitatory postsynaptic potential as the normalized response and the amplitude of the fiber valley (presynaptic action potential) as the normalized input stimulus for WT and Tg groups, and we found these to be very similar, with no significant differences at any level of stimulation (P < 0.05) (Fig. 3C). This indicates that overexpression of βCaMKII-F90G did not produce any gross alterations in membrane excitability and the baseline efficacy of glutamatergic neurotransmission. Next we examined the short-term plasticity elicited by paired-pulse stimulation. It has been shown that dentate granule cell in response to medial perforant pathway input characteristically exhibits paired-pulse depression (16, 17). We observed paired-pulse depression responses to stimulation applied to the mid-s. moleculaire; however, there is no difference in paired-pulse depression between the Tg animals and the WT littermates (Fig. 3D).
We then investigated LTP by applying high-frequency stimulation that is known to elicit LTP (16). We first measured and compared LTP in the CA1 Schaffer collateral path in the Tg mice with that of their control littermates. Using the same age groups of mice (PN 60–85 days), we found that both WT and Tg slices exhibited comparable LTP, thereby confirming the spatial specificity of βCaMKII overexpression being limited to the dentate gyrus (Fig. 3E). When high-frequency stimulation was used to elicit LTP in the perforant pathway, the same stimuli elicited significantly smaller LTP in the Tg slices compared with the WT slices (Fig. 3F). Moreover, we determined that impaired LTP was caused by overexpressed βCaMKII activity because the impairment can be reversed readily by NM-PP1 perfusion. As shown in Fig. 3G, 0.5 μM NM-PP1 fully restored the level of perforant path–dentate gyrus LTP in the Tg slices to the WT level. Taken together, the above electrophysiological experiments demonstrate that the elevated βCaMKII Tg activity in the dentate gyrus results in selective impairment in dentate LTP.
Effects of Dentate-Specific βCaMKII-F90G Overexpression on Memory Function.
One key advantage of the chemical genetic technique over the traditional Tg overexpression method is the ability to rapidly and selectively manipulate the critical enzymatic activity, rather than to merely increase the physical amount, of the targeted protein. To determine whether dentate-specific overexpression of βCaMKII-F90G has any effect on learning and memory function in Tg mice, we first measured the performance of recognition memory using a novel object recognition test over a 1-day retention period in 60- to 85-day-old mice. During training, both WT and Tg animals showed an equal preference index for the objects used in training (Fig. 4A) [50.15 ± 2.38% and 49.37 ± 1.35% for WT and Tg, respectively (mean ± SEM)]. Interestingly, the Tg animals showed an elevated preference to the novel object over the familiar one during the retention test at a level comparable to that of the WT littermates' performances and do not show any statistical significance (Fig. 4A) [66.97 ± 1.75% and 64.95 ± 2.10% for WT and Tg, respectively (mean ± SEM)]. We then used classical contextual and cued fear conditioning (14, 18, 19) to determine whether the fear memory function in βCaMKII-F90G mice also held intact for the 1 day in duration. Both Tg and WT mice showed comparable performances in 1-day recollection of both contextual (Fig. 4B) and cued (Fig. 4C) memory. Above data, therefore, suggest that the elevated βCaMKII activity had no effect on the acquisition, retention, and recall of those 1-day-old long-term memories.
Fig. 4.
βCaMKII-F90G Tg animals show normal 1-day memory, but long-term memory lasting 10 days is impaired by the elevated level of βCaMKII and is reversible by NM-PP1. (A) One-day novel object recognition test of βCaMKII-F90G Tg shows comparable performance compared with the WT littermates. WT, n = 16; Tg, n = 14; all mice 3–3.5 months old (P = 0.467). All values are mean (± SEM) percentage of time explored to the novel object. (B and C) One-day fear conditioning paradigm of βCaMKII-F90G Tg also shows comparable performance in both contextual (B) and cued (C) memory tests. WT, n = 12; Tg, n = 11 (P = 0.778 for contextual freezing, and P = 0.210 for cued freezing). (D and E) At the same age of animals as above, βCaMKII-F90G Tg line 8 shows a performance deficit in 10-day contextual recall (D) while retaining a statistically comparable cued fear recall performance (E). WT, n = 14; Tg, n = 15 (P = 0.000287 for contextual freezing, and P = 0.307 for cued freezing). (F and G) The long-term memory deficit seen in 10-day recall is reversible when βCaMKII-F90G Tg activity was suppressed from the training through recall session by oral feeding of 5 μM NM-PP1 in drinking water from day −1 until the test day. WT, n = 14; Tg, n = 11 (P = 0.395 for contextual freezing, and P = 0.717 for cued freezing). All mice were 60–80 days old, and a single pairing was used in training for all. All values are mean percentage freezing ± SEM.
In considering the role of the NMDA receptor and αCaMKII reactivations in a gradual consolidation of longer-lasting memories of 10 days or more (5, 14, 20), we asked whether increased βCaMKII activity would adversely affect the retention of memory over a longer period. Using the same training protocol for establishing 1-day fear memory, we trained groups of Tg and WT mice when they were 70 days old and then measured 10-day memory retention. Indeed, Tg animals exhibited significantly less freezing compared with WT counterparts during the recall test of 10-day contextual fear memory, suggesting a deficit in memory over a longer period (Fig. 4D). Importantly, we did not find any alternation in the recollection of 10-day cued fear conditioning memory (Fig. 4E). This is consistent with in situ visualization because the Tg animals at this age (60–85 days) do not express the βCaMKII-F90G transgene in any other brain regions (Fig. 3B).
We next sought to determine the effects of inhibiting Tg βCaMKII-F90G on the 10-day memory in Tg animals. In determining in vivo pharmacokinetics of NM-PP1, we found the forebrain homogenate prepared from the Tg animals having significantly higher CaMKII activity that can be inhibited to the WT level upon chronic administration of 5 μM NM-PP1 [see supporting information (SI) Fig. 7]. Importantly, the WT animals receiving the identical NM-PP1 treatment did not show any discernible change in the total level of CaMKII activity, and CaMKII activity in the Tg forebrains returned to its pre-drug treatment level within 24 h once the 5 μM NM-PP1-containing water was removed on the last day of the 10-day treatment (SI Fig. 7).
Both Tg and WT were provided with 5 μM NM-PP1 in their drinking water 1 day before training, and the chronic inhibition was maintained throughout the acquisition, 10-day retention/consolidation, and recall periods. While untreated Tg mice exhibited significant impairment in the 10-day contextual fear memory retention (Fig. 4D), NM-PP1-treated Tg mice showed normal performances in the 10-day contextual fear memory test (Fig. 4F). In addition, both NM-PP1-treated and untreated mice exhibited comparable 10-day cued fear memory retention (Fig. 4G). These data provide strong evidence that the 10-day contextual fear memory deficit observed in βCaMKII-F90G animals directly resulted from increased βCaMKII enzymatic activity in the dentate gyrus.
Elevated Dentate βCaMKII Activity Disrupts LTM Consolidation, Not Acquisition and Recall.
To study further the effects of altered CaMKII signaling in the granule cells of the dentate gyrus on acquisition, consolidation, and retrieval of the hippocampal-dependent contextual fear memory, we conducted a temporal analysis in which Tg βCaMKII activity was elevated only during learning. Using a combination of acute i.p. injections and chronic NM-PP1 administrations (see Fig. 5A legend for details), we found that the Tg mice showed completely normal retention of 10-day contextual fear memories (Fig. 5A Left). We have previously shown that the inhibition by i.p. injection of NM-PP1 at this dosage lasts ≈30 min in vivo (5). It is important to note that our drug treatment regimen did not have any effect on the relative amount of freezing in WT animals, again indicating that neither NM-PP1 nor the prerequisite handling for i.p. injections has any detrimental effect on the 10-day memory itself. Equally important, we did not observe any significant difference in the cued memory performance in WT and Tg animals (Fig. 5A Right), further indicating that the NM-PP1 alone has no effect in consolidation of long-term memory. These experiments, therefore, suggest that the elevated dentate βCaMKII expression does not affect the acquisition of 10-day contextual memory.
Fig. 5.
Consolidation of memory during the first day after training is sensitive to the elevated level of βCaMKII, yet the elevated level of βCaMKII activity during initial training is not detrimental to long-term memory. (A) Ten-day fear conditioning paradigm of βCaMKII-F90G Tg line 8 when the Tg βCaMKII activity is elevated during training only by i.p. injection of 16.57 ng/g of body weight at 3 min before training, 30, 60, and 90 min after training, and oral feeding of 5 μM NM-PP1 in drinking water from hour 2 after training until the recall session after 6–8 h of water deprivation before training. WT, n = 12; Tg, n = 15; all mice 65–80 days old (P = 0.639 for contextual freezing, and P = 0.733 for cued freezing). (B) Ten-day fear conditioning paradigm of βCaMKII-F90G Tg line 8 when βCaMKII is elevated during the first 8 days of consolidation by a single i.p. injection of 16.57 ng of NM-PP1 per gram of body weight 10 min before training and oral feeding of 5 μM NM-PP1 in drinking water from day 8 until the recall session. WT, n = 16; Tg, n = 14; all mice 60–80 days old [P = 0.014 for contextual freezing (Fig. 6B Left), and P = 0.11 for cued freezing (Fig. 6B Right)]. (C) Ten-day fear conditioning paradigm of βCaMKII-F90G Tg line 8 when the Tg activity is elevated during the initial day after training by a single i.p. injection of NM-PP1 (16.57 ng/g of body weight) 25 min before training, the same i.p. injection of NM-PP1 at hours 8 and 8.5 after training, and oral feeding of 5 μM NM-PP1 in drinking water from hour 8 after training until recall. WT, n = 11; Tg, n = 13; all mice 60–85 days old (P = 0.023 for contextual freezing, and P = 0.237 for cued freezing). The timeline and a gray bar above it indicate the relative level of βCaMKII activity. A single pairing was used in training for all. All values are mean percentage freezing ± SEM.
We then carried out a second set of experiments in which βCaMKII-F90G in the dentate gyrus was suppressed to the normal WT level during the learning and recall phase but was elevated during the initial following 8 days of the consolidation period (Fig. 5B). Interestingly, we observed the Tg mice exhibiting a significant deficit in the 10-day contextual fear memory retention tests (Fig. 5B Left). As expected, the same mice showed normal performances in the 10-day cued fear memory retention tests (Fig. 5B Right). For a fine-tuning of temporal window in which the excessive βCaMKII is affecting the consolidation of LTM, another set of temporal experiments was carried out in which the elevation of βCaMKII-F90G is restricted to the initial 8 h of the consolidation. As shown in Fig. 5C, the perturbation of βCaMKII activity in the initial 8 h immediately after the training resulting in a memory performance deficit when Tg mice were tested 10 days later (Fig. 5C Left). Again, the retention of 10-day cued fear memories in Tg mice is comparable to that of their WT littermates receiving the identical treatments (Fig. 5C Right).
We carried out a further experiment in which we allowed for the temporal elevation of βCaMKII activity restricted to days 3–7 after training. Under this regimen, Tg animals were statistically indistinguishable in the 10-day contextual memory performance when compared with the WT (Fig. 6A Left). These mice also showed comparable responses in the 10-day cued fear memory tests (Fig. 6A Right). Finally, we sought to determine whether overexpression of βCaMKII activity during the retrieval period affects long-term memory processes (see the Fig. 6 legend for detailed inhibitor regimen). Interestingly, the Tg animals again had performances comparable to WT animals in 10-day contextual tests as well as 10-day cued tests (Fig. 6B). Therefore, our above temporal analyses suggest that the elevated βCaMKII activity in the dentate gyrus leads to selective disturbance in the consolidation of long-term memory and that only the initial day of consolidation after training is amendable to such action by excessive βCaMKII activity.
Fig. 6.
Elevated level of βCaMKII activity during the middle of the consolidation period or at the time of recall is not detrimental to long-term memory. (A) Ten-day fear conditioning paradigm of βCaMKII-F90G Tg line 8 when the Tg βCaMKII activity is elevated during consolidation days 3–7 by oral feeding of 5 μM NM-PP1 in drinking water 1 day before training, discontinued oral feeding of 5 μM NM-PP1 in drinking water from day 3 until day 7, and oral feeding of 5 μM NM-PP1 in drinking water from day 7 until the recall session. WT, n = 13; Tg, n = 14; all mice 60–85 days old (P = 0.770 for contextual freezing, and P = 0.280 for cued freezing). (B) Ten-day fear conditioning paradigm of βCaMKII-F90G Tg line 8 when βCaMKII activity is elevated during consolidation day 7 to the recall session on day 10 by oral feeding of 5 μM NM-PP1 in drinking water from day −1 until day 7. WT, n = 11; Tg, n = 11; all mice 60–80 days old (P = 0.719 for contextual freezing, and P = 0.749 for cued freezing). The timeline and a gray bar above it indicate the relative level of βCaMKII activity. A single pairing was used in training for all. All values are mean percentage freezing ± SEM.
Discussion
Here we used an inducible chemical genetic method for in vivo manipulation of βCaMKII activity in the dentate gyrus of the hippocampus of freely behaving mice. Because the target protein is engineered to accept a modified inhibitor that is otherwise inert to other WT kinases, this method of conditional protein manipulation combines a specificity of genetics with a temporal kinetics of pharmacology. This is quite useful to tease out the differences of two similar kinases that also exist in the same protein complex (as in this case of αCaMKII and βCaMKII).
Molecular and physiological characterizations of the CaMKII holoenzyme in our Tg mice show that elevated βCaMKII activity leads to reduced LTP in the perforant path of the dentate gyrus and selective deficits in the consolidation of long-term contextual memories. Although there is an emerging consensus that in vitro LTP does not accurately and fully match with in vivo memory function (21), it is nonetheless useful for the investigation of molecular action on synaptic plasticity. Because the β-subunit has an F-actin binding module that confers anchoring of the CaMKII complex at the base of the synaptic spine, previous efforts suggest that the numerical increase in βCaMKII results in an altered biophysical property of the holoenzyme complex, (i.e., a complex would be slower to translocate from the base of dendritic spines or to return faster from the postsynaptic density because of the holoenzyme complex containing more β-subunits) (6, 8, 9). This altered kinetics of the resultant CaMKII holoenzyme is well in line with our electrophysiological finding of the faster decay in LTP strength in the dentate gyrus (Fig. 3F).
Because the subunit ratio between αCaMKII and βCaMKII has been suggested to be important for determining the biochemical functions of CaMKII holoenzyme complex (8), we have determined the subunit ratio of αCaMKII to βCaMKII in our Tg mice. A crude separation of holoenzyme complex using size-exclusion liquid chromatography was used to isolate α:β multimeric holoenzyme from forebrain homogenates, and the determined ratio of α:β in WT forebrain of 3:1 is in agreement with previous findings (α:β = 3.034; see SI Fig. 8) (7). Interestingly, the fraction from βCaMKII-F90G Tg forebrain show a lowered ratio of α:β immunoreactivity when compared with the WT, and the composition of α and β has been shifted toward β to approximately half of the WT ratio (α:β = 1.568; see SI Text for an expanded discussion on the altered α:β ratio and the consolidation deficit).
In addition to previous findings of βCaMKII subunit's role in regulating the subcellular translocation of the holoenzyme (6, 8) and its ability to regulate the frequency and decay kinetics of miniature excitatory postsynaptic currents in cultured neurons (7), our observation has extended the role of βCaMKII to long-term synaptic plasticity in the hippocampal pathway. Interestingly, the reduced dentate LTP did not appear to be correlated with the acquisition and retrieval of hippocampal-dependent contextual memories but is correlated with long-term memory consolidation deficits. Memories lasting >10 days have been shown to require the repeated reactivations of the NMDA receptors or αCaMKII during the postlearning period especially during the first week after training (5, 14). Our present study indicates that the differential levels of CaMKII activities between the acquisition and consolidation stage in βCaMKII-F90G Tg mice may cause consolidation impairment.
Recent findings of the spontaneous reactivations of memory coding patterns during the posttraining stage suggest the existence of a neural mechanism for reactivating NMDA receptors between activated neurons (22, 23). Those findings collectively lead us to postulate that the reduced reactivation due to increased βCaMKII activity may be the cause for the impairment of memory consolidation. The mismatch between the actual learning pattern and reactivation patterns in the dentate gyrus can underlie the memory consolidation deficit we observed in βCaMKII-F90G Tg mice (see SI Text for further discussion).
Materials and Methods
Generation of βCaMKII-F90G and Its Tg Expression.
A site-directed mutagenesis was performed to introduce point mutation F90G in wild-type βCaMKII cDNA. Transgenic injection method is the same as previously described (14). See SI Methods.
Kinase Assays, Western Blotting, and in Situ Hybridization.
Assay of CaMKII activity was carried out by monitoring phosphorylation of the specific peptide substrate AutoCamtide II (Upstate Biotechnology, Lake Placid, NY). The in situ hybridization probe was 5′-TAATACGACTGACTATAGGGCCATCCATC A C A C TGGCGGCCGGCCGCTCGAGCATGCATCTA G A G G G C CCTATTCTATAGTTGCACCTAAAT-3′. For details, see SI Methods.
Hippocampal Slice Recording and Behavioral Tasks.
Coronal slices of the hippocampus from Tg and wild-type littermate mice (60–85 days old) were rapidly prepared and maintained in a submerge chamber containing artificial cerebrospinal fluid (ACSF). For details, see SI Methods.
Supplementary Material
Acknowledgments
We thank Huimin Wang for her initial technical assistance and discussion. This research was supported by funds from the National Institute of Mental Health, the National Institute on Aging, the Beckman Foundation, the Burroughs Wellcome Fund, and the W. M. Keck Foundation (all to J.Z.T.).
Abbreviations
- LTP
long-term potentiation
- CaMKII
Ca2+/calmodulin-dependent kinase II
- Tg
transgenic
- PP1
4-amino-1-tert-butyl-3-(p-methylphenyl)pyrazolo[3,4-d]pyrimidine
- NM-PP1
1-napthylmethyl-PP1
- PN
postnatal.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703344104/DC1.
References
- 1.Bliss TV, Lomo T. J Physiol (London) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy MB, Bennett MK, Erondu NE. Proc Natl Acad Sci USA. 1983;80:7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elgersma Y, Sweatt JD, Giese KP. J Neurosci. 2004;24:8410–8415. doi: 10.1523/JNEUROSCI.3622-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva AJ, Stevens CF, Tonegawa S, Wang Y. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Shimizu E, Tang YP, Cho M, Kyin M, Zuo W, Robinson DA, Alaimo PJ, Zhang C, Morimoto H, et al. Proc Natl Acad Sci USA. 2003;100:4287–4292. doi: 10.1073/pnas.0636870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen K, Teruel MN, Subramanian K, Meyer T. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- 7.Thiagarajan TC, Piedras-Renteria ES, Tsien RW. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 8.Shen K, Teruel MN, Connor JH, Shenolikar S, Meyer T. Nat Neurosci. 2000;3:881–886. doi: 10.1038/78783. [DOI] [PubMed] [Google Scholar]
- 9.Shen K, Meyer T. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 10.Brocke L, Chiang LW, Wagner PD, Schulman H. J Biol Chem. 1999;274:22713–22722. doi: 10.1074/jbc.274.32.22713. [DOI] [PubMed] [Google Scholar]
- 11.De Koninck P, Schulman H. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 12.Alaimo PJ, Shogren-Knaak MA, Shokat KM. Curr Opin Chem Biol. 2001;5:360–367. doi: 10.1016/s1367-5931(00)00215-5. [DOI] [PubMed] [Google Scholar]
- 13.Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, et al. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu E, Tang YP, Rampon C, Tsien JZ. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- 15.Tsien JZ, Huerta PT, Tonegawa S. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 16.Colino A, Malenka RC. J Neurophysiol. 1993;69:1150–1159. doi: 10.1152/jn.1993.69.4.1150. [DOI] [PubMed] [Google Scholar]
- 17.McNaughton BL. Brain Res. 1980;199:1–19. doi: 10.1016/0006-8993(80)90226-7. [DOI] [PubMed] [Google Scholar]
- 18.Kim JJ, Rison RA, Fanselow MS. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 19.Phillips RG, LeDoux JE. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 20.Frankland PW, O'Brien C, Ohno M, Kirkwood A, Silva AJ. Nature. 2001;411:309–313. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- 21.Tsien JZ. Curr Opin Neurobiol. 2000;10:266–273. doi: 10.1016/s0959-4388(00)00070-2. [DOI] [PubMed] [Google Scholar]
- 22.Lin L, Osan R, Shoham S, Jin W, Zuo W, Tsien JZ. Proc Natl Acad Sci USA. 2005;102:6125–6130. doi: 10.1073/pnas.0408233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsien JZ. Sci Am. 2007;297:52–59. doi: 10.1038/scientificamerican0707-52. [DOI] [PubMed] [Google Scholar]
- 24.Zheng J, Trafny EA, Knighton DR, Xuong N-H, Taylor SS, Teneyck LF, Sowadski JM. Acta Crystallogr D. 1993;49:362–365. doi: 10.1107/S0907444993000423. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg OS, Deindl S, Sung RJ, Nairn AC, Kuriyan J. Cell. 2005;123:849–860. doi: 10.1016/j.cell.2005.10.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.