Abstract
Nerve growth factor (NGF) binds to TrkA receptor and triggers activation of numerous signaling cascades, which play critical roles in neuronal plasticity, survival, and neurite outgrowth. To mimic NGF functions pharmacologically, we developed a high-throughput screening assay to identify small-molecule agonists for TrkA receptor. The most potent compound, gambogic amide, selectively binds to TrkA, but not TrkB or TrkC, and robustly induces its tyrosine phosphorylation and downstream signaling activation, including Akt and MAPKs. Further, it strongly prevents glutamate-induced neuronal cell death and provokes prominent neurite outgrowth in PC12 cells. Gambogic amide specifically interacts with the cytoplasmic juxtamembrane domain of TrkA receptor and triggers its dimerization. Administration of this molecule in mice substantially diminishes kainic acid-triggered neuronal cell death and decreases infarct volume in the transient middle cerebral artery occlusion model of stroke. Thus, gambogic amide might not only establish a powerful platform for dissection of the physiological roles of NGF and TrkA receptor but also provide effective treatments for neurodegenerative diseases and stroke.
Keywords: neurotrophic effect, nerve growth factor, ligand, receptor dimerization
Neurotrophins play an essential role in the development and maintenance of the peripheral nervous system and CNS. The receptors for neurotrophins are members of a family of highly similar transmembrane tyrosine kinases (TrkA, TrkB, and TrkC). Each neurotrophin binds to a preferred receptor in the family: Nerve growth factor (NGF) binds mainly TrkA, brain-derived neurotrophic factor (BDNF) and neurotrophin-4 bind TrkB, and neurotrophin-3 binds TrkC, whereas p75NTR receptor nonselectively interacts with all members of the neurotrophins with similar affinity. Docking of TrkA by NGF initiates receptor dimerization, phosphorylation of cytoplasmic tyrosine residues on the receptor, and a cascade of cell-signaling events, including activation of phosphatidylinositol 3-kinase (PI3-kinase)/Akt, MAPK, and phospholipase C-γ1 signaling pathways (1). These signals lead to the prevention of apoptotic cell death, promotion of cellular differentiation and axon elongation, and up-regulation of choline acetyl transferase. Several neuronal cell types implicated in various diseases express TrkA and therefore respond to NGF (2). In neurodegenerative processes such as mild cognitive impairment, loss of TrkA density correlates with neuronal atrophy and precedes neuronal death and severe cognitive impairment in Alzheimer's disease (AD) (3). In mild cognitive impairment and AD progression, loss of TrkA correlates with cognitive decline. In basal forebrain neurons of the aged rat, the expression of NGF receptors is decreased, but can be reversed by NGF administration (4). It has been suggested that NGF therapy may delay the onset of AD (5, 6) and ameliorate peripheral diabetic neuropathies (7). Both in vitro and animal model studies demonstrate that NGF might be clinically useful for treatment of CNS diseases (8). Preclinical and clinical findings also suggest that s.c. or i.v. administration of neurotrophins may be an effective treatment for peripheral neurodegenerative disorders (9, 10). Despite the therapeutic potential of NGF, clinical trials have been disappointing (11), which is due to inherent drawbacks associated with the use of polypeptides applied as drugs (12), in vivo instability (5), and pleiotropic effects. To circumvent these curbs, substantial efforts have been made to design small, proteolytically stable molecules with neurotrophic activity, selective for cells expressing TrkA (12–14). They present partial agonistic activity in the absence of NGF by activating TrkA. Although they do not directly dimerize the receptor, the compounds cause conformational changes on TrkA that stabilize its dimerization. Thus, they act more as potentiators of NGF action, rather than as robust TrkA agonists.
Gambogic acid (GA) is the major active ingredient of gamboge, a brownish to orange resin exuded from the Garcinia hanburryi tree in southeast Asia. The resin has been used in traditional Chinese medicine for the treatment of cancers (15). It has potent anticancer activity because of its activation of the apoptotic pathways in cancerous cells (16–19). Recently, it has been reported that GA triggers apoptosis upon binding to the transferrin receptor (20).
Here we describe a cell-based chemical genetic screen for TrkA agonists. We found that gambogic amide selectively binds to TrkA, triggers its tyrosine phosphorylation, elicits PI3-kinase/Akt and MAPK activation, provokes neurite outgrowth in PC12 cells, and prevents neuronal cell death. Moreover, it substantially decreases the infarct volume after middle cerebral artery occlusion (MCAO). Thus, gambogic amide mimics NGF and possesses potent neurotrophic activities.
Results
A Cell-Based Screen for TrkA-Expressing Cells.
To identify small molecules that mimic NGF and activate TrkA, we developed a cell-based apoptotic assay by using a cell-permeant fluorescent dye, MR(DEVD)2, which turns red upon caspase-3 cleavage in apoptotic cells. We used a murine cell line T17, which was derived from basal forebrain SN56 cells. T17 cells are TrkA stably transfected SN56 cells. The candidates selectively protecting T17 cells, but not SN56 cells, from the first screen are then subjected to neurite outgrowth assay for the second screen. The positive compounds were analyzed for TrkA tyrosine phosphorylation, Akt, and MAPK signaling cascade activation. The screening strategy scheme is depicted in supporting information (SI) Fig. 6. Using the caspase-3-activated fluorescent dye as a visual assay, we screened 2,000 biologically active compounds from the Spectrum Collection Library. Thirty-one compounds selectively protected T17 cells, but not SN56 cells, from staurosporine-initiated apoptosis, indicating that these compounds might act either directly through TrkA receptor or its downstream signaling effectors. The positive compounds were further validated by an independent cell viability assay (data not shown).
GA Binds the Cytoplasmic Juxtamembrane Domain of TrkA Receptor.
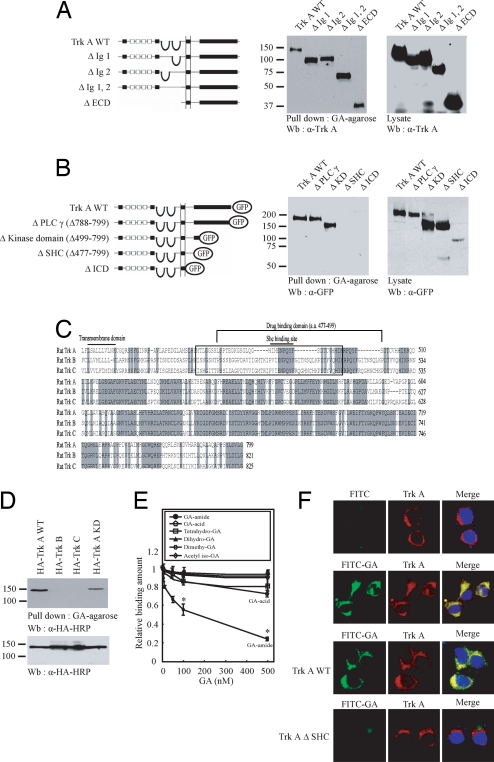
The Ig-like domain (TrkA-d5 domain) in the extracellular region of TrkA proximal to the membrane is required for specific binding of NGF (21). To investigate which portion of TrkA receptor binds to gambogic amide, we conducted an in vitro binding assay with immobilized gambogic amide through covalent linkage of Affi-Gel 102. We generated numerous GFP-tagged TrkA truncates and transfected them into HEK293 cells. The binding assay showed that the extracellular domain was not required for the association (Fig. 1A). Interestingly, the cytoplasmic juxtamembrane domain was critical for the ligand binding (Fig. 1B). Although the intracellular domain (ICD) of Trk family members shares great homology, the juxtamembrane regions are different among the members (Fig. 1C). We conducted an in vitro binding assay using Affi-Gel 102 coupled to GA through amide bond and found that gambogic amide selectively bound to both wild-type and kinase-dead TrkA, but not to TrkB or TrkC. Noticeably, TrkA-KD exhibited weaker affinity to gambogic amide than did wild-type TrkA (Fig. 1D Upper). It did not bind to p75NTR, ErbB3, or EGF receptor (data not shown), underscoring the finding that gambogic amide specifically associates with TrkA, but not other neurotrophin receptors or transmembrane tyrosine kinase receptors. To determine the binding constant between GA and TrkA, we conducted a competition assay with GFP-TrkA-bound gambogic amide beads. The beads-associated TrkA was gradually decreased as the free gambogic amide concentrations increased. In contrast, the inactive GA derivatives failed. Quantitative analysis of the competition data revealed that the Kd is ≈75 nM (Fig. 1E). Incubation of FITC-conjugated gambogic amide with PC12 cells for 10 min elicited its tight association with TrkA receptor, whereas FITC alone failed to penetrate into cells (Fig. 1F Upper). Truncation of the SHC (amino acids 477–499) drug-binding domain abolishes GA-FITC membrane penetration (Fig. 1F Lower). Thus, gambogic amide penetrates the cell membrane and binds tightly to TrkA receptor through a different region than NGF.
Fig. 1.
The juxtamembrane domain of TrkA binds GA. (A) The extracellular domain-truncated TrkA mutants potently bind to gambogic amide. The expression of transfected various GFP-TrkA mutants by immunoblotting with anti-GFP antibody. (B) Some of the ICD-truncated TrkA mutants, including the whole ICD (ΔICD) and SHC-binding domain- deleted (ΔSHC) mutants, failed to bind GA. (C) Sequence alignment of the ICD of TrkA, TrkB, and TrkC. (D) Gambogic amide selectively bound to TrkA, but not TrkB or TrkC receptor. (Upper) The tyrosine kinase-dead TrkA decreased its binding affinity to gambogic amide. (Lower) The expression of transfected constructs was verified. (E) Competition assay for the Kd. GFP-TrkA-associated gambogic amide beads were incubated with increased gambogic amide and various GA derivatives. After extensive washing, the beads-bound proteins were monitored by immunoblotting and quantitated by National Institutes of Health Image software. The Kd for the gambogic amide to TrkA is ≈75 nM. The inactive control GA derivatives did not compete for binding at all (Student's t test; *, P < 0.05). (F) FITC-gambogic amide penetrates cell membrane and binds to TrkA receptor. PC12 cells were incubated for 10 min with 0.5 μM FLTC-gambogic amide and control FITC, respectively. (Upper) After washing and fixing, the cells were stained with TrkA antibody. (Lower) Depletion of SHC drug-binding domain abolishes GA-FITC membrane penetration.
GA Derivatives Prevent Apoptosis.
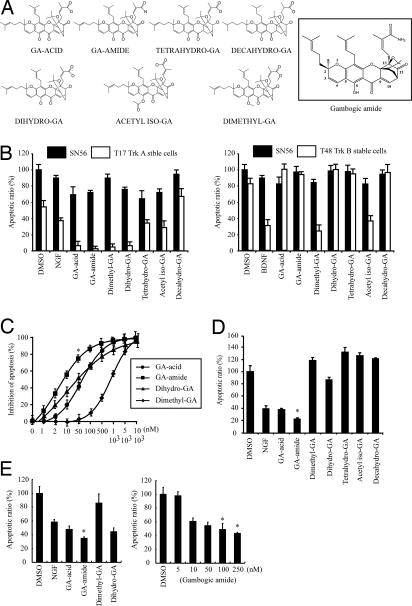
In our initial screening, 4 of 31 compounds are GA derivatives. The library also contains three more GA derivatives that are inactive. The chemical structures of the seven compounds are depicted in Fig. 2A. To compare the apoptosis inhibitory activity, we preincubated 0.5 μM GA derivatives with T17 and SN56 cells, followed by 1 μM staurosporine for 9 h. Quantitative analysis of the apoptosis inhibitory activities revealed that all these compounds barely protected SN56 cells from apoptosis. By contrast, GA, gambogic amide, dimethyl-GA, and dihydro-GA strongly suppressed apoptosis in T17 cells with protective activities even stronger than NGF. However, tetrahydro-GA and acetyl iso-GA slightly blocked staurosporine-initiated apoptosis. Decahydro-GA even increased apoptosis in T17 cells (Fig. 2B Left). Interestingly, dimethyl-GA and acetyl iso-GA exhibited a protective effect on TrkB-stable cells as did BDNF (Fig. 2B Right). We observed the similar effect on TrkC-stable cells as well (data not shown), indicating that these two compounds block apoptotic machinery independent of Trk receptors. These findings suggest that some GA derivatives can trigger programmed cell death, which is in agreement with a previous report that they possess anticancer activity (20). Quantitative analysis of the antiapoptotic activity reveals that the EC50 values for gambogic amide, GA, dimethyl-GA, and dihydro-GA on T17 cells are 5, 20, 120, and 200 nM, respectively (Fig. 2C).
Fig. 2.
Gambogic amide protects hippocampal neurons from apoptosis. (A) (Left) Chemical structures of GA derivatives. (Right) The chemical structure of gambogic amide with the numeric positions labeled. (B) (Left) Some GA derivatives prevent apoptosis in TrkA-expressing T17 cells, but not control SN56 cells. (Right) Interestingly, dimethyl-GA and acetyl iso-GA also protect TrkB-stable cells from apoptosis as BDNF. (C) Gambogic amide exhibits the strongest antiapoptotitc activity in T17 cells. EC50 values for GA derivatives in preventing apoptosis in T17 cells are 10 nM for gambogic amide, 50 nM for dihydro-GA, 55 nM for GA, and 750 nM for dimethyl-GA. (D) Gambogic amide protects hippocampal neurons from glutamate-triggered apoptosis. (E) (Left) Gambogic amide protects neurons from apoptosis triggered by OGD. (Right) Gambogic amide displayed a dose-dependent protective manner on neurons in OGD. All results from three independent experiments were expressed as mean ± SD (Student's t test; *, P < 0.05).
TrkA is highly expressed in hippocampal neurons (22–24). TrkA and p75NTR are up-regulated in hippocampal and cortical neurons under pathophysiological conditions (25, 26). Moreover, neuroprotective effects of NGF in hippocampal and cortical neurons have been demonstrated in vitro and in vivo (22, 27). To examine whether these compounds could promote neuronal survival, we prepared hippocampal neurons and pretreated the primary neurons with various GA derivatives for 30 min, followed by 50 μM glutamate treatment for 16 h. Quantitative apoptosis assay with MR(DEVD)2 demonstrated that GA displayed the same protective effect as the positive control NGF, and gambogic amide exhibited even stronger apoptosis inhibitory activity. Surprisingly, dimethyl-GA, tetrahydro-GA, acetyl iso-GA, and decahydro-GA even slightly enhanced apoptosis compared with control, and dihydro-GA weakly protected neurons from glutamate-incurred cell death (Fig. 2D). NGF overexpression decreased infarct volume and neuronal apoptosis in transgenic mice or intraventricular-injected mice (28, 29). NGF also potently protected PC12 cells from apoptosis in an oxygen–glucose-deprivation (OGD) model (30). To explore whether gambogic amide exerts any protective effect on hippocampal neurons in OGD, we pretreated the primary preparations with NGF or various compounds 30 min before OGD stimulation. In 3 h, apoptotic analysis showed that gambogic amide exhibited the most potent protective effects (Fig. 2E Left). Titration assay revealed that it protected neurons in a dose-dependent manner (Fig. 2E Right). Therefore, gambogic amide selectively protects TrkA expression cells and primary neurons from apoptosis.
Gambogic Amide Triggers TrkA Activation.
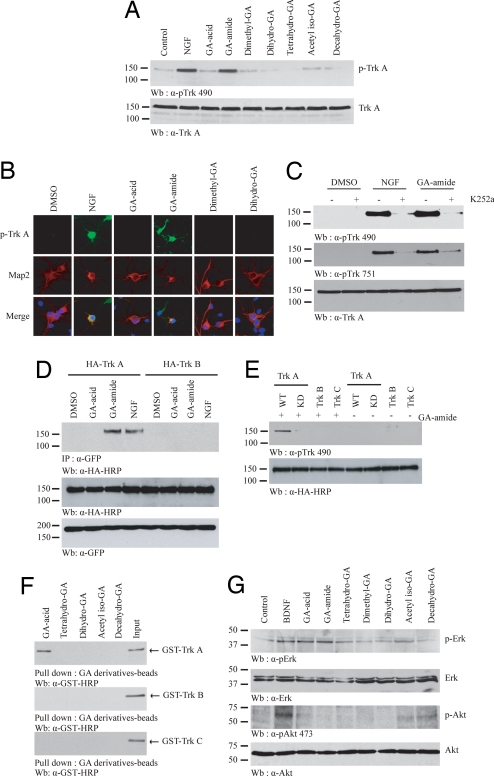
NGF binds to receptor TrkA and elicits its dimerization and autophosphorylation on tyrosine residues. Numerous tyrosine residues on TrkA are phosphorylated upon NGF stimulation. For example, Y490 phosphorylation is required for Shc association and activation of MAPK signaling cascade. Y751 phosphorylation is essential for PI3-kinase docking and activation. To evaluate whether GA compounds also could trigger TrkA tyrosine phosphorylation, we treated primary hippocampal neurons with various drugs at 0.5 μM for 30 min. NGF induced potent TrkA phosphorylation. Surprisingly, only gambogic amide, but not other compounds, elicited TrkA phosphorylation (Fig. 3A Upper). Gambogic amide also initiated the pronounced TrkA tyrosine phosphorylation in hippocampal neurons, as demonstrated by immunofluorescent staining with anti-TrkA Y490-specific antibody (Fig. 3B). In addition, gambogic amide triggered Y751 tyrosine phosphorylation. Both Y490 and Y751 phosphorylation by NGF can be completely inhibited by 30 nM K252a, a TrkA tyrosine kinase inhibitor. K252a also can substantially decrease TrkA phosphorylation elicited by gambogic amide (Fig. 3C), which indicates that the stimulatory effect by gambogic amide is mainly mediated through TrkA autophosphorylation. A coimmunoprecipitation assay demonstrated that gambogic amide provoked TrkA dimerization even more strongly than NGF, whereas GA and DMSO failed to do so. By contrast, the cotransfected TrkB and TrkA receptors failed to dimerize regardless of NGF or gambogic amide treatment (Fig. 3D). Gambogic amide also elicited tyrosine phosphorylation in TrkA, but not in TrkB or TrkC receptor. TrkA-KD displayed negligible tyrosine phosphorylation compared with wild-type TrkA (Fig. 3E), indicating that TrkA autophosphorylation activated by gambogic amide contribute to Y490 phosphorylation. An in vitro binding assay with Affi-Gel 102-conjugated GA derivatives, which form an amide bond between GAs and NH2-Affi-Gel 102, revealed that only GA selectively associated with TrkA, but not TrkB or TrkC (Fig. 3F). GA and gambogic amide provoked Erk1/2 phosphorylation, but failed to activate Akt in TrkB-stable cells (Fig. 3G). Similar effects occurred to TrkC-stable cells. GA derivatives failed to initiate TrkB or TrkC tyrosine phosphorylation (data not shown). Hence, these results demonstrate that gambogic amide mimics NGF and selectively provokes TrkA dimerization and tyrosine phosphorylation.
Fig. 3.
Gambogic amide elicits TrkA tyrosine phosphorylation in hippocampal neurons. (A) Gambogic amide induces TrkA tyrosine phosphorylation in primary neurons. Immunoblotting analysis with anti-phospho-Trk490 of hippocampal neurons treated with 0.5 μM GA derivatives for 30 min. (B) Immunofluorescent staining of GA derivative-treated hippocampal neurons with anti-phospho-Trk490 antibody. Both NGF and gambogic amide selectively triggered TrkA Y490 phosphorylation in neurons. (C) K252a decreases gambogic amide-triggered TrkA tyrosine phosphorylation. (D) Gambogic amide provokes TrkA dimerization. GFP-TrkA and HA-TrkA or HA-TrkB were, respectively, cotransfected into HEK293 cells and treated with 0.5 μM GA or gambogic amide for 30 min. GFP-TrkA was immunoprecipitated with anti-GFP antibody, and the coprecipitated proteins were analyzed with anti-HA antibody. Gambogic amide, but not GA, evidently stimulated TrkA dimerization. The stimulatory effect was even stronger than NGF. (Upper) By contrast, TrkA did not bind to TrkB regardless of NGF or gambogic amide treatment. (E) Gambogic amide triggered TrkA, but not TrkB or TrkC, tyrosine phosphorylation in transfected HEK293 cells. Kinase-dead TrkA displayed negligible tyrosine phosphorylation. (F) In vitro binding assay with GST-Trk recombinant proteins. Gambogic amide, but not other GA derivatives, selectively binds TrkA, but not TrkB or TrkC. (G) GA derivatives are unable to trigger both PI3-kinase and MAPK signaling activation in TrkB-stable cells. The experiments and immunoblotting gels in this study were repeated more than three times.
Gambogic Amide Provokes Akt and MAPK Activation and Elicits Neurite Outgrowth.
NGF triggers PI3-kinase/Akt and Ras/MAPK signaling cascades activation through TrkA receptor. To explore whether the GA derivatives possess similar mitogenic effects, we treated T17 cells with various GA derivatives for 30 min. NGF treatment stimulated demonstrable Erk1/2 and Akt phosphorylation. Strikingly, gambogic amide provoked robust phosphorylation of both Erk1/2 and Akt, whereas GA, tetrahydro-GA, and decahydro-GA failed to activate MAPKs. Dimethyl-GA, dihydro-GA, and acetyl iso-GA weakly agitated MAPK activation. Although GA and decahydro-GA were unable to activate MAPK, they strongly provoked Akt activation (Fig. 4A), suggesting that these compounds might differentially regulate PI3-kinase/Akt and Ras/MAPK pathways either through TrkA receptor or its downstream cellular targets. We also extended the assays into primary hippocampal neurons. NGF, GA, gambogic amide, and tetrahydro-GA evidently activated Akt, whereas other gambogic derivatives slightly up-regulated Akt phosphorylation (Fig. 4B). A time course assay with hippocampal neurons showed that 0.5 μM gambogic amide elicited Akt phosphorylation after a 5-min treatment, and Akt activation increased after 10 min and sustained for 60 min. The signal faded away after 180 min (Fig. 4C Left). Gambogic amide also provoked Akt activation in a dose-dependent manner, and 50 nM gambogic amide induced Akt activation as strongly as 50 ng/ml NGF (Fig. 4C Right). Taken together, these observations support the hypothesis that gambogic amide mimics NGF and potently activates Akt and MAPK activation in neurons.
Fig. 4.
Gambogic amide provokes MAPK and Akt kinase activation and neurite outgrowth. (A) Gambogic amide selectively provokes Erk phosphorylation in T17 cells, whereas other derivatives exhibited weakly stimulatory effects. By contrast, in addition to gambogic amide, GA and decahydro-GA also triggered robust Akt activation. (B) GA derivatives initiate Akt activation in hippocampal neurons. (C) Characterization of gambogic amide's stimulatory effect on Akt phosphorylation. (Left) Then 500 nM gambogic amide elicited Akt activation in a time-dependent manner. (Right) Gambogic amide induced Akt activation in a dose-dependent manner during 30-min treatment. (D) PC12 cells were treated with NGF or 0.5 μM GA derivatives for 5 days. Gambogic amide triggered neurite outgrowth as potently as NGF, and dihydro-GA also displayed weak stimulatory activity. By contrast, other derivatives had no effect. (E) Dose-dependent effect of neurite outgrowth. Further, 10–50 nM gambogic amide can provoke neurite outgrowth in PC12 cells. (F) K252a abolishes gambogic amide-provoked neurite outgrowth in PC12 cells.
One of most prominent neurotrophic effects of NGF is to trigger neurite outgrowth in neuronal cells and incur differentiation. To explore whether GA compounds possess this activity, we incubated PC12 cells with 0.5 μM compounds for 5 days. The cell medium containing the compounds was replenished every other day. As expected, NGF elicited pronounced neurite sprouting in PC12 cells after 5 days of treatment. Both gambogic amide and dihydro-GA induced evident neurite outgrowth in PC12 cells, whereas GA and dimethyl GA failed. The neurite network generated by gambogic amide was much more demonstrable than that generated by dihydro-GA, suggesting that gambogic amide might possess stronger neurotrophic activity than the latter (Fig. 4D). A dose-dependent assay revealed that 10–50 nM gambogic amide was sufficient to provoke substantial neurite sprouting in PC12 cells (Fig. 4E). This effect can be blocked by 30 nM K252a (Fig. 4F), indicating that gambogic amide-induced neurite outgrowth is TrkA-dependent. Thus, gambogic amide possesses potent neurotrophic activity at a concentration comparable to NGF and robustly provokes neurite outgrowth.
Gambogic Amide Prevents Neuronal Apoptosis and Decreases Infarct Volume.
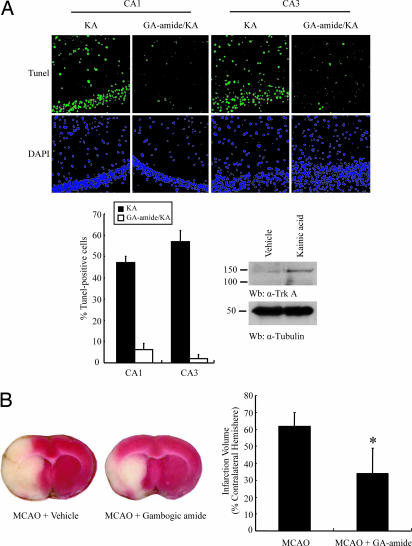
Kainic acid (KA) is a potent agonist for the AMPA receptor. Peripheral injections of KA result in recurrent seizures and the subsequent degeneration of select populations of neurons in the hippocampus (31–33). KA induces neuronal cell death in caspase-dependent and caspase-independent manners (34, 35). To explore whether gambogic amide can block the neurotoxicity initiated by KA, we s.c. injected 2 mg/kg gambogic amide into C57BL/6 mice, followed by 25 mg/kg KA. In 5 days, the mice were perfused, and the brains were cut to a thickness of 5 μm and mounted onto slides. TUNEL staining revealed that KA provoked enormous apoptosis in the hippocampus, and this finding was substantially diminished by gambogic amide (Fig. 5A Upper). Quantitative analysis of apoptosis in the hippocampus revealed that KA induced 47% and 57% cell death in the CA1 and CA3 regions, respectively, whereas gambogic amide strongly decreased apoptosis to 6.3% and 1.8% in these two regions, respectively (Fig. 5A Lower Left). Interestingly, we also found that the TrkA expression level was substantially increased after KA administration (Fig. 5A Lower Right), which fits with previous reports that TrkA is up-regulated in hippocampal and cortical neurons under pathophysiological conditions (25, 26).
Fig. 5.
Gambogic amide prevents neuronal cell death and decreases infarct volume in MCAO animal model. (A) Gambogic amide diminished Kainic acid-triggered hippocampal neuronal cell death. The brain slides were analyzed with TUNEL assay and stained with DAPI. Green stands for apoptotic nuclei, which also were stained with DAPI. Kainic acid evidently initiated devastating apoptosis in hippocampal CA3 region, which was substantially blocked by gambogic amide (Upper). (Lower Left) Quantitative analysis of apoptosis in the hippocampal neurons. (Lower Right) KA treatment enhanced TrkA expression in hippocampus and cortex. (B) Gambogic amide reduces infarct volume in MCAO rat brain. Tetanus toxin-stained coronal section from representative animals given either vehicle or gambogic amide and had brains harvested at 24 h postocclusion. (Left) The infarct area in gambogic amide-treated animals is substantially reduced. Infarct volumes after 24 h MCAO. Compared with vehicle alone, gambogic amide significantly reduced total infarct volumes (percentage of contralateral hemisphere). (Right) The data are represented as mean ± SD; *, P < 0.05.
To further determine the neuroprotective potential in vivo, gambogic amide was tested in a transient MCAO stroke model in adult male rats. After 2 h of MCAO followed by reperfusion, the animals received vehicle or 2 mg/kg gambogic amide 5 min before the onset of reperfusion. All animals included in the study survived the ischemic insult and treatment with gambogic amide. A representative brain slice stained with tetanus toxin 24 h after MCAO in vehicle- and gambogic amide-treated rats is shown (Fig. 5B Left).
Area and volume measurements from tetanus toxin-stained sections indicated that GA treatments substantially reduced infarct volumes in this transient ischemic model of stroke (Fig. 5B Right). These results concur with a previous report that NGF reduces infarct volume and apoptosis in focal ischemia (28). Laser-Doppler flowmetry was measured over the ipsilateral parietal cortex. Relative cerebral blood flow was reduced to 24.41% (±3.78%) and 25.09% (±5.73%) within 5 min of MCAO in rats that subsequently received GA or vehicle treatment. After 120-min filament withdrawal, relative cerebral blood flow increased to 85.83% (±4.94%) in the GA-treated group and to 87.81% (±2.23%) in the vehicle-treated group compared with preischemic levels. There were no significant differences between groups, suggesting that the relative ischemic insult was equivalent among all groups. The TrkA expression level was increased after ischemia (data not shown). Taken together, gambogic amide is a potent TrkA agonist, prevents neuronal cell death, and protects the neurodegeneration elicited by excitatory neurotoxicity and stroke.
Discussion
In the present study, we demonstrate that gambogic amide mimics NGF and acts as a robust TrkA agonist. However, gambogic amide and NGF bind to different regions on TrkA. NGF is a homodimer polypeptide, which associates with the Ig-like domain (TrkA-d5 domain) proximal to the membrane, whereas gambogic amide robustly binds the cytoplasmic juxtamembrane region. NGF triggers TrkA activation through promoting its dimerization and autophosphorylation. Gambogic amide, but not GA, also stimulates TrkA's dimerization and tyrosine phosphorylation (Fig. 1). It remains unknown exactly how gambogic amide provokes TrkA dimerization and autophosphorylation. Conceivably, it provokes TrkA conformational change and reduces the autoinhibitory effect by the Ig2 domain, which can block TrkA dimerization in the absence of NGF (36). It is worth noting that gambogic amide provokes TrkA tyrosine phosphorylation, which can be blocked by K252a (Fig. 3), suggesting that Y490 and Y751 phosphorylation is TrkA-dependent. Interestingly, gambogic amide, but not GA, possesses evident neurotrophic activities. For example, gambogic amide strongly stimulates both Akt and MAPK activation and triggers TrkA tyrosine phosphorylation in hippocampal neurons. In contrast, GA can elicit only Akt, but not MAPK, activation and fails to induce TrkA tyrosine phosphorylation (Figs. 3 and 4). These results are consistent with the observation that gambogic amide, but not gambogic GA, enhances TrkA dimerization (Fig. 1). However, it is unclear how GA protects hippocampal neurons from apoptosis (Fig. 2). Presumably, this effect results from its potent effect on Akt activation (Fig. 4A).
The structure-activity study with T17 cells and hippocampal neurons reveals that the carboxyl group can tolerate different modifications, suggesting that the carboxyl side chain might not be critical for binding to its biological targets. Therefore, the carboxyl group can be used to prepare compounds to optimize the apoptosis-inhibitory efficacy. Reduction of the unsaturated prenyl side chains or C9–C10 α,β-unsaturated ketone and C12 ketone (Fig. 2A) completely abolishes the protective effect. On the contrary, it even triggers substantial apoptosis in hippocampal neurons. These results demonstrate that the spatial structure of prenyl side chains and unsaturated double bonds in the caged polycyclic rings are essential for its neurotrophic effects. Notably, some of the GA derivatives exhibit robust antiapoptotic activity in T17, but not in hippocampal neurons. This effect is probably due to TrkA overexpression in T17 cells because T17 cells display much stronger antiapoptotic effects compared with their parental SN56 cells even in the absence of any drugs (Fig. 2B).
GA has been used in traditional Chinese medicine to treat cancers. It potently blocks human cancer proliferation in vitro and in animals (17, 18). Recently, it has been shown that the transferrin receptor functions as a cellular target for GA to exert its anticancer activity. Presumably, gambogic amide, the most potent TrkA agonist we identified, might bind transferrin receptor as well as TrkA. GA associates with transferrin with a Kd of 2.2 μM (20). Conceivably, gambogic amide binds to the TrkA receptor much more specifically and tightly than to the transferrin receptor. Consequently, it might exert neurotrophic activity more robustly and selectively than apoptotic effects. GA can be dissolved in 0.9% saline at low concentrations. GA is stable, and a pharmacokinetic study shows that the t1/2 of GA in human plasma is ≈15.54 ± 1.3 h (37). NGF may be useful for the treatment of neurodegenerative disorders such as Alzheimer's disease and stroke (28, 38). Thus, our findings that gambogic amide is a TrkA agonist that prevents neuronal cell death may be clinically important for the treatment of various neurodegenerative diseases and stroke.
Materials and Methods
Cells and Reagents.
Mouse septal neuron × neuroblastoma hybrids SN56 cells were created by fusing N18TG2 neuroblastoma cells with murine (strain C57BL/6) neurons from postnatal day 21. SN56 cells were maintained at 37°C with 5% CO2 atmosphere in DMEM containing 1 mM pyruvate and 10% FBS. T17 cells stably transfected with rat TrkA were cultured in the same medium containing 300 μg/ml G418. The cells were gifts from Brygida Berse (Boston University, Boston, MA). NGF was purchased from Roche Diagnostics (Indianapolis, IN). Phospho-Akt-473 or -308, Akt, and lamin A/C antibodies were from Cell Signaling Technology (Danvers, MA). Anti-phospho-Erk1/2, anti-phospho-TrkA Y490, anti-phospho-TrkA Y751, and anti-phospho-Akt 473 antibodies were from Upstate Biotechnology (Lake Placid, NY). The chemical library containing 2,000 biologically active compounds was from the Spectrum Collection (MicroSource Discovery System, Gaylordsville, CT). All chemicals not included herein were purchased from Sigma–Aldrich (St. Louis, MO).
Cell-Based Screen.
T17 cells were seeded in a 96-well plate at 10,000 cells per well in 100 μl of complete medium. Cells were incubated overnight, followed by 30-min pretreatment with 10 μM compounds in DMSO (10 mM stock concentration from the Spectrum Collection Library). The cells were then treated with 1 μM staurosporine for 9 h. One hour before the termination of the experiment, 10 μM MR(DEVD)2, a cell-permeant, caspase-3-activated fluorescent dye, was introduced. Cells were fixed with 4% paraformaldehyde for 15 min. Cells were washed with PBS and incubated with 1 μg/ml Hoechst 33342 for 10 min. Cover slides were washed with PBS, mounted, and examined using a fluorescence microscope (see SI Text).
Immobilization of GA and Synthesis of FITC-GA.
GA was immobilized to Affi-Gel 102 (Bio-Rad, Hercules, CA), which contains a free amine group, by using N′-(3-dimethylaminopropyl)-N-ethylcarbodiimide (EDC) and N-hydroxysuccinimide (NHS). The amidation reaction was carried out at room temperature. In brief, a mixture of 6.288 mg of reduced GA (10 μmol), 1.1 mg of NHS (5 μmol), 0.4 mg of EDC (2 μmol), and 1 ml of Affi-Gel 102 in ethanol was stirred at room temperature for 5 h. The reaction mixture was washed three times with a large volume of 100% ethanol. The GA-conjugated beads were kept in 1× PBS at 4°C in the dark. For synthesizing FITC-conjugated GA, GA was activated with EDC and NHS in ethanol. The reaction mixture was introduced into 1 ml of FITC (3.89 mg, 10 μmol) in ethanol. After a 2-h incubation at room temperature, the reaction solution was poured into 10 ml of water and extracted with 3× 10 ml of ethyl acetate. The combined organic layer was dried and concentrated to yield the crude product, which was purified by chromatography (SiO2, ethyl acetate/hexane) to yield the desired compounds.
Neurite Outgrowth Assay.
In a typical neurite-induction experiment, PC12 cells were seeded in the growth medium at 1 × 104 cells per well in poly(l-lysine)-coated 12-well culture plates (BD Biosciences, San Jose, CA), allowed to grow for 24 h, and supplemented with each of the various drugs or NGF. After 5 days, neurite outgrowth was measured as the distance between the cell periphery and the tip of neurite, and neuritic processes longer than two cell bodies in length were calculated as neurites. Unless otherwise mentioned, results were represented as the three independent experiments.
Statistical Analysis.
All results were expressed as mean ± SD. Mean ischemic laser-doppler flowmetry and lesion volume were analyzed using Student's t test. The criterion for statistical significance was set at P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. P. Perez (Universidad de Salamanca, Salamanca, Spain) and Dr. Y. Barde (Max Planck Institute of Neurobiology, Munich, Germany) for various Trk constructs. This work was supported by National Institutes of Health Grants R01 NS045627 (to K.Y.) and R01 NS04851 (to D.S.).
Abbreviations
- BDNF
brain-derived neurotrophic factor
- GA
gambogic acid
- KA
kainic acid
- MCAO
middle cerebral artery occlusion
- NGF
nerve growth factor
- OGD
oxygen–glucose deprivation
- PI3-kinase
phosphatidylinositol 3-kinase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706662104/DC1.
References
- 1.Kaplan DR, Stephens RM. J Neurobiol. 1994;25:1404–1417. doi: 10.1002/neu.480251108. [DOI] [PubMed] [Google Scholar]
- 2.Mufson EJ, Lavine N, Jaffar S, Kordower JH, Quirion R, Saragovi HU. Exp Neurol. 1997;146:91–103. doi: 10.1006/exnr.1997.6504. [DOI] [PubMed] [Google Scholar]
- 3.Counts SE, Nadeem M, Wuu J, Ginsberg SD, Saragovi HU, Mufson EJ. Ann Neurol. 2004;56:520–531. doi: 10.1002/ana.20233. [DOI] [PubMed] [Google Scholar]
- 4.Backman C, Rose GM, Bartus RT, Hoffer BJ, Mufson EJ, Granholm AC. J Comp Neurol. 1997;387:1–11. [PubMed] [Google Scholar]
- 5.Barinaga M. Science. 1994;266:973. doi: 10.1126/science.7973680. [DOI] [PubMed] [Google Scholar]
- 6.Lindsay RM. Philos Trans R Soc Lond B Biol Sci. 1996;351:365–373. doi: 10.1098/rstb.1996.0030. [DOI] [PubMed] [Google Scholar]
- 7.Ebadi M, Bashir RM, Heidrick ML, Hamada FM, Refaey HE, Hamed A, Helal G, Baxi MD, Cerutis DR, Lassi NK. Neurochem Int. 1997;30:347–374. doi: 10.1016/s0197-0186(96)00071-x. [DOI] [PubMed] [Google Scholar]
- 8.Connor B, Dragunow M. Brain Res Brain Res Rev. 1998;27:1–39. doi: 10.1016/s0165-0173(98)00004-6. [DOI] [PubMed] [Google Scholar]
- 9.McMahon SB, Priestley JV. Curr Opin Neurobiol. 1995;5:616–624. doi: 10.1016/0959-4388(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 10.McArthur JC, Yiannoutsos C, Simpson DM, Adornato BT, Singer EJ, Hollander H, Marra C, Rubin M, Cohen BA, Tucker T, et al. Neurology. 2000;54:1080–1088. doi: 10.1212/wnl.54.5.1080. [DOI] [PubMed] [Google Scholar]
- 10.Verrall M. Nature. 1994;370:6. doi: 10.1038/370006a0. [DOI] [PubMed] [Google Scholar]
- 11.Saragovi HU, Greene MI, Chrusciel RA, Kahn M. Biotechnology (NY) 1992;10:773–778. doi: 10.1038/nbt0792-773. [DOI] [PubMed] [Google Scholar]
- 12.Saragovi HU, Fitzpatrick D, Raktabutr A, Nakanishi H, Kahn M, Greene MI. Science. 1991;253:792–795. doi: 10.1126/science.1876837. [DOI] [PubMed] [Google Scholar]
- 13.Lee FS, Chao MV. Proc Natl Acad Sci USA. 2001;98:3555–3560. doi: 10.1073/pnas.061020198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asano J, Chiba K, Tada M, Yoshii T. Phytochemistry. 1996;41:815–820. doi: 10.1016/0031-9422(95)00682-6. [DOI] [PubMed] [Google Scholar]
- 15.Guo QL, You QD, Wu ZQ, Yuan ST, Zhao L. Acta Pharmacol Sin/I. 2004;25:769–774. [PubMed] [Google Scholar]
- 16.Wu ZQ, Guo QL, You QD, Zhao L, Gu HY. Biol Pharm Bull. 2004;27:1769–1774. doi: 10.1248/bpb.27.1769. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Guo QL, You QD, Wu ZQ, Gu HY. Biol Pharm Bull. 2004;27:998–1003. doi: 10.1248/bpb.27.998. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Guo QL, You QD, Zhao L, Gu HY, Yang Y, Zhang HW, Tan Z, Wang X. Carcinogenesis. 2007;28:632–638. doi: 10.1093/carcin/bgl168. [DOI] [PubMed] [Google Scholar]
- 19.Kasibhatla S, Jessen KA, Maliartchouk S, Wang JY, English NM, Drewe J, Qiu L, Archer SP, Ponce AE, Sirisoma N, et al. Proc Natl Acad Sci USA. 2005;102:12095–12100. doi: 10.1073/pnas.0406731102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urfer R, Tsoulfas P, O'Connell L, Shelton DL, Parada LF, Presta LG. EMBO J. 1995;14:2795–2805. doi: 10.1002/j.1460-2075.1995.tb07279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Tatsuno T, Carney JM, Mattson MP. J Cereb Blood Flow Metab. 1993;13:378–388. doi: 10.1038/jcbfm.1993.51. [DOI] [PubMed] [Google Scholar]
- 22.Kume T, Nishikawa H, Tomioka H, Katsuki H, Akaike A, Kaneko S, Maeda T, Kihara T, Shimohama S. Brain Res. 2000;852:279–289. doi: 10.1016/s0006-8993(99)02226-x. [DOI] [PubMed] [Google Scholar]
- 23.Culmsee C, Gerling N, Lehmann M, Nikolova-Karakashian M, Prehn JH, Mattson MP, Krieglstein J. Neuroscience. 2002;115:1089–1108. doi: 10.1016/s0306-4522(02)00539-0. [DOI] [PubMed] [Google Scholar]
- 24.Kokaia Z, Andsberg G, Martinez-Serrano A, Lindvall O. Neuroscience. 1998;84:1113–1125. doi: 10.1016/s0306-4522(97)00579-4. [DOI] [PubMed] [Google Scholar]
- 25.Lee TH, Kato H, Chen ST, Kogure K, Itoyama Y. Stroke. 1998;29:1687–1696. doi: 10.1161/01.str.29.8.1687. [DOI] [PubMed] [Google Scholar]
- 27.Culmsee C, Stumm RK, Schafer MK, Weihe E, Krieglstein J. Eur J Pharmacol. 1999;379:33–45. doi: 10.1016/s0014-2999(99)00452-5. [DOI] [PubMed] [Google Scholar]
- 28.Guegan C, Onteniente B, Makiura Y, Merad-Boudia M, Ceballos-Picot I, Sola B. Brain Res Mol Brain Res. 1998;55:133–140. doi: 10.1016/s0169-328x(97)00372-0. [DOI] [PubMed] [Google Scholar]
- 29.Luk YO, Chen WY, Wong WJ, Hu HH, Hsu LC, Chern CM, Huang KJ, Law SL. Drug Deliv. 2004;11:319–324. doi: 10.1080/10717540490494104. [DOI] [PubMed] [Google Scholar]
- 30.Tabakman R, Jiang H, Shahar I, Arien-Zakay H, Levine RA, Lazarovici P. Ann NY Acad Sci. 2005;1053:84–96. doi: 10.1196/annals.1344.008. [DOI] [PubMed] [Google Scholar]
- 31.Nadler JV, Perry BW, Gentry C, Cotman CW. J Comp Neurol. 1980;192:333–359. doi: 10.1002/cne.901920209. [DOI] [PubMed] [Google Scholar]
- 32.Sperk G, Lassmann H, Baran H, Kish SJ, Seitelberger F, Hornykiewicz O. Neuroscience. 1983;10:1301–1315. doi: 10.1016/0306-4522(83)90113-6. [DOI] [PubMed] [Google Scholar]
- 33.Schauwecker PE, Steward O. Proc Natl Acad Sci USA. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faherty CJ, Xanthoudakis S, Smeyne RJ. Brain Res Mol Brain Res. 1999;70:159–163. doi: 10.1016/s0169-328x(99)00143-6. [DOI] [PubMed] [Google Scholar]
- 35.Glassford A, Lee JE, Xu L, Giffard RG. Neurol Res. 2002;24:796–800. doi: 10.1179/016164102101200915. [DOI] [PubMed] [Google Scholar]
- 36.Arevalo JC, Conde B, Hempstead BL, Chao MV, Martin-Zanca D, Perez P. Mol Cell Biol. 2000;20:5908–5916. doi: 10.1128/mcb.20.16.5908-5916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding L, Huang D, Wang J, Li S. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;846:112–118. doi: 10.1016/j.jchromb.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 38.Olson L. Exp Neurol. 1993;124:5–15. doi: 10.1006/exnr.1993.1167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.