Abstract
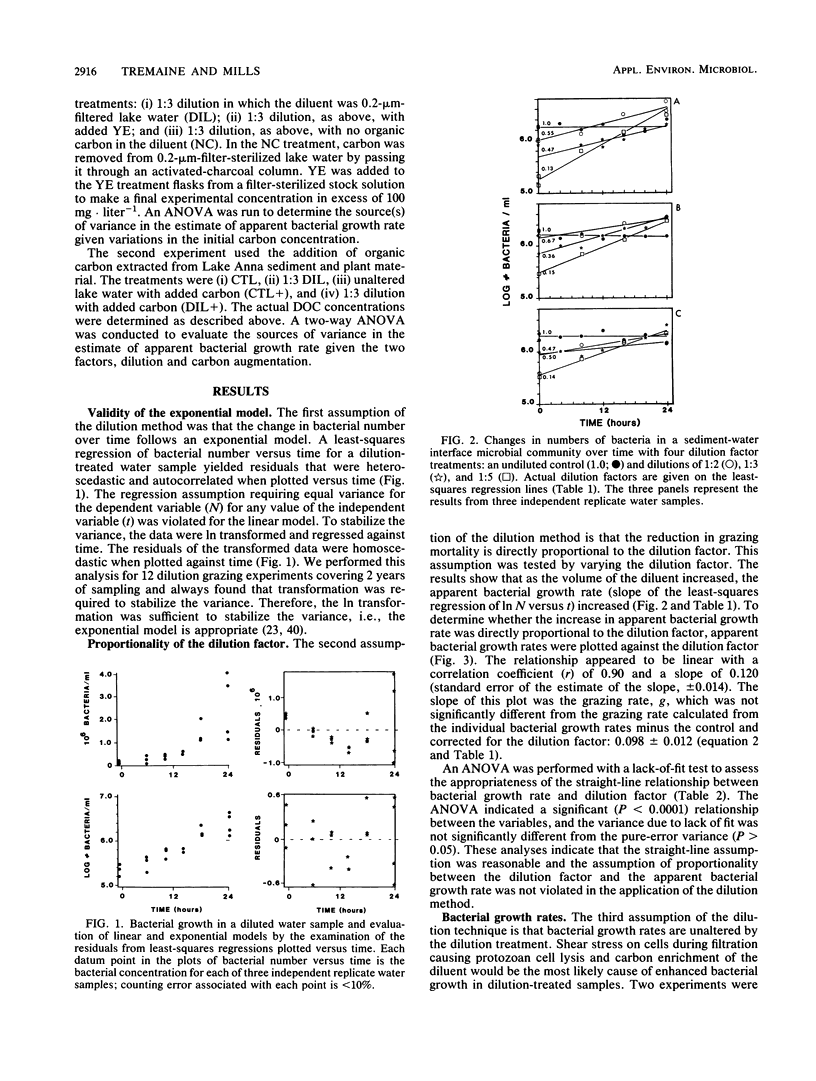
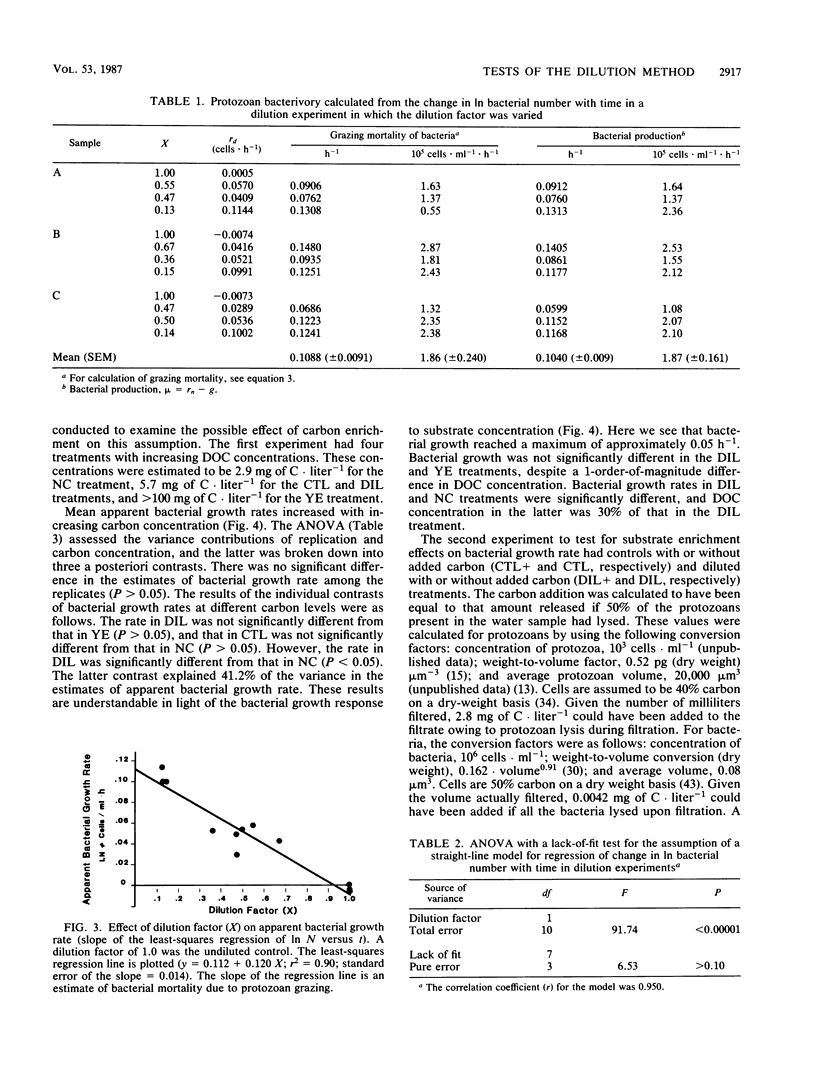
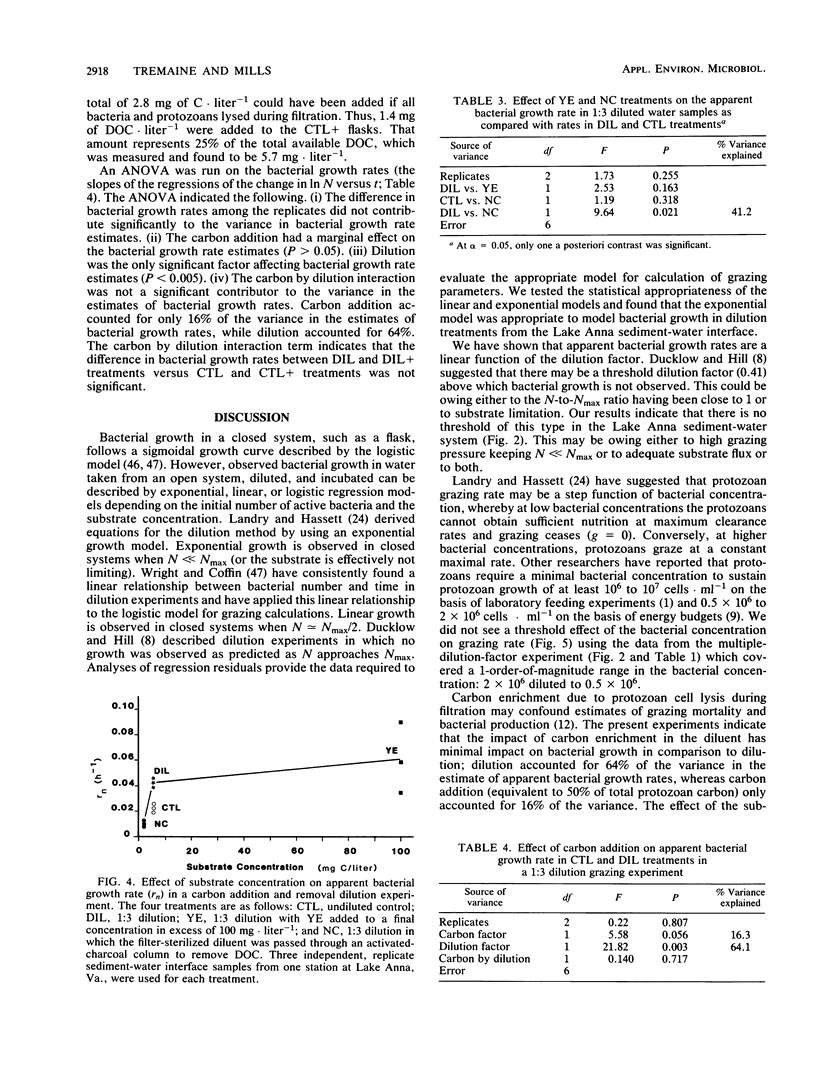
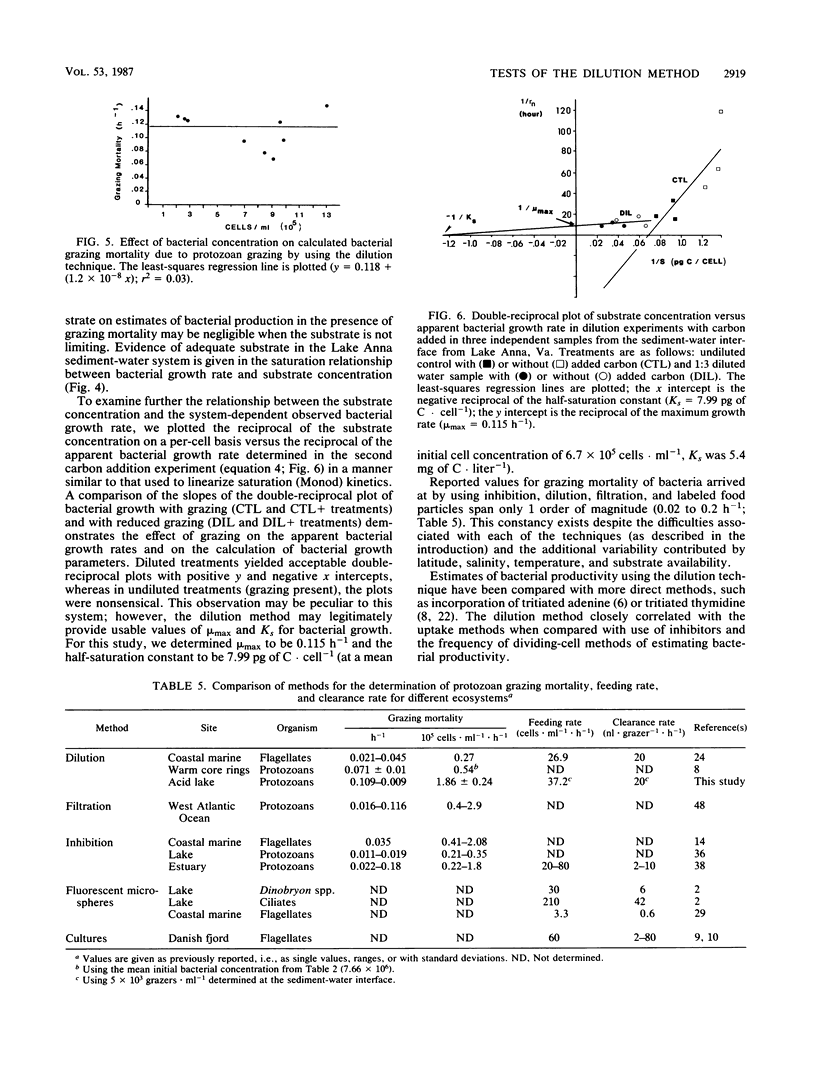
The critical assumptions of the dilution method for estimating grazing rates of microzooplankton were tested by using a community from the sediment-water interface of Lake Anna, Va. Determination of the appropriate computational model was achieved by regression analysis; the exponential model was appropriate for bacterial growth at Lake Anna. The assumption that the change in grazing pressure is linearly proportional to the dilution factor was tested by analysis of variance with a lack-of-fit test. There was a significant (P < 0.0001) linear (P > 0.05) relationship between the dilution factor and time-dependent change in ln bacterial abundance. The assumption that bacterial growth is not altered by possible substrate enrichment in the dilution treatment was tested by amending diluted water with various amounts of dissolved organic carbon (either yeast extract or extracted carbon from lake sediments). Additions of carbon did not significantly alter bacterial growth rates during the incubation period (24 h). On the basis of these results, the assumptions of the dilution method proved to be valid for the system examined.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird D. F., Kalff J. Bacterial grazing by planktonic lake algae. Science. 1986 Jan 31;231(4737):493–495. doi: 10.1126/science.231.4737.493. [DOI] [PubMed] [Google Scholar]
- Caron D. A. Technique for enumeration of heterotrophic and phototrophic nanoplankton, using epifluorescence microscopy, and comparison with other procedures. Appl Environ Microbiol. 1983 Aug;46(2):491–498. doi: 10.1128/aem.46.2.491-498.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian R. R., Hanson R. B., Newell S. Y. Comparison of methods for measurement of bacterial growth rates in mixed batch cultures. Appl Environ Microbiol. 1982 May;43(5):1160–1165. doi: 10.1128/aem.43.5.1160-1165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson R. L., Buckley E. N., Palumbo A. V. Response of marine bacterioplankton to differential filtration and confinement. Appl Environ Microbiol. 1984 Jan;47(1):49–55. doi: 10.1128/aem.47.1.49-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman J. A., McManus G. B. Do bacteria-sized marine eukaryotes consume significant bacterial production? Science. 1984 Jun 15;224(4654):1257–1260. doi: 10.1126/science.224.4654.1257. [DOI] [PubMed] [Google Scholar]
- Herlihy A. T., Mills A. L. Sulfate reduction in freshwater sediments receiving Acid mine drainage. Appl Environ Microbiol. 1985 Jan;49(1):179–186. doi: 10.1128/aem.49.1.179-186.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Ducklow H., Mitchell R. Estimates of bacterial growth from changes in uptake rates and biomass. Appl Environ Microbiol. 1982 Dec;44(6):1296–1307. doi: 10.1128/aem.44.6.1296-1307.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez C., Alexander M. Evidence Suggesting Protozoan Predation on Rhizobium Associated with Germinating Seeds and in the Rhizosphere of Beans (Phaseolus vulgaris L.). Appl Environ Microbiol. 1980 Sep;40(3):492–499. doi: 10.1128/aem.40.3.492-499.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders R. W., Porter K. G. Use of metabolic inhibitors to estimate protozooplankton grazing and bacterial production in a monomictic eutrophic lake with an anaerobic hypolimnion. Appl Environ Microbiol. 1986 Jul;52(1):101–107. doi: 10.1128/aem.52.1.101-107.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Fallon R. D. Use of monodispersed, fluorescently labeled bacteria to estimate in situ protozoan bacterivory. Appl Environ Microbiol. 1987 May;53(5):958–965. doi: 10.1128/aem.53.5.958-965.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. T., Pace M. L. Validity of eucaryote inhibitors for assessing production and grazing mortality of marine bacterioplankton. Appl Environ Microbiol. 1987 Jan;53(1):119–128. doi: 10.1128/aem.53.1.119-128.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaine S. C., Mills A. L. Inadequacy of the eucaryote inhibitor cycloheximide in studies of protozoan grazing on bacteria at the freshwater-sediment interface. Appl Environ Microbiol. 1987 Aug;53(8):1969–1972. doi: 10.1128/aem.53.8.1969-1972.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Novitsky T. J., Quinby H. L., Valois F. W. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetka J. E., Wiebe W. J. Ecological application of antibiotics as respiratory inhibitors of bacterial populations. Appl Microbiol. 1974 Dec;28(6):1033–1039. doi: 10.1128/am.28.6.1033-1039.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


