Abstract
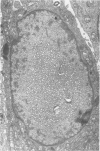
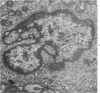
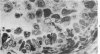
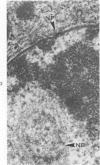
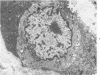
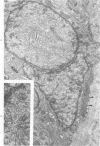
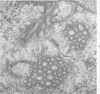
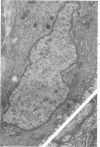
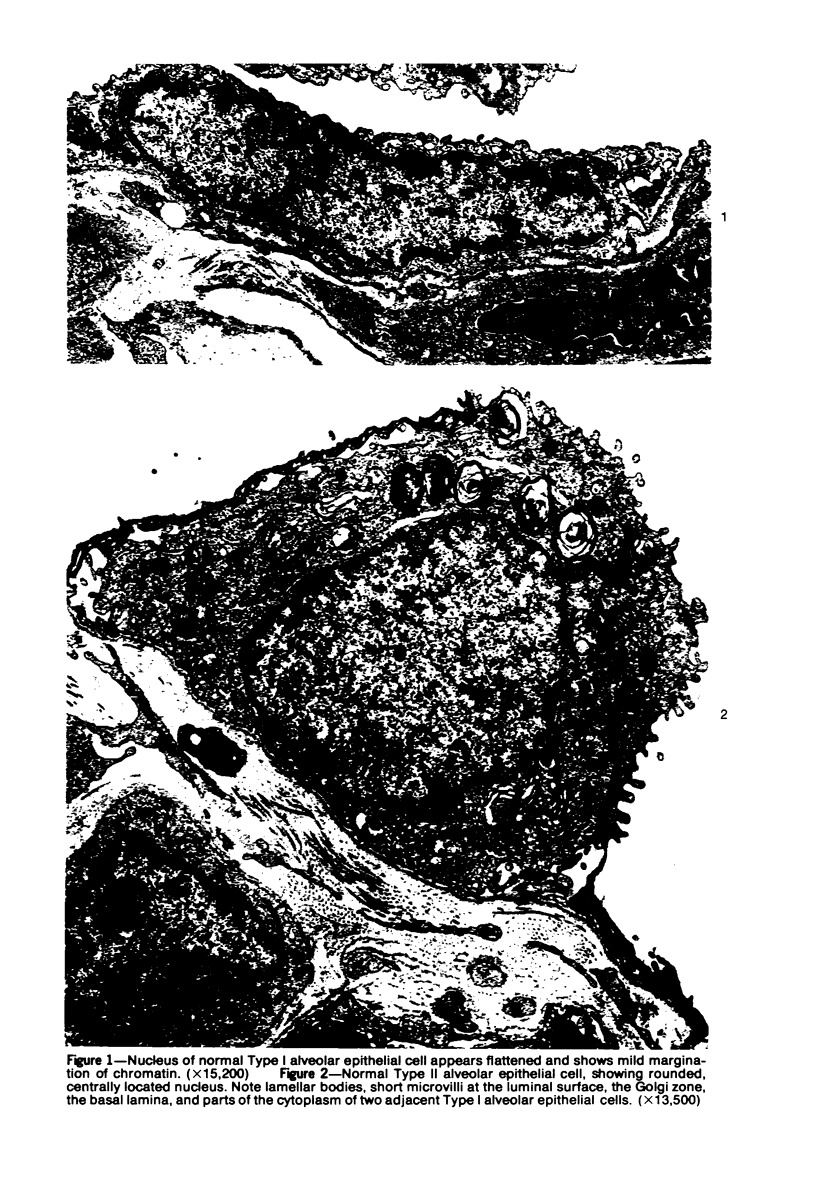
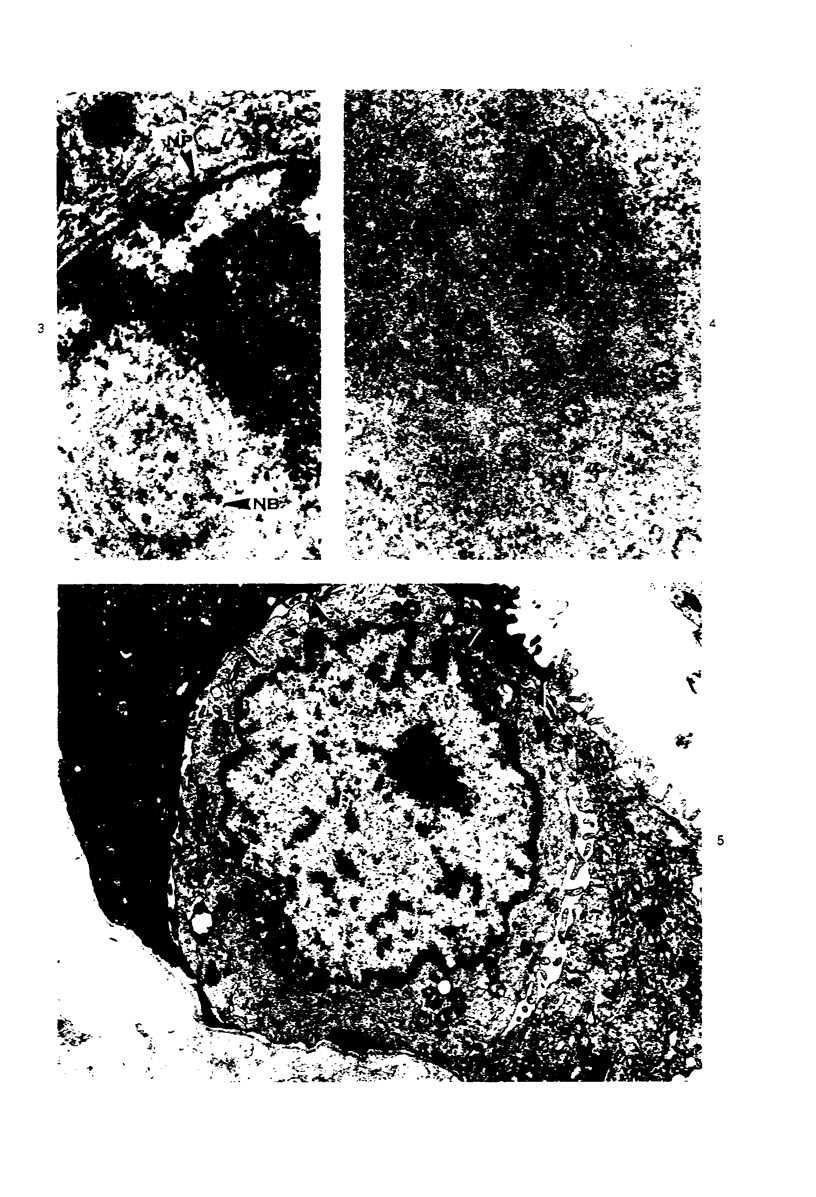
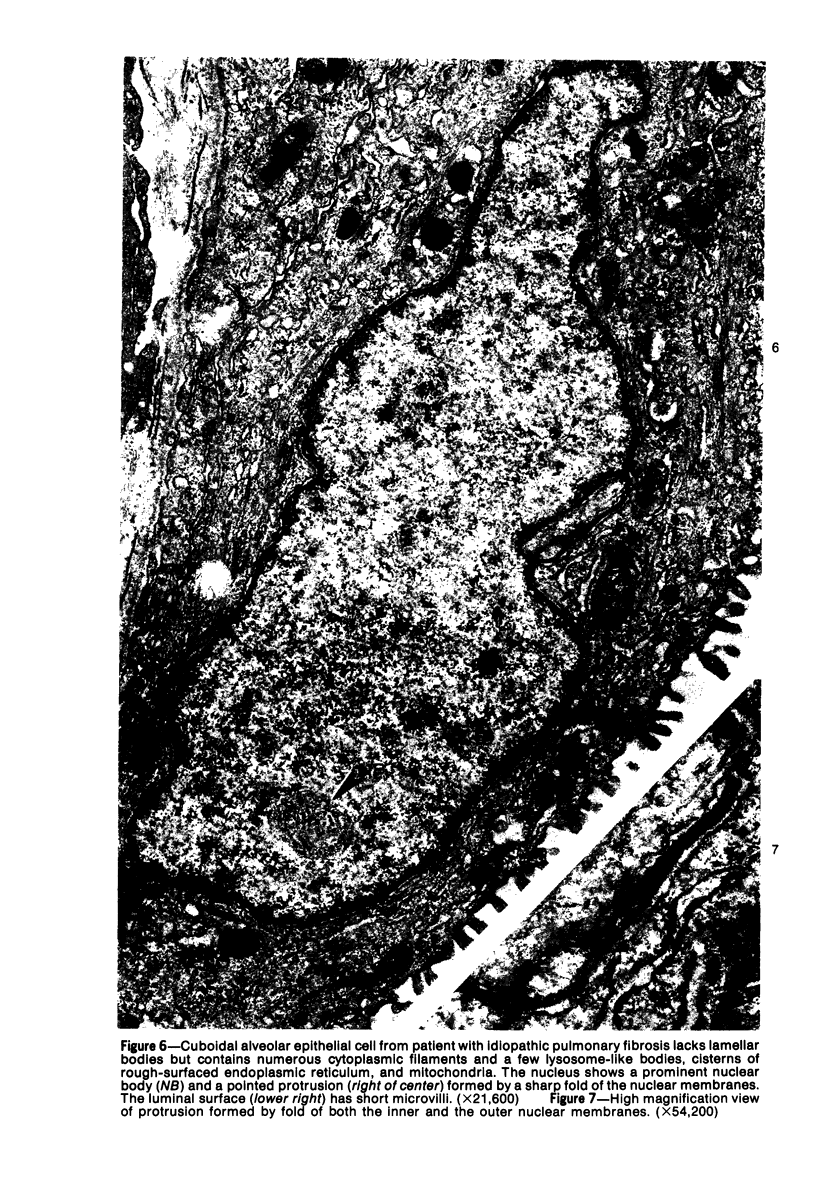
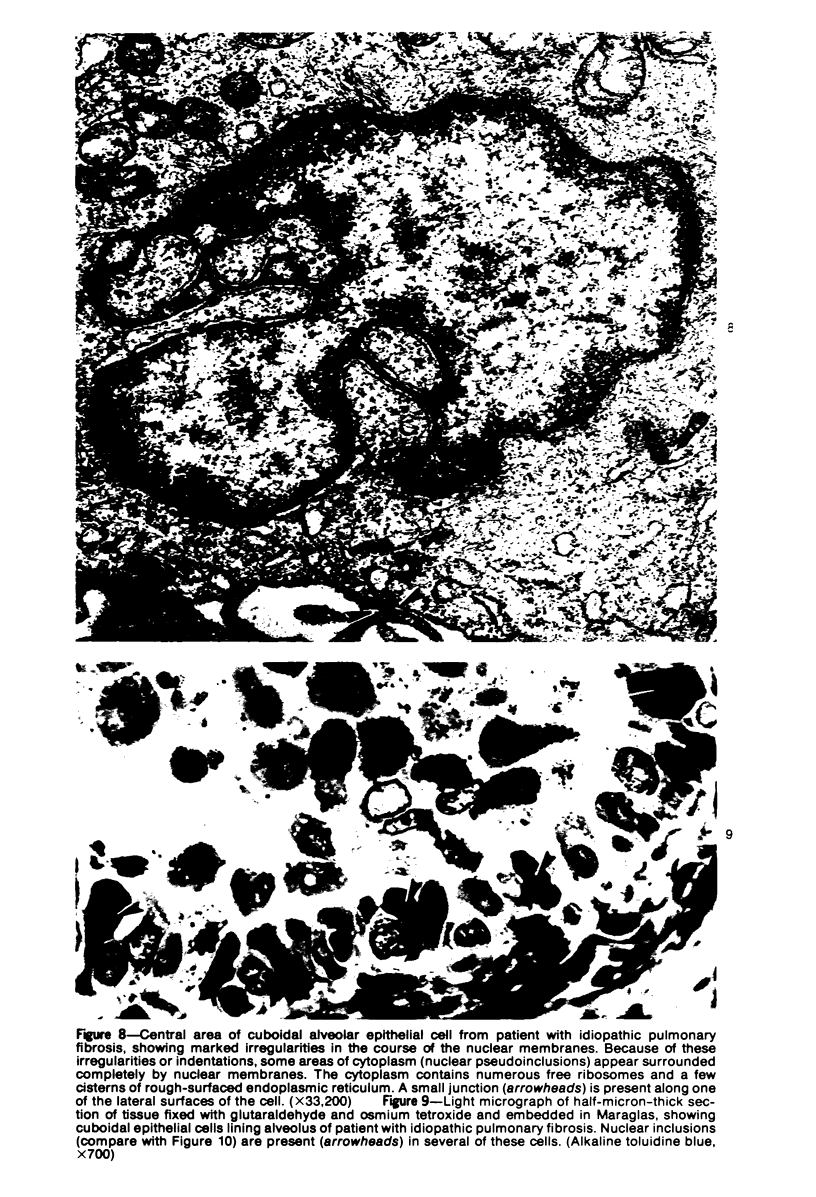
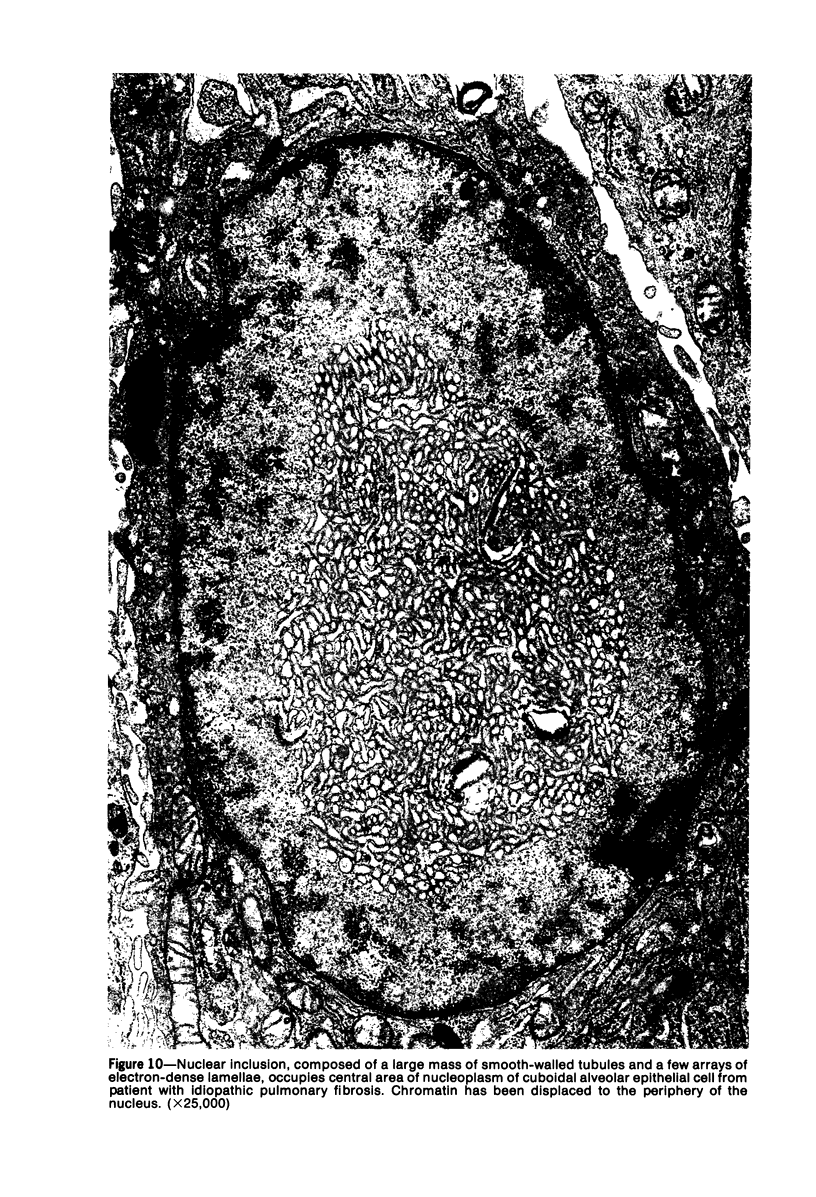
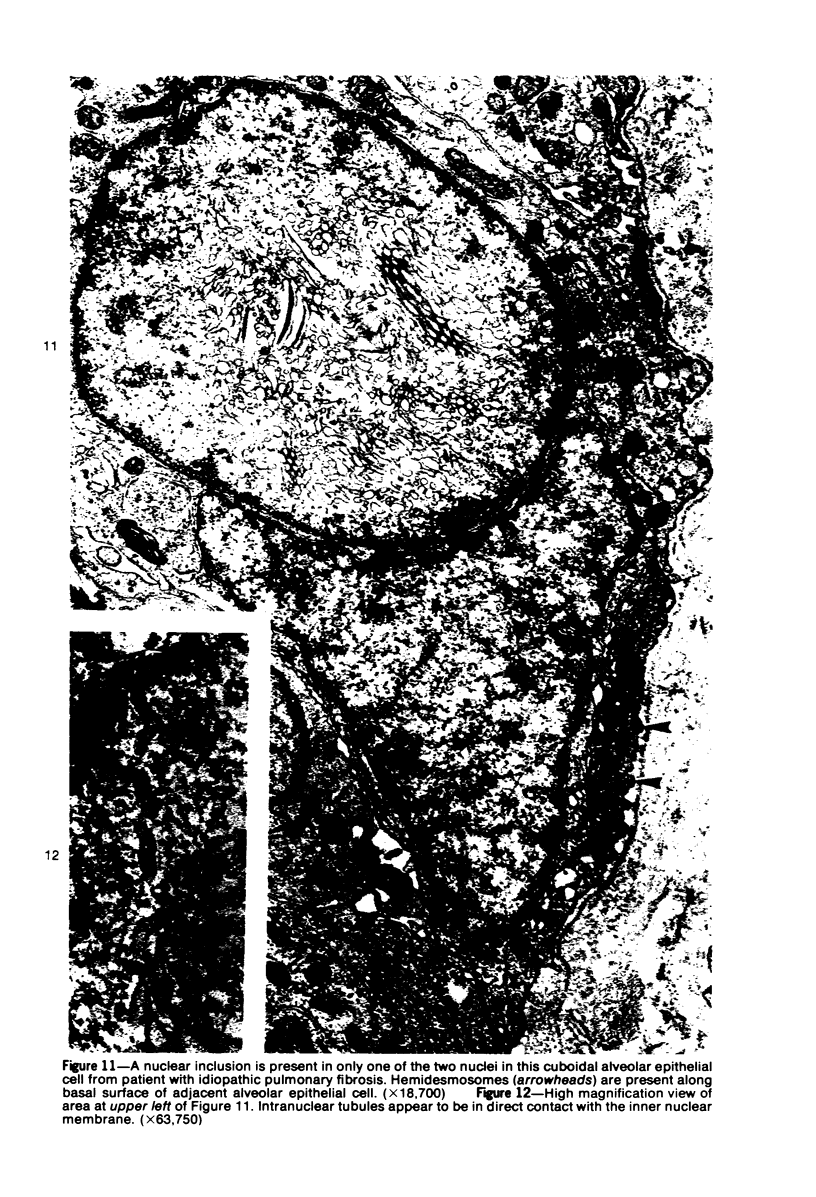
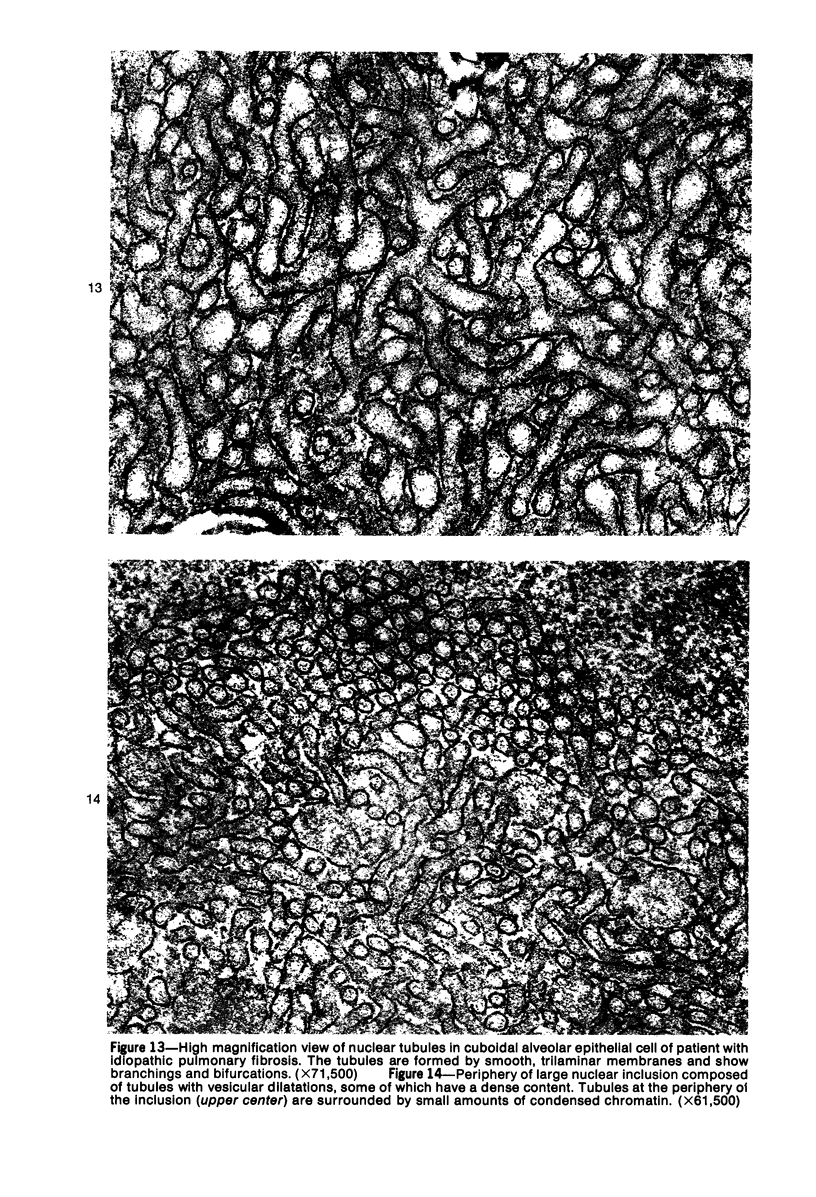
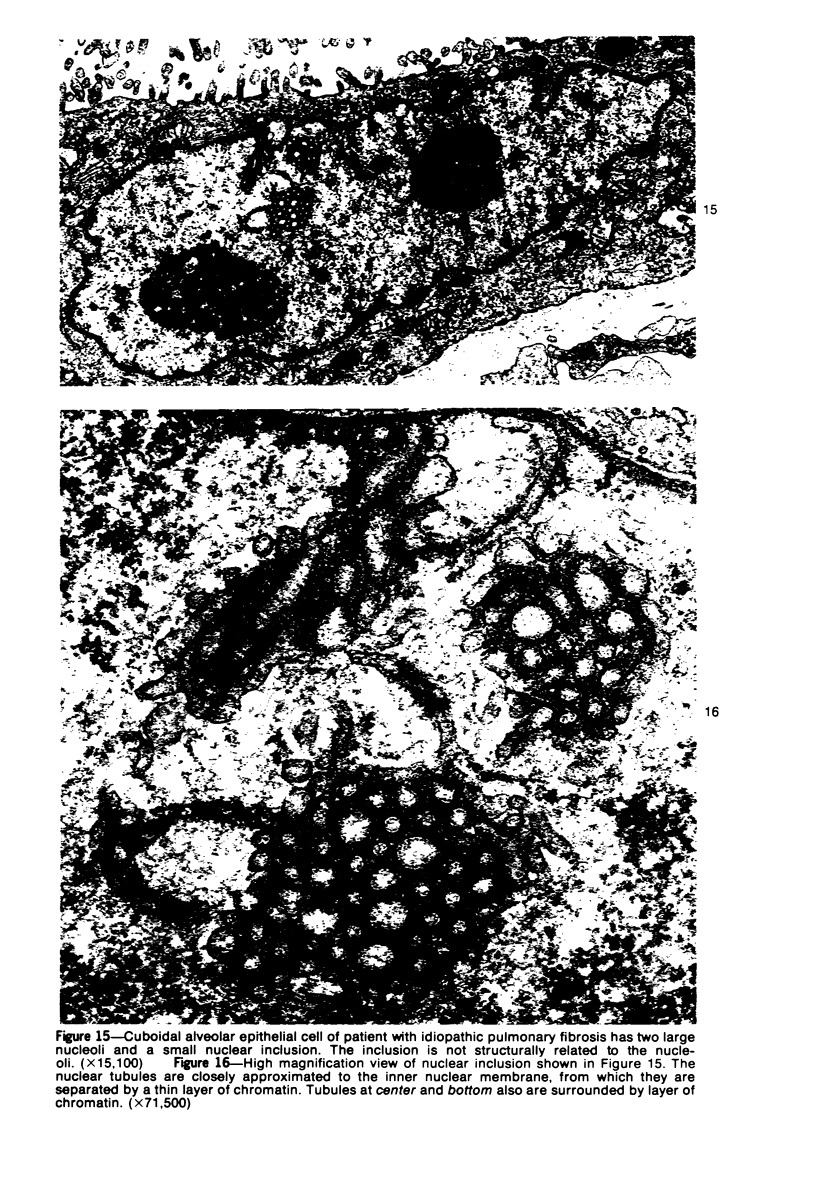
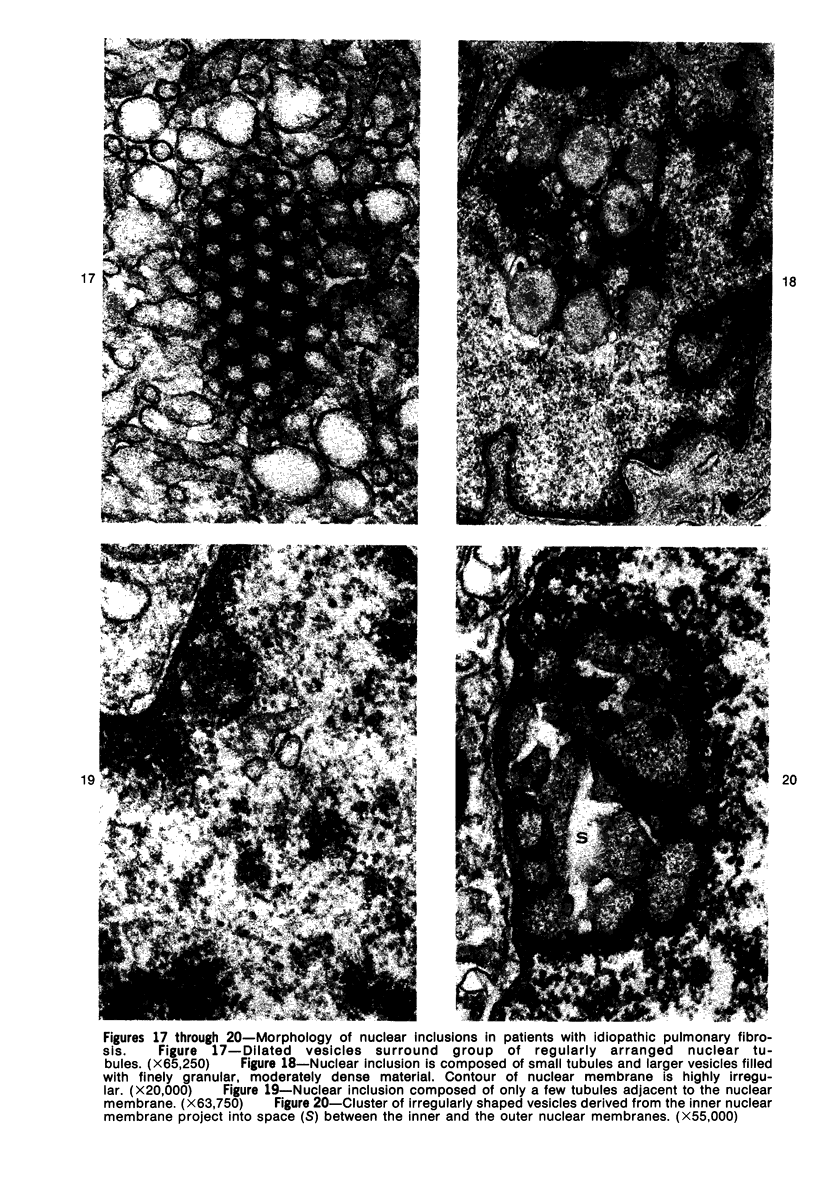
Ultrastructural study of pulmonary biopsy specimens from patients with fibrotic lung disease disclosed the presence of nuclear inclusions in 1% or less of cuboidal alveolar epithelial cells in 9 of 19 patients, including 6 of 12 patients with idiopathic pulmonary fibrosis, 2 of 3 patients with collagen-vascular disease, and 1 of 3 patients with sarcoidosis. Nuclear inclusions were not observed by ultrastructural study in 5 control patients. The inclusions consisted of masses or aggregates of tubules which probably were derived from the inner nuclear membranes. These tubules were smooth-walled, showed branchings and bifurcations, were composed of single trilaminar membranes, usually had a clear content, and ranged from 500 to 1000 Å in diameter. They resembled nuclear tubules which occur in other cell types under conditions of rapid growth or specific hormonal stimulation. Statistically significant differences between the groups of patients with and without nuclear inclusions in cuboidal alveolar epithelial cells were not found with respect to smoking history, degree of fibrosis in the lung biopsy specimen, or the degree of pulmonary physiologic impairment. However, the average age of the patients having nuclear inclusions was significantly greater than that of patients not having nuclear inclusions. In addition, the frequency of indentations in the nuclei of cuboidal alveolar epithelial cells was greater in patients with nuclear inclusions than in patients without nuclear inclusions. Highly significant correlations were observed between the presence of nuclear inclusions and the presence of a) anchoring fibrils and hemidesmosomes along the basal surfaces of alveolar epithelial cells and b) multilayering of the alveolar epithelium.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong E. M., More I. A., McSeveny D., Chatfield W. R. Reappraisal of the ultrastructure of the human endometrial glandular cell. J Obstet Gynaecol Br Commonw. 1973 May;80(5):446–460. doi: 10.1111/j.1471-0528.1973.tb15961.x. [DOI] [PubMed] [Google Scholar]
- Babai F., Tremblay G., Dumont A. Intranuclear and intranucleolar tubular structures in Novikoff hepatoma cells. J Ultrastruct Res. 1969 Jul;28(1):125–130. doi: 10.1016/s0022-5320(69)90010-0. [DOI] [PubMed] [Google Scholar]
- Bouteille M., Kalifat S. R., Delarue J. Ultrastructural variations of nuclear bodies in human diseases. J Ultrastruct Res. 1967 Aug 30;19(5):474–486. doi: 10.1016/s0022-5320(67)80074-1. [DOI] [PubMed] [Google Scholar]
- Bucciarelli E. Intranuclear cisternae resembling structures of the Golgi complex. J Cell Biol. 1966 Sep;30(3):664–665. doi: 10.1083/jcb.30.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLYMAN M. J. A new structure observed in the nucleolus of the human endometrial epithelial cell. Am J Obstet Gynecol. 1963 Jun 15;86:430–432. doi: 10.1016/0002-9378(63)90166-2. [DOI] [PubMed] [Google Scholar]
- Carlson E. C., Ollerich D. A. Intranuclear tubules in trophoblast 3 of rat and mouse chorioallantoic placenta. J Ultrastruct Res. 1969 Jul;28(1):150–160. doi: 10.1016/s0022-5320(69)90013-6. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Fulmer J. D., Roberts W. C., Moss M. L., Line B. R., Reynolds H. Y. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med. 1976 Dec;85(6):769–788. doi: 10.7326/0003-4819-85-6-769. [DOI] [PubMed] [Google Scholar]
- DUBRAUSZKY V., POHLMANN G. [The ultra-structure of the corpus endometrium during the cycle]. Arch Gynakol. 1961;196:180–199. doi: 10.1007/BF00669449. [DOI] [PubMed] [Google Scholar]
- Engedal H., Jensen H., Saetersdal T. S. Ultrastructure of abnormal membrane inclusions in nuclei of human myocardial cells. Br Heart J. 1977 Feb;39(2):145–151. doi: 10.1136/hrt.39.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrans V. J., Jones M., Maron B. J., Roberts W. C. The nuclear membranes in hypertrophied human cardiac muscle cells. Am J Pathol. 1975 Mar;78(3):427–460. [PMC free article] [PubMed] [Google Scholar]
- Gaensler E. A., Goff A. M., Prowse C. M. Desquamative interstitial pneumonia. N Engl J Med. 1966 Jan 20;274(3):113–128. doi: 10.1056/NEJM196601202740301. [DOI] [PubMed] [Google Scholar]
- HOSHINO M. The deep invagination of the inner nuclear membrane into the nucleoplasm in the ascites hepatoma cells. Exp Cell Res. 1961 Sep;24:606–609. doi: 10.1016/0014-4827(61)90465-7. [DOI] [PubMed] [Google Scholar]
- Henry K., Petts V. Nuclear bodies in human thymus. J Ultrastruct Res. 1969 May;27(3):330–343. doi: 10.1016/s0022-5320(69)80021-3. [DOI] [PubMed] [Google Scholar]
- Hruban Z., Mochizuki Y., Slesers A., Morris H. P. A comparative study of cellular organelles of Morris hepatomas. Cancer Res. 1972 Apr;32(4):853–867. [PubMed] [Google Scholar]
- Karasaki S. An electron microscope study of intranuclear canaliculi in Novikoff hepatoma cells. Cancer Res. 1970 Jun;30(6):1736–1742. [PubMed] [Google Scholar]
- Karasaki S. Passage of cytoplasmic lipid into interphase nuclei in preneoplastic rat liver. J Ultrastruct Res. 1973 Mar;42(5):463–478. doi: 10.1016/s0022-5320(73)80020-6. [DOI] [PubMed] [Google Scholar]
- Kawanami O., Ferrans V. J., Roberts W. C., Crystal R. G., Fulmer J. D. Anchoring fibrils. A new connective tissue structure in fibrotic lung disease. Am J Pathol. 1978 Aug;92(2):389–410. [PMC free article] [PubMed] [Google Scholar]
- Kessel R. G., Beams H. W. Intranucleolar membranes and nuclear-cytoplasmic exchange in young crayfish oocytes. J Cell Biol. 1968 Dec;39(3):735–741. doi: 10.1083/jcb.39.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn E. I., Rice S. I., Gordon M. In vitro production of nucleolar channel system by progesterone in human endometrium. Nature. 1970 Nov 14;228(5272):671–672. doi: 10.1038/228671a0. [DOI] [PubMed] [Google Scholar]
- Kohorn E. I., Rice S. I., Hemperly S., Gordon M. The relation of the structure of progestational steroids to nucleolar differentiation in human endometrium. J Clin Endocrinol Metab. 1972 Feb;34(2):257–264. doi: 10.1210/jcem-34-2-257. [DOI] [PubMed] [Google Scholar]
- Kuhn C. Nuclear bodies and intranuclear globulin inclusions in Waldenström's macroglobulinemia. Lab Invest. 1967 Oct;17(4):404–415. [PubMed] [Google Scholar]
- LIEBOW A. A., STEER A., BILLINGSLEY J. G. DESQUAMATIVE INTERSTITIAL PNEUMONIA. Am J Med. 1965 Sep;39:369–404. doi: 10.1016/0002-9343(65)90206-8. [DOI] [PubMed] [Google Scholar]
- Levine A. S., Nesbit M. E., White J. G., Yarbro J. W. Effects of fractionated histones on nucleic acid synthesis in 6C3HED mouse ascites tumor cells and in normal spleen cells. Cancer Res. 1968 May;28(5):831–844. [PubMed] [Google Scholar]
- Levy B. M., Mirkovic R. R. An epizootic of measles in a marmoset colony. Lab Anim Sci. 1971 Feb;21(1):33–39. [PubMed] [Google Scholar]
- Lipsey A. I., Kahn M. J., Bolande R. P. Pathologic variants of congenital hypogamma-globulinemia: an analysis of 3 patients dying of measles. Pediatrics. 1967 May;39(5):659–674. [PubMed] [Google Scholar]
- Luginbuhl W. H. Electron microscopic study of the effects of tissue culture on human endometrium. Am J Obstet Gynecol. 1968 Sep 15;102(2):192–201. doi: 10.1016/0002-9378(68)90318-9. [DOI] [PubMed] [Google Scholar]
- MORICARD R., MORICARD F. MODIFICATIONS CYTOPLASMIQUES ET NUCL'EAIRES ULTRASTRUCTURALES UT'ERINES AU COURS DE L''ETAT FOLLICO-LUT'EINIQUE 'A GLYCOG'ENE MASSIF. (MITOCHONDRIES--GLYCOG'ENE--NUCL'EOLON'EMA--CHROMATINE SEXUELLE--FORMATIONS TUBALAIRES NUCL'EAIRES) Gynecol Obstet (Paris) 1964 Apr-May;63:203–220. [PubMed] [Google Scholar]
- McNary W. F., Jr, Gaensler E. A. Intranuclear inclusion bodies in desquamative interstitial pneumonia. Electron microscopic observations. Ann Intern Med. 1971 Mar;74(3):404–407. doi: 10.7326/0003-4819-74-3-404. [DOI] [PubMed] [Google Scholar]
- More I. A., Armstrong E. M., McSeveney D., Chatfield W. R. The morphogenesis and fate of the nucleolar channel system in the human endometrial glandular cell. J Ultrastruct Res. 1974 Apr;47(20):74–85. doi: 10.1016/s0022-5320(74)90027-6. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B. A transplantable rat liver tumor induced by 4-dimethylaminoazobenzene. Cancer Res. 1957 Nov;17(10):1010–1027. [PubMed] [Google Scholar]
- Nakayama I., Nickerson P. A. Intranuclear inclusions in mammotrophs of the female Mongolian gerbil. Am J Anat. 1972 Sep;135(1):93–104. doi: 10.1002/aja.1001350108. [DOI] [PubMed] [Google Scholar]
- Nickerson P. A. Effect of partial thyroidectomy, propylthiouracil or thyroxine on estrogen-induced intranuclear inclusions in mammotrophs of the Mongolian gerbil. Tissue Cell. 1975;7(4):763–772. doi: 10.1016/0040-8166(75)90042-7. [DOI] [PubMed] [Google Scholar]
- Nickerson P. A. Induction of intranuclear inclusions by estrogen in mammotrophs of the Mongolian gerbil. J Ultrastruct Res. 1973 Jul;44(1):41–48. doi: 10.1016/s0022-5320(73)90039-7. [DOI] [PubMed] [Google Scholar]
- Nickerson P. A. Intranuclear inclusions in mammotrophs of the Mongolian gerbil: effect of low doses of estradiol benzoate and a study of females before weaning. Tissue Cell. 1975;7(4):773–776. doi: 10.1016/0040-8166(75)90043-9. [DOI] [PubMed] [Google Scholar]
- Patchefsky A. S., Banner M., Freundlich I. M. Desquamative interstitial pneumonia. Significance of intranuclear viral-like inclusion bodies. Ann Intern Med. 1971 Mar;74(3):322–327. doi: 10.7326/0003-4819-74-3-322. [DOI] [PubMed] [Google Scholar]
- Terzakis J. A. The nucleolar channel system of human endometrium. J Cell Biol. 1965 Nov;27(2):293–304. doi: 10.1083/jcb.27.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER A., WHIPP S., USENIK E., FROMMES S. STRUCTURAL CHANGES IN THE NUCLEAR BODY IN THE ADRENAL ZONA FASCICULATA OF THE CALF FOLLOWING THE ADMINISTRATION OF ACTH. J Ultrastruct Res. 1964 Dec;11:564–576. doi: 10.1016/s0022-5320(64)80082-4. [DOI] [PubMed] [Google Scholar]