Abstract
Anorexia nervosa is a growing concern in mental health, often inducing death. The potential neuronal deficits that may underlie abnormal inhibitions of food intake, however, remain largely unexplored. We hypothesized that anorexia may involve altered signaling events within the nucleus accumbens (NAc), a brain structure involved in reward. We show here that direct stimulation of serotonin (5-hydroxytryptamine, 5-HT) 4 receptors (5-HT4R) in the NAc reduces the physiological drive to eat and increases CART (cocaine- and amphetamine-regulated transcript) mRNA levels in fed and food-deprived mice. It further shows that injecting 5-HT4R antagonist or siRNA-mediated 5-HT4R knockdown into the NAc induced hyperphagia only in fed mice. This hyperphagia was not associated with changes in CART mRNA expression in the NAc in fed and food-deprived mice. Results include that 5-HT4R control CART mRNA expression into the NAc via a cAMP/PKA signaling pathway. Considering that CART may interfere with food- and drug-related rewards, we tested whether the appetite suppressant properties of 3,4-N-methylenedioxymethamphetamine (MDMA, ecstasy) involve the 5-HT4R. Using 5-HT4R knockout mice, we demonstrate that 5-HT4R are required for the anorectic effect of MDMA as well as for the MDMA-induced enhancement of CART mRNA expression in the NAc. Directly injecting CART peptide or CART siRNA into the NAc reduces or increases food consumption, respectively. Finally, stimulating 5-HT4R- and MDMA-induced anorexia were both reduced by injecting CART siRNA into the NAc. Collectively, these results demonstrate that 5-HT4R-mediated up-regulation of CART in the NAc triggers the appetite-suppressant effects of ecstasy.
Keywords: eating; knockout; siRNA; 3,4-methylenedioxymethamphetamine (MDMA)
Anorexia nervosa is one of the mental diseases exhibiting the highest mortality rates in industrialized countries (1, 2). No effective strategies for treating this disorder are available. If one defines anorexia as self-imposed deprivation despite an energy demand, similar behavior can be provoked by treatments that increase serotonin (5-hydroxytryptamine, 5-HT) neuromodulation (3). For instance, fenfluramine, which increases synaptic 5-HT levels, lowers the consumption of food in humans and rodents (4, 5). Similarly, amphetamine and 3,4-N-methylenedioxymethamphetamine (MDMA, ecstasy) diminish food consumption in humans (6) and rats (7) and reduce deprivation-induced eating in mice (8). 5-HT-induced hypophagia is mediated by both 5-HT1B and 5-HT2C receptors (5-HT1BR and 5-HT2CR) (9–11). In particular, 5-HT2CR in the hypothalamus contributes to fenfluramine- and MDMA-induced anorexia-like behavior (5, 8, 12). Moreover, 5-HT1BR and 5-HT2CR knockout (KO) mice are less sensitive to fenfluramine (4, 5).
Stress and anxiety can also induce anorexia (13), and increases in 5-HT neuromodulation are known to participate in the decreased food intake caused by stress (14–16). We have demonstrated that 5-HT4R KO displays attenuated responses to stress-induced hypophagia (17). Because MDMA is a rewarding drug that reduces the intake of food despite an energy demand (8), mimicking anorexia-like behavior, we reasoned that the activation of 5-HT4R into the nucleus accumbens (NAc) may be essential for MDMA-induced anorexia. Indeed, the NAc, as a brain reward center, may influence the physiological drive to eat (18–21), which also contains high densities of 5-HT4R (22).
To address this possibility, we examined the effects on food intake of directly stimulating or inactivating 5-HT4R in the NAc, and we also investigated whether these receptors are further involved in the anorectic effect of MDMA by using a combination of pharmacological, biochemical, immunocytochemical, and molecular biology techniques that include intracerebral injection of siRNA-mediated 5-HT4R (si5-HT4R) knockdown into the NAc. Using 5-HT4R KO mice, we further determined that 5-HT4R contribute to the appetite-suppressant effect of MDMA. Additional experiments revealed that the activation of accumbal 5-HT4R increases the mRNA level of the satiety factor cocaine- and amphetamine-regulated transcript (CART) via a cAMP/PKA signaling pathway. Finally, we provide evidence that increased CART mRNA expression mediates the appetite-suppressant effects of both accumbal 5-HT4R stimulation and MDMA, the psychogenic compound of ecstasy.
Results
Stimulation of Accumbal 5-HT4R Inhibits Food Intake and Increases the Level of CART mRNA.
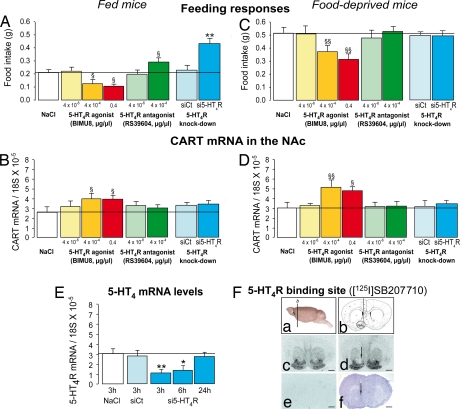
Food intake was measured over 2 h, starting 1 h after intraaccumbal injections of NaCl or endo-N-8-methyl-8-azabicyclo[3.2.1]oct-3-yl)-2,3-dihydro-3-isopropyl-2-oxo-1H-benzimidazol-1-carboxamide hydrochloride (BIMU8), a 5-HT4R agonist. Two different doses of BIMU8 reduced food intake in both fed (42–46%, Fig. 1A) and food-deprived mice (27–33%, Fig. 1C). As expected, no significant effect of BIMU8 (4 × 10−4 μg/μl) was observed in food-deprived 5-HT4R KO [total food intake in g: wild type (WT), 0.58 ± 0.05; KO, 0.60 ± 0.06 (mean ± SEM)]. BIMU8-induced hypophagia is then mediated specifically by the activation of 5-HT4R.
Fig. 1.
Influence of the 5-HT4R on food intake and CART mRNA expression into the NAc. (A and C) The data are means ± SEM of total food intake in fed and food-deprived WT mice treated with an intraaccumbal infusion of 1 μl/min NaCl (n = 11–12), BIMU8 (n = 6–11 per each dose), RS39604 (n = 5–11 per each dose), si5-HT4R control (siCt, 0.05 μg/μl, n = 5–6), or si5-HT4R (0.05 μg/μl, n = 5–7). Food intake was measured between 1 and 3 h after treatments. (B–D) Number of CART mRNA copies in the NAc of identical mice over 3 h. (E) si5-HT4R-mediated decreases in the levels of 5-HT4R mRNA over 3 and 6 h, which is restored for 24 h. (F) Autoradiographs of 5-HT4R-binding sites labeled with [125I]SB207710 in transverse midbrain sections (a) at the level of the NAc (b) from mice treated with siCt (c) and si5-HT4R (d) over 6 h, into the NAc. (e) Nonspecific binding (see Experimental Procedures). Arrows point to decreases in 5-HT4R binding sites (d) and the injection site in a midbrain section labeled with hematoxylin (f), which labeled the nuclei and illustrates that siRNA did not induce any tissue damage. A significant treatment effect is noted (§, P < 0.05; §§, P < 0.01 compared with NaCl. *, P < 0.05; **, P < 0.01 compared with siCt). (Scale bar: 1 mm.)
The animals used in the experiments presented in Fig. 1 A and C were killed 3 h after the injections and the levels of CART mRNA determined in the NAc. The levels of CART mRNA were higher in the BIMU8-injected than in the NaCl-treated mice. Both doses of BIMU8 induced increases in CART mRNA levels in both fed (57–58%, Fig. 1B) and food-deprived (67–56%, Fig. 1D) mice. In neither group were changes in CART mRNA levels observed in the hypothalamus (data not shown).
Inactivation of Accumbal 5-HT4R or siRNA-Mediated Knockdown of 5-HT4R Elevates Food Intake and Does Not Change the Levels of CART mRNA.
Infusing RS39604, a 5-HT4R antagonist, into the NAc, increased food intake in fed (+39%, Fig. 1A), but not in food-deprived mice (Fig. 1C). To further demonstrate that 5-HT4R into the NAc regulates food intake, we performed a knockdown of 5-HT4R into the NAc, using siRNA. Infusing si5-HT4R into the NAc reduced the level of both 5-HT4R mRNA over 6 h (Fig. 1E) and binding sites in the NAc (Fig. 1F). As well as RS39604, injecting si5-HT4R into the NAc increased food intake in fed (+89% vs. si5-HT4R control, +92% vs. NaCl, Fig. 1A) and not in food-deprived mice (Fig. 1C) compared with controls. In all groups, there were no changes in CART mRNA levels observed in the NAc (Fig. 1 B and D) and the hypothalamus (data not shown).
Intracellular Signaling Events Involved in 5-HT4R-Induced CART mRNA Expression.
We hypothesized that the observed 5-HT4R-mediated up-regulation of CART mRNA expression in the NAc may involve the Gs/cAMP/PKA pathway for two reasons. First, stimulating 5-HT4R in cultured neurons induces the production of cAMP and the activation of PKA (23). Second, forskolin, an adenylyl cyclase activator, increases CART mRNA levels in the NAc (24). Injecting BIMU8 into the NAc potently activated cAMP production in this brain structure, compared with saline-injected mice (Fig. 2A). This increase was not detected when BIMU8 was combined with N-(2-[p-bromocinnamylamino]ethyl)-5-isoquinoline-sulfonamide hydrochloride (H89) (Fig. 2B), used at a dose of 2 μg/μl, which inhibits the PKA- and forskolin-induced increase in accumbal CART mRNA levels (24).
Fig. 2.
In the NAc, the 5-HT4R/cAMP/PKA signaling pathway positively controls CART mRNA levels. (A) The data are means ± SEM of tissue cAMP levels in the NAc of WT mice 30 min after intraaccumbal injection of BIMU8 compared with NaCl-treated mice (n = 4 each). (B) The data are means ± SEM of CART mRNA copies in the NAc of WT mice 3 h after intraaccumbal injection of NaCl (n = 8), BIMU8 (n = 8), H89 (n = 6), or BIMU8/H89 (n = 5). H89 inhibited BIMU8-induced increases in the levels of CART mRNA. Treatments that differ significantly from saline are marked (§, P < 0.05; §§, P < 0.01).
Accumbal 5-HT4R Are Involved in Both the Appetite-Suppressant Effect of MDMA and in Its Ability to Increase CART mRNA Expression.
No difference in body weight was detected between WT and 5-HT4R KO mice for a habituation period of 3 days in metabolic cages and after a 24-h deprivation period (data not shown). We then compared the appetite-suppressant effects of i.p. injections of MDMA in food-deprived WT or 5-HT4R KO mice. We found that the appetite-suppressant effect of MDMA was weaker in 5-HT4R KO mice than in WT mice 1 h after the MDMA injection (Fig. 3A). By 3 h after injection, the anorectic effect of MDMA was entirely suppressed in 5-HT4R KO mice (Fig. 3B).
Fig. 3.
5-HT4R KO mice are less sensitive to MDMA-induced anorexia-like behavior. Data are means ± SEM of total food intake in starved WT and KO mice. Animals received an i.p. injection of NaCl (WT, n = 20; KO, n = 10) or MDMA (WT, n = 10; KO, n = 10). MDMA-treated WT mice displayed hypophagia over 1 h (A) and 3 h (B), which was less marked in 5-HT4R KO mice. Significant differences between saline and MDMA-treated animals were detected (§§§, P < 0.0001; §§, P < 0.01). The significance between genotypes and genotype × treatment interaction is noted (***, P < 0.0001; **, P < 0.01; ##, P < 0.01; #, P < 0.05, respectively).
The i.p. injection of MDMA also increased the levels of CART mRNA in the NAc of WT mice (Fig. 4). When MDMA was injected into 5-HT4R KO mice, however, CART mRNA levels were the same as in saline-treated KO mice (Fig. 4). No differences in CART mRNA expression were detected between the NAc of saline-treated mice of both genotypes (Fig. 4). Again, CART mRNA expression was unchanged in the hypothalamus after the MDMA injection (data not shown).
Fig. 4.
MDMA lost its ability to increase CART mRNA expression in the NAc of 5-HT4R KO mice. Using QR-PCR (A and B), in eight MDMA-treated WT mice, the levels of CART mRNA were higher than in seven saline-treated WT animals (F1,13 = 4.7, P < 0.05). This effect was not apparent in 5-HT4R KO mice (F1,10 = 0.8, n = 5–7). A significant difference between NaCl and MDMA is noted (*, P < 0.05), and a significant genotype × treatment interaction is marked (# P < 0.05). Using in situ hybridization (C–F), MDMA-induced increases in CART mRNA levels in the NAc were also observed in coronal brain sections in WT (C and D), but not in KO mice (E and F). (Scale bar: 700 μm.)
Knockdown of CART in the NAc Induces Overeating in Fed Mice and Inhibits Stimulating 5-HT4R- and MDMA-Induced Anorexia.
As reported previously (25), intraaccumbal injection of the CART 55–102 peptide reduced food deprivation-induced eating (Fig. 5). We next examined whether CART mRNA in the NAc could mediate the anorectic effects of MDMA. For this purpose, we performed a tissue-specific knockdown of CART with siRNA (siCART).
Fig. 5.
Injection of CART 55–102 into the NAc diminished deprivation-induced eating. Data are means ± SEM of total food intake in starved WT mice treated with 1 μl of NaCl (n = 7) or CART 55–102 at a dose of 1 μg/μl (n = 9) or 5 μg/μl (n = 7) or untreated (controls, n = 7). Surgical manipulations did not modify feeding responses compared with mice not submitted to any surgical procedure (controls) (P = 0.19). Treatments that differ significantly from saline are noted (§§, P < 0.01; §§§, P < 0.001).
Injecting siCART into the NAc reduced both CART mRNA and peptide levels in the NAc after 3 days (Fig. 6B and darkfield photomicrographs in B c and e). CART immunoreactivity (Ir) indicated that the peptide level was unchanged in the arcuate nucleus, but was lower in the lateral hypothalamus (Fig. 6B d and f). Injection of siCART into the NAc increased the level of food intake in fed mice compared with control mice (Fig. 6A). To test for possible nonspecific effects of siCART, substance P-Ir was examined on serial brain sections because both peptides are colocalized in the NAc (26). No changes in substance P-immunolabeled fibers were detected in brain sections from siCART-treated or control mice (Fig. 6B g, i and h, j).
Fig. 6.
siCART effect on mice. (A) Overeating after intraaccumbal injection of siCART in fed WT mice. Reduced labeling of both CART mRNA and peptide on the third day plus 3 h after the final intracerebral injection of siCART into the NAc of WT compared with the siCART control-treated mice. Data are means ± SEM of total food intake in fed WT mice treated with NaCl (n = 6), siCART control (0.05 μg/μl, n = 5; 0.1 μg/μl, n = 7), or siCART (0.05 μg/μl, n = 6; 0.1 μg/μl, n = 7) measured 3 h after injection. Treatments that differ significantly from control are noted (§, P < 0.05; §§, P < 0.01; §§§, P < 0.001). (B) Darkfield photomicrographs show CART mRNA labeling on coronal sections taken at the level of NAc in control and siCART-treated mice. (Scale bar: 850 μm.) (a and b) Observed labeling after intraaccumbal injection of a fluorescent siRNA (siGlo) to visualize the diffusion of 1 μl. (c–f) CART peptide-Ir fibers on coronal sections at the levels of the NAc (c and e) and lateral hypothalamus (d and f). Arrows point to decreases in CART-Ir (e and f). Substance P-Ir fibers (g and h) were visualized at similar levels of the NAc from identical animals (arrow marks the injection site). aca, anterior commissure, anterior. (Scale bar: 300 μm.)
In contrast to what was observed in fed mice (Fig. 6A), no differences were observed between the food intake of food-deprived mice receiving intraaccumbal injections of either siCART or siCART control (Fig. 7 A and B). In addition, siRNA knockdown of CART in the NAc abolished the appetite-suppressant effect of injected BIMU8 (Fig. 7A). Finally, CART knockdown in the NAc partially restored deprivation-induced eating in mice treated with MDMA (Fig. 7B).
Fig. 7.
MDMA-induced anorexia-like behavior in starved mice was reduced when CART was knocked down in the NAc by using siCART. Data are means ± SEM of total food intake for 1 h. (A) WT mice treated with saline or BIMU8 injected alone (n = 14–14) or combined with the siCART control (n = 15) or siCART (n = 10) into the NAc. (B) WT mice treated with an i.p. injection of saline or MDMA plus an intraaccumbal infusion of siCART control (n = 8 and 14, respectively) or siCART (n = 14 and 12, respectively). Treatments that differ significantly from NaCl are noted (§, P < 0.05; §§§, P < 0.0001). Significant differences between siCART and siCART control are marked (*, P < 0.05; **, P < 0.01). There was a significant interaction between both modes of injection (#, P < 0.05).
Discussion
Over the last six decades, studies have demonstrated that the hypothalamus influences feeding behavior through multiple neuronal messengers, including 5-HT (11, 27). In particular, 5-HT-induced hypophagia is known to involve the hypothalamic receptors 5-HT1BR and 5-HT2CR (11, 28). At the same time, the hypothalamus is probably not the only brain structure through which 5-HT influences food intake. Although several results have suggested that the NAc is crucial for regulating feeding behavior (18–20, 29), the involvement of specific 5-HT receptor subtypes present in this structure and involved in the modulation of feeding behavior remained to be explored fully.
This work reports one example of a 5-HT receptor subtype being expressed in the NAc, which influences the physiological drive to eat. Specifically, we observed that stimulating accumbal 5-HT4R diminishes the intake of food in fed mice (increased satiety) and reduces the physiological drive to eat after food deprivation. It provides a further demonstration that 5-HT reduced appetite in the NAc via the 5-HT4R because blocking (antagonist) or inactivating (si5-HT4R) accumbal 5-HT4R elevates food intake in fed mice (decreased satiety) but not in motivated mice (food-deprived). Results are consistent with studies indicating that 5-HT in the hypothalamus induces satiety (30). For example, the i.p. injection of the 5-HT2CR antagonist RS102221 does not modify food deprivation-induced eating although it increases food intake when mice are fed ad libitum (31).
Stimulation of 5-HT4R also increases the levels of mRNA encoding the anorectic peptide CART in the NAc. Further, consistent with the well known coupling of adenylyl cyclase with 5-HT4R (23), activating 5-HT4R increases cAMP production and up-regulates CART mRNA expression via PKA in the NAc. These results are consistent with those of Jones and Kuhar (24), who demonstrated that the injection of forskolin increases CART mRNA levels in a cAMP/PKA pathway-dependent manner. In parallel, injecting acutely 5-HT4R antagonist or si5-HT4R did not change CART mRNA levels into the NAc, as well as observed in 5-HT4R KO mice. Together, these results suggest that 5-HT4R exert a phasic and positive control of CART mRNA expression via the cAMP/PKA pathway in the NAc.
Using siRNA technology, we found that the selective knockdown of CART in the NAc suppresses the 5-HT4R-mediated inhibition of deprivation-induced eating. It is noteworthy that only fed, but not food-deprived, mice consumed more food after intraaccumbal injections of siCART. This result indicates that basal CART levels in the NAc do not produce a significant anorectic effect when a positive drive to eat is reinforced (i.e., with food deprivation). Interestingly, CART peptide-Ir was lower in the lateral hypothalamus after the injection of siCART into the NAc, consistent with the possible presence of CART/GABA neuronal projections from the NAc to the hypothalamus (25). Therefore, we cannot exclude the possibility that the observed effects of siCART partly involve CART-expressing accumbal–hypothalamus neurons. In any case, the local presence of CART peptide within the NAc influences the consumption of food, as evidenced by the appetite-suppressant effect of intraaccumbal injection of the CART 55–102 peptide. Two additional results further support the notion that CART acts locally to control appetite within the NAc: (i) i.p. injection of MDMA increased CART mRNA expression in the NAc, but not in the hypothalamus; and (ii) the selective knockdown of CART in the NAc diminished the inhibitory effect that i.p. injection of MDMA has on the physiological drive to eat.
The final experiment in our series bore out our hypothesis that MDMA-induced anorexia involves the activation of 5-HT4R in the NAc. Indeed, both anorexia and the increased levels of CART mRNA in the NAc resulting from i.p. injection of MDMA were reduced in 5-HT4R KO mice. The decrease in food intake that normally occurs in response to MDMA treatment was completely blocked in 5-HT4R KO mice 3 h after the i.p. MDMA injection. In contrast, in the 1st h after MDMA administration, the appetite-suppressant effect of the drug was only reduced in 5-HT4R KO mice (but not in WT mice), which suggests that there may be additional short-lasting mechanisms (e.g., 5-HT2CR-dependent pathways, contributing to MDMA-induced anorexia; see ref. 8).
We have shown previously that the 5-HT4R KO mouse is one animal model that displays decreased anorexia-like behavior in response to stress (17). Again, the hyposensitivity of 5-HT4R KO mice to MDMA raises the possibility that the absence of 5-HT4R results in a general inadaptability to stressors, as defined previously (32), which may include psychostimulants such as MDMA. Given the fact that stress is widely suspected to influence feeding disorders in humans, a pathology associated with anxiety and depression (13, 33), it is conceivable that, under stress, genetic predispositions related to 5-HT4R and CART may be involved in feeding anomalies.
Experimental Procedures
Animals.
All experiments were performed on 4- to 6-month-old male WT and 5-HT4R KO mice on a 129/Sv genetic background. Mice were obtained from heterozygous breeding (17) and housed (n = 5 per cage) with food and water available ad libitum in a temperature-controlled environment with a 12-h light/12-h dark cycle (light onset at 0700). We performed experiments with different groups of WT and KO mice in accordance with the Guide for Care and Use of Laboratory Animals established by the Centre National de la Recherche Scientifique.
Surgery.
WT mice were anesthetized by i.p. injection of ketamine (60 mg/kg) and xylasine (15 mg/kg) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Each compound used (see below) was dissolved in NaCl (9‰) and stored at 4°C until use. A sterile 26-gauge stainless steel guide was unilaterally implanted in the left-shell NAc at the following coordinates from the bregma: anterior, +1.6 mm, height, −4.3 mm; lateral, +0.7 mm according to the brain atlas (34). The localization of the injection sites was systematically assessed for each mouse.
Feeding Tests and Treatments.
We performed experiments using a classic feeding paradigm including (or not) food deprivation for 24 h (8). First, a series of WT mice received, in the NAc (i) an acute injection of 1 μl containing NaCl (9‰), the 5-HT4R agonist BIMU8 (4 × 10−6, 4 × 10−4, 0.4 μg/μl; Boehringer Ingelheim, Ingelheim, Germany), the antagonist RS39604 (4 × 10 −6, 4 × 10−4; Tocris, Ellisville, MO), double-stranded si5-HT4R (0.05 μg/μl, mouse mRNA: sense, 5′-AUGAUGGCAACUGAUCGAC99-3′; antisense, 5′-GUCGAUCAGUUGCCAUCAU99-3′), si5-HT4R control, or the CART 55–102 peptide (1.0 μg/μl; Bachem AG); (ii) a chronic injection (3 days, 1 μl) of double-stranded siCART (0.05, 0.1 μg/μl, mouse mRNA: sense, 5′-CCUGAAUAGACCAUUCGCG99-3′; antisense, 5′-CGCGAAUGGUCUAUUCAGG99-3′) or siCART control. Both siCART and si5-HT4R controls (Eurogentec, San Diego, CA) contained identical nucleotides but in random order and had no significant homology with any known rodent mRNAs. Second, a series of WT and 5-HT4R KO mice received an i.p. injection containing a single dose of NaCl (9‰) or MDMA (10 mg/kg; Sigma, see ref. 8). Third, WT mice were injected i.p. with a single dose of NaCl (9‰) or MDMA (10 mg/kg) combined with a chronic intraaccumbal injection of siCART, siCART control (0.1 μg/μl), or NaCl (9‰). Finally, another set of WT mice received, in the NAc, 1 μl of BIMU8 (4 × 10−4 μg/μl), combined or not with siCART, siCART control (0.1 μg/μl), or NaCl (9‰). In the latter combined treatment experiments, animals were food-deprived for 18 h. Each compound was infused into the NAc of conscious mice at a rate of 1 μl/min with a microsyringe nanopump (CE; myNeuroLab, St. Louis, MO). After a recovery period of 24 h, mice were isolated in individual metabolic cages for a baseline period of 3 days. Classic food (16.5% crude protein/3.6% crude fat/4.6% crude fiber/5.2% ash) was provided, as described previously (8, 17).
Immunocytochemistry.
Mice were anesthetized 3 h after the final injection of siCART or siCART control. Brains were fixed in paraformaldehyde (4%, 24 h, 4°C) and cryoprotected for 24 h in 20% (wt/vol) sucrose/0.1 M phosphate buffer (35). Serial 30-μm-thick coronal sections were labeled with a rabbit polyclonal primary antibody against CART (1/2,000; Phœnix Pharmaceuticals, St. Joseph, MO) or substance P (1/1,000; DiaSorin, Inc., Stillwater, MN) and incubated for 2 h with a goat anti-rabbit IgG conjugated to horseradish peroxidase (1/1,000; AbCys, Paris, France). An average of five to eight serial coronal sections were examined, respectively, at the levels of the NAc (anterior +1.6 mm) and the hypothalamus (anterior −1.5 mm) from bregma (34).
Receptor Autoradiography.
Frozen serial coronal brain sections (12 μm thick) from mice were processed for ligand-binding autoradiography of 5-HT4R with the antagonist 125I-SB207710 (36, 37). Briefly, sections were incubated in the appropriate buffer supplemented with 10 μM pargyline/0.01% ascorbic acid/125I-SB207710 (specific activity, 2,000 Ci/mmol; final concentration, 0.02 nM; Amersham, Piscataway, NJ) at 37°C for 30 min. Nonspecific binding was determined on consecutive sections incubated in the presence of 1 μM GR113808 [Sigma–Aldrich, St. Louis, MO (35, 36)]. All of the labeled sections were exposed to Kodak X-Omat Blue (PerkinElmer, Waltham, MA) for 5 days at 4°C (36, 37). Sections from mice of both groups were processed together to obtain corresponding radiograms on the same films.
Quantitative Real-Time PCR (QR-PCR).
Animals were killed 3 h after the various treatments, and the NAc (1.2 mm3) and hypothalamus (2 × 2.4 mm3) were microdissected from 1-mm-thick sections at −20°C by using a micropunch following the landmarks of the stereotaxic atlas [NAc, anterior +1.6 mm; hypothalamus, anterior +0.58 and −1.58 mm, from bregma (34)]. As described (38), total mRNA was isolated, treated with DNase, and reverse-transcribed. First-strand cDNA was used as a template for QR-PCR amplification (ABI Prism 7000; Applied Biosystems, Foster City, CA) in 10-μl reactions containing a 300 nM concentration of each of the CART primers [sense and antisense primers, respectively, hybridized to nucleotides 246–269 and 296–314 of the mRNA sequence of mouse CART (NM_013732)] or 5-HT4R mRNA primers [sense and antisense primers, respectively, hybridized to nucleotides 688–705 and 631–654 of the mRNA sequence of mouse 5-HT4R (NM_008313)] and a master mix (SybR Green) including Taq polymerase. Samples were successively incubated (50°C, 2 min; 95°C, 10 min) followed by 40 cycles of denaturation (95°C, 15 s), annealing, and extension (60°C, 1 min). The size of fragments was assessed by using electrophoretic separation in nusive 3:1 agarose gel. The relative expression level of the CART gene was evaluated by QR-PCR of two housekeeping genes (aldolase 3 and 18S rRNA). Their expression was not significantly different between genotypes or treatments (data not shown).
In Situ Hybridization Histochemistry.
A 389-bp segment of the mouse CART gene in pCR4-TOPO (Invitrogen, Carlsbad, CA) was used for the synthesis of riboprobes in the presence of [35S]UTP, using in vitro transcription (Maxiscript T3/T7; Ambion AMS, Abingdon, U.K.). As we have reported (39), the primers used for PCR covered the CART mRNA coding sequence (NM_013732). Coronal sections (16 μm thick) from fixed brain (see Immunocytochemistry), taken from representative planes of the NAc (anterior, +1.6 to 0.98 mm) from bregma (34), were incubated with radiolabeled antisense or sense (control section) riboprobe. The hybridization signal was detected by autoradiography with MR1 film (Kodak, Rochester, NY) (39).
Biochemical Analysis of Tissue cAMP Levels.
The levels of cAMP were analyzed after the injection of NaCl (9‰) or BIMU8 (4 × 10−4 μg/μl) into the NAc of WT mice. The protocol (40) included a combined i.p. injection of the phosphodiesterase inhibitor rolipram (0.2 mg/kg). Thirty minutes later, the brains were quickly frozen in isopentane cooled with liquid nitrogen. Brains were sliced into 1 mm, and tissue samples were microdissected as above [see Quantitative Real-Time PCR (QR-PCR)]. Quantification of cAMP production was performed by using a homogeneous time-resolved fluorescence-based kit (cAMP Dynamic kit; CisBio International, Bedford, MA).
Statistical Analysis.
Data were analyzed with STATVIEW 5 software. A repeated-measures ANOVA was performed on the data, which were obtained in multiple sessions over time (food intake). Genotype and treatment were used as independent variables. When significant effects of genotype, treatment, or genotype × treatment were found, the independent variables were split for a two-way (genotype and treatment) or one-way ANOVA (genotype or treatment) analysis. For multiple comparisons, the Scheffé F test was used. Differences with P < 0.05 were considered significant.
Acknowledgments
We are grateful to B. Greggio and G. Erba, who partly analyzed CART mRNA levels. We thank C. Bobo, L. Forichon, and A. Delalbre for help in breeding the mice, and we acknowledge the assistance of A. Turner-Madeuf and F. Bertaso in editing the manuscript. We acknowledge the assistance of E. Trinquet and H. Ansanay (CisBio International) and F. Gaven in using the cAMP kit. This work was supported by grants from the French government (Centre National de la Recherche Scientifique, Action Concertée Incitative (ACI) Biologie du développement et physiologie intégrée).
Abbreviations
- BIMU8
endo-N-8-methyl-8-azabicyclo[3.2.1]oct-3-yl)-2,3-dihydro-3-isopropyl-2-oxo-1H-benzimidazol-1-carboxamide hydrochloride
- CART
cocaine- and amphetamine-regulated transcript
- H89
N-(2-[p-bromocinnamylamino]ethyl)-5-isoquinoline-sulfonamide hydrochloride
- 5-HT
serotonin
- 5-HT4R
serotonin receptor
- Ir
immunoreactivity
- MDMA
3,4-N-methylenedioxymethamphetamine
- NAc
nucleus accumbens
- QR-PCR
quantitative real-time PCR
- si5-HT4R
siRNA-mediated 5-HT4R knockdown
- siCART
tissue-specific knockdown of CART with siRNA.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Sullivan PF. Am J Psychiatry. 1995;152:1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- 2.Signorini A, De Filippo E, Panico S, De Caprio C, Pasanisi F, Contaldo F. Eur J Clin Nutr. 2007;61:119–122. doi: 10.1038/sj.ejcn.1602491. [DOI] [PubMed] [Google Scholar]
- 3.Compan V. In: Serotonin Receptors in Neurobiology. Chattopadhyay A, editor. Boca Raton, FL: RC; 2007. pp. 157–180. [Google Scholar]
- 4.Lucas JJ, Yamamoto A, Scearce-Levie K, Saudou F, Hen R. J Neurosci. 1998;18:5537–5544. doi: 10.1523/JNEUROSCI.18-14-05537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vickers SP, Clifton PG, Dourish CT, Tecott LH. Psychopharmacology. 1999;143:309–314. doi: 10.1007/s002130050952. [DOI] [PubMed] [Google Scholar]
- 6.Rochester JA, Kirchner JT. J Am Board Fam Pract. 1999;12:137–142. doi: 10.3122/jabfm.12.2.137. [DOI] [PubMed] [Google Scholar]
- 7.Frith CH, Chang LW, Lattin DL, Walls RC, Hamm J, Doblin R. Fundam Appl Toxicol. 1987;9:110–119. doi: 10.1016/0272-0590(87)90158-8. [DOI] [PubMed] [Google Scholar]
- 8.Conductier G, Crosson C, Hen R, Bockaert J, Compan V. Neuropsychopharmacology. 2005;30:1056–1063. doi: 10.1038/sj.npp.1300662. [DOI] [PubMed] [Google Scholar]
- 9.Kennett GA, Curzon G. Psychopharmacology. 1988;96:93–100. doi: 10.1007/BF02431539. [DOI] [PubMed] [Google Scholar]
- 10.Klodzinska A, Chojnacka-Wojcik E. Pol J Pharmacol Pharm. 1990;42:13–17. [PubMed] [Google Scholar]
- 11.Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, et al. Neuron. 2006;51:239–249. doi: 10.1016/j.neuron.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Vickers SP, Dourish CT, Kennett GA. Neuropharmacology. 2001;41:200–209. doi: 10.1016/s0028-3908(01)00063-6. [DOI] [PubMed] [Google Scholar]
- 13.Godart NT, Flament MF, Lecrubier Y, Jeammet P. Eur Psychiatry. 2000;15:38–45. doi: 10.1016/s0924-9338(00)00212-1. [DOI] [PubMed] [Google Scholar]
- 14.Ge J, Barnes NM, Costall B, Naylor RJ. Pharmacol Biochem Behav. 1997;58:775–783. doi: 10.1016/s0091-3057(97)00024-5. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Tsuchiya K, Koyama T. Pharmacol Biochem Behav. 1994;49:911–920. doi: 10.1016/0091-3057(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 16.Konstandi M, Johnson E, Lang MA, Malamas M, Marselos M. Pharmacol Res. 2000;41:341–346. doi: 10.1006/phrs.1999.0597. [DOI] [PubMed] [Google Scholar]
- 17.Compan V, Zhou M, Grailhe R, Gazzara RA, Martin R, Gingrich J, Dumuis A, Brunner D, Bockaert J, Hen R. J Neurosci. 2004;24:412–419. doi: 10.1523/JNEUROSCI.2806-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stratford TR, Kelley AE. J Neurosci. 1997;17:4434–4440. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassareo V, Di Chiara G. Eur J Neurosci. 1999;11:4389–4397. doi: 10.1046/j.1460-9568.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds SM, Berridge KC. J Neurosci. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Compan V, Daszuta A, Salin P, Sebben M, Bockaert J, Dumuis A. Eur J Neurosci. 1996;8:2591–2598. doi: 10.1111/j.1460-9568.1996.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 23.Dumuis A, Bouhelal R, Sebben M, Cory R, Bockaert J. Mol Pharmacol. 1988;34:880–887. [PubMed] [Google Scholar]
- 24.Jones DC, Kuhar MJ. J Pharmacol Exp Ther. 2006;317:454–461. doi: 10.1124/jpet.105.096123. [DOI] [PubMed] [Google Scholar]
- 25.Yang SC, Shieh KR, Li HY. Neuroscience. 2005;133:841–851. doi: 10.1016/j.neuroscience.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Hubert GW, Kuhar MJ. Brain Res. 2005;1050:8–14. doi: 10.1016/j.brainres.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, Rossi-Fanelli F. Nutrition. 2000;16:843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- 28.Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, et al. Science. 2002;297:609–611. doi: 10.1126/science.1072327. [DOI] [PubMed] [Google Scholar]
- 29.Stratford TR, Kelley AE. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- 31.Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, et al. Neuropharmacology. 1997;36:621–629. doi: 10.1016/s0028-3908(97)00049-x. [DOI] [PubMed] [Google Scholar]
- 32.Caamano CA, Morano MI, Akil H. Psychopharmacol Bull. 2001;35:6–23. [PubMed] [Google Scholar]
- 33.Casper RC. Depress Anxiety. 1998;8(Suppl 1):96–104. [PubMed] [Google Scholar]
- 34.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [Google Scholar]
- 35.Compan V, Dusticier N, Nieoullon A, Daszuta A. Synapse. 1996;24:87–96. doi: 10.1002/(SICI)1098-2396(199609)24:1<87::AID-SYN9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 36.Compan V, Segu L, Buhot MC, Daszuta A. Brain Res. 1998;795:264–276. doi: 10.1016/s0006-8993(98)00316-3. [DOI] [PubMed] [Google Scholar]
- 37.Vilaro MT, Cortes R, Mengod G. J Comp Neurol. 2005;484:418–439. doi: 10.1002/cne.20447. [DOI] [PubMed] [Google Scholar]
- 38.Conductier G, Dusticier N, Lucas G, Cote F, Debonnel G, Daszuta A, Dumuis A, Nieoullon A, Hen R, Bockaert J, Compan V. Eur J Neurosci. 2006;24:1053–1062. doi: 10.1111/j.1460-9568.2006.04943.x. [DOI] [PubMed] [Google Scholar]
- 39.Lucas G, Compan V, Charnay Y, Neve RL, Nestler EJ, Bockaert J, Barrot M, Debonnel G. Biol Psychiatry. 2005;57:918–925. doi: 10.1016/j.biopsych.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 40.Barthet G, Gaven F, Framery B, Shinjo K, Nakamura T, Claeysen S, Bockaert J, Dumuis A. J Biol Chem. 2005;280:27924–27934. doi: 10.1074/jbc.M502272200. [DOI] [PubMed] [Google Scholar]