Abstract
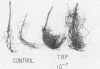
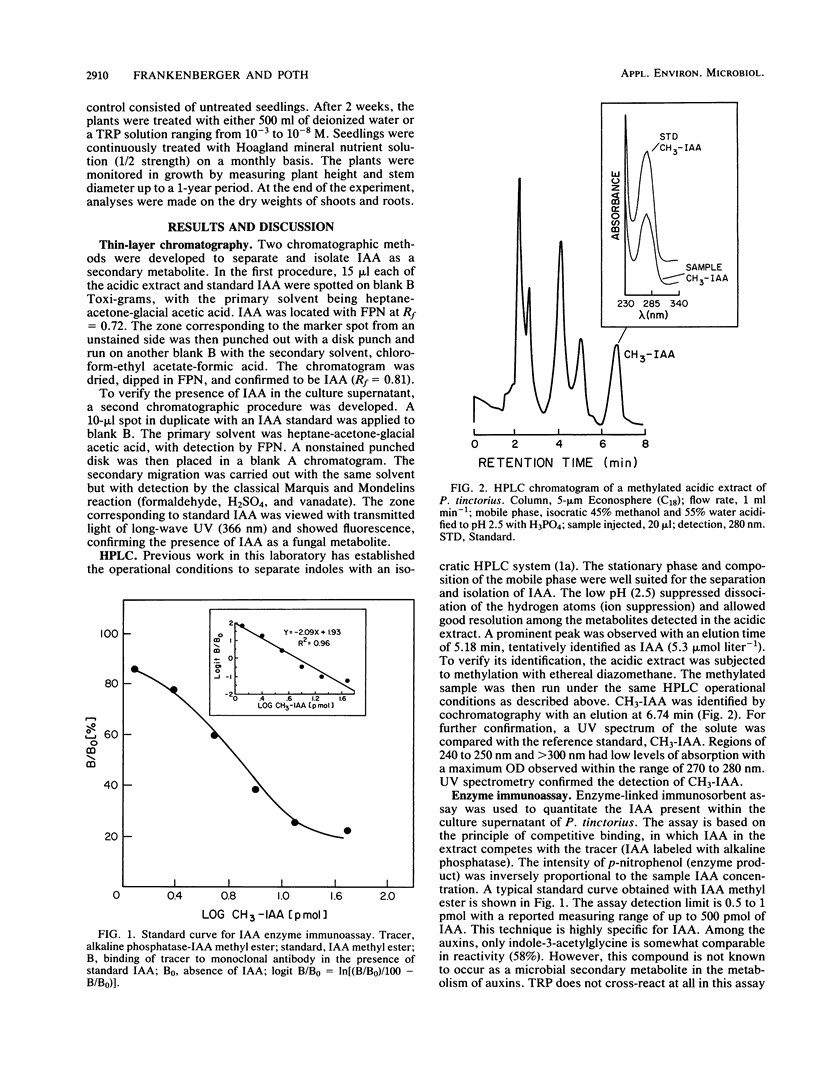
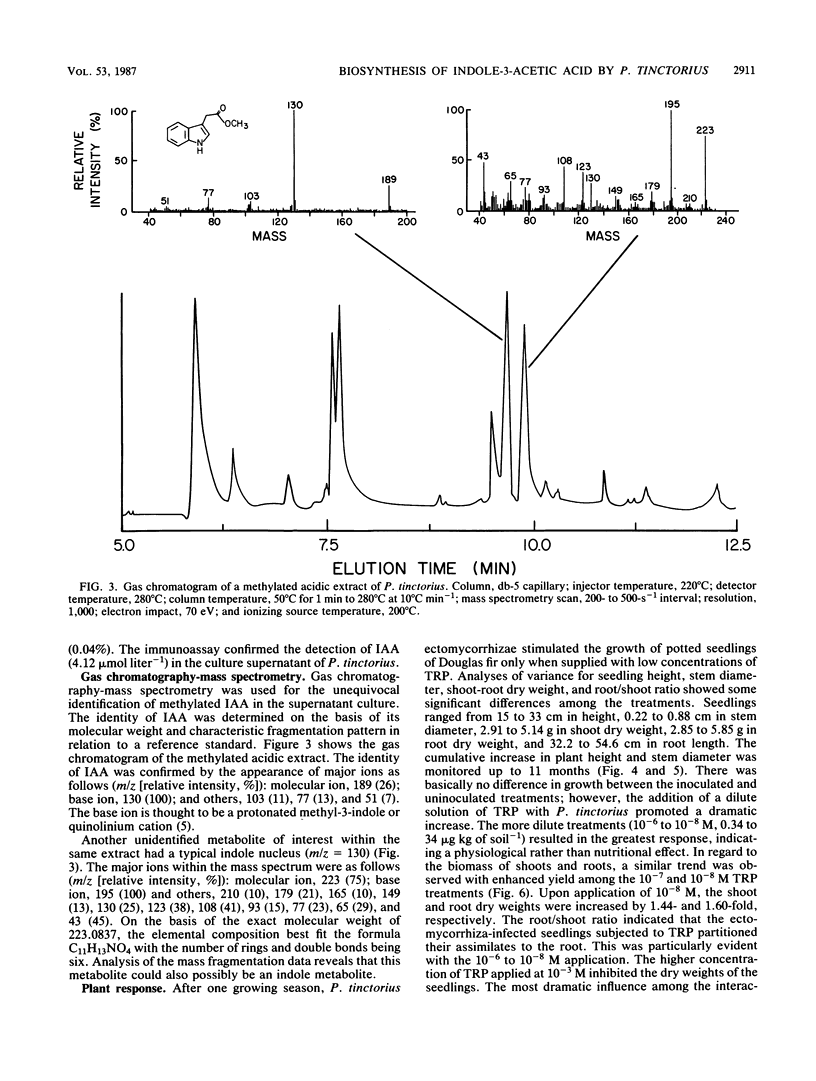
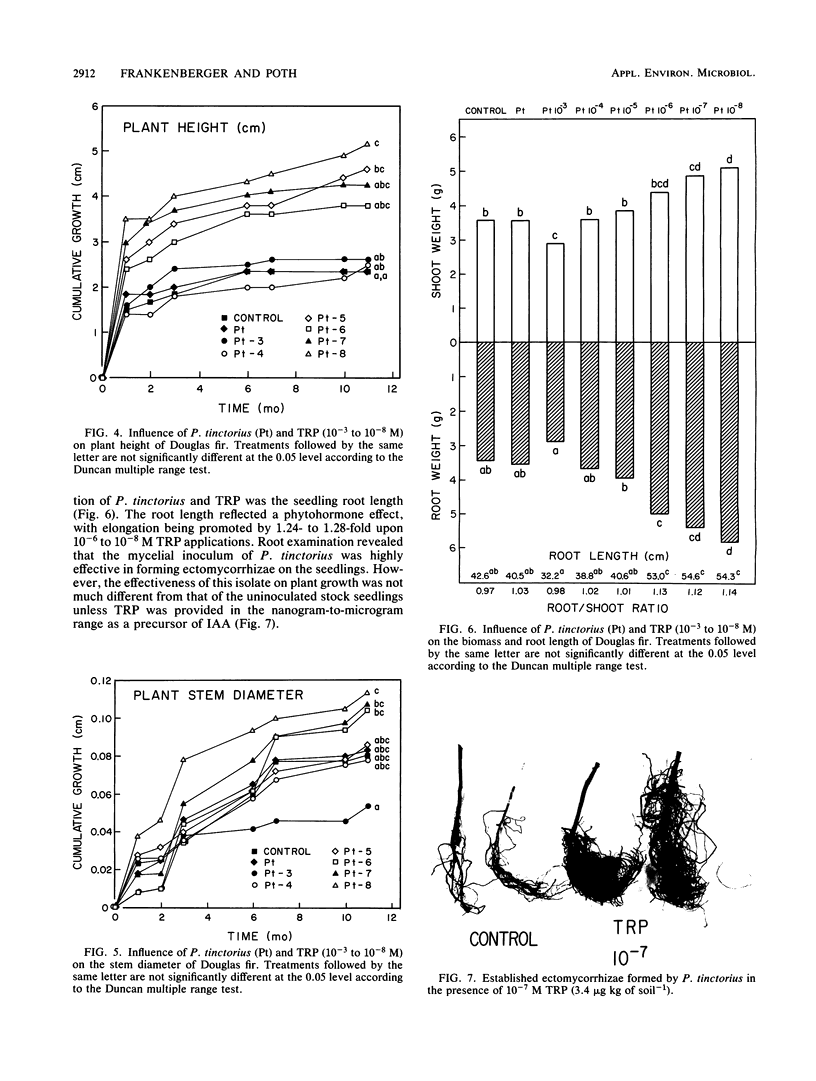
Previous work has indicated that anatomical and morphological changes (stunting and dichotomy) in roots of various conifers may be influenced by plant-growth-regulating substances secreted by mycorrhizae. Indole-3-acetic acid (IAA) has been tentatively identified as a major auxin produced by some selected ectomycorrhizae. We report the isolation and detection of IAA as a secondary metabolite from Pisolithus tinctorius by thin-layer chromatography, high-performance liquid chromatography (HPLC), enzyme-linked immunosorbent (monoclonal antibody) assay (ELISA), and unequivocal identification by gas chromatography-mass spectrometry (GC-MS). The thin-layer chromatography methods for auxin isolation described here are novel, with the use of heptane-acetone-glacial acetic acid as the migrating solvent and formaldehyde, H2SO4, and vanadate in detection. The acidic extract of the culture supernatant was methylated with ethereal diazomethane to detect IAA as methyl-3-IAA by HPLC, ELISA, and GC-MS. The quantitative amount of IAA detected ranged from 4 to 5 μmol liter−1 by HPLC and ELISA. Another unidentified metabolite was detected by GC-MS with a typical indole nucleus (m/z = 130), indicating that it could be an intermediate in auxin metabolism. Plant response (Pseudotsuga menziesii, Douglas fir) was monitored upon inoculation of P. tinctorius and l-tryptophan. There was a consistent increase in plant height and stem diameter as a result of the two treatments, with statistical differences in dry weights of the shoots and roots.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frankenberger W. T., Jr, Poth M. Determination of substituted indole derivatives by ion suppression-reverse-phase high-performance liquid chromatography. Anal Biochem. 1987 Sep;165(2):300–308. doi: 10.1016/0003-2697(87)90273-9. [DOI] [PubMed] [Google Scholar]
- MOSER M. [Contributions tio the recognition of growth substance relationships in the area of ectotrophic mycorrhiza I]. Arch Mikrobiol. 1959;34:251–269. [PubMed] [Google Scholar]
- Marx D. H. Tree host range and world distribution of the extomycorrhizal fungus Pisolithus tinctorius. Can J Microbiol. 1977 Mar;23(3):217–223. doi: 10.1139/m77-033. [DOI] [PubMed] [Google Scholar]
- Strzelczyk E., Sitek J. M., Kowalski S. Synthesis of auxins from tryptophan and tryptophan-precursors by fungi isolated from mycorrhizae of pine (Pinus silvestris L.). Acta Microbiol Pol. 1977;26(3):255–264. [PubMed] [Google Scholar]