Abstract
The stress-activated protein kinase (SAPK, alternatively JNK) is activated rapidly by cell stress stimuli such as inflammatory cytokines and oxidative stress, and more slowly by the initiation of the apoptotic cell death response by events such as ligation of the Fas protein. Mitogen-activated protein kinase/Erk kinase kinase-1 (MEKK1) is an activator of SAPK, serving as a SAPK-kinase-kinase through intermediate phosphorylation of the SAPK kinase SEK1. By sequencing proteolytic cleavage products of MEKK1, we found that the proapoptotic protease caspase 3 (CPP32) cleaves MEKK1 after residue D68 both in vivo and in vitro. Cleavage of MEKK1 after D68 is blocked by viral and chemical protease inhibitors. Cleavage of MEKK1 at D68 changes the intracellular distribution of the protein from a Triton-insoluble compartment to a Triton-soluble compartment, reflected in a redistribution from a particulate to a diffuse cytoplasmic staining seen by immunofluorescence. Activation of both SAPK and MEKK1 after Fas ligation is prevented by both viral and chemical caspase 3 inhibitors, which in contrast fail to block activation of SAPK by rapidly acting cell stresses. Stress factor-induced SAPK signaling is not dependent on caspase 3 function. We propose that two mechanisms of stress signaling through MEKK1 exist. One is rapid, independent of proteases, and occurs in the particulate Triton-insoluble compartment. The other is more slowly activated and involves liberation of particulate MEKK1 by proteolytic cleavage and activation by caspase 3.
The stress-activated protein kinase (SAPK, alternatively JNK) (1–3) is activated rapidly by cell stress stimuli such as inflammatory cytokines and oxidative stress. Activation of SAPK involves a protein kinase cascade wherein mitogen-activated protein kinase/Erk kinase kinase-1 (MEKK1) (4) (or similar kinases) activate SAPK/Erk kinase-1 (SEK1) (5) and ultimately activate SAPK (6). The SAPK activation cascade parallels the cascade that activates the mitogen-activated protein kinase (MAPK). In addition to SAPK, a second stress-induced MAPK homolog kinase termed RK, mHOG, or p38, is similarly activated by cell stress (7).
Induction of apoptosis by antibody crosslinking of Fas (CD95) induces stress-activated kinases gradually over a period of hours (8, 9). Fas receptor activation induces recruitment of the adapter molecule FADD, which engages a cascade of aspartate-directed cysteine proteases collectively referred to as caspases (for reviews, see ref. 10 and other review articles in that journal issue). Fas-induced activation of SAPK is inhibited by the caspase inhibitor z-VAD-fmk and the viral protease inhibitor CrmA (11).
The SAPK activator MEKK1 is a large protein with a C-terminal catalytic domain and a large amino terminal regulatory domain (4). The murine cDNA used in this study encodes 687 amino acids from the first methionine codon. A cDNA encoding additional amino acids at the amino terminus has been identified from rat (12). MEKK1 gene products with masses ranging from 60 to 195 kDa have been identified in the literature (4, 12). Similarly, expression of the full-length murine cDNA in human embryonic kidney 293 cells results in C-terminal peptides of varying sizes (6). We isolated and sequenced the amino termini of these MEKK1 fragments. We found that one prominent fragment represents a cleavage product of the apoptotic protease caspase 3. Here we show that proteolysis of MEKK1 accompanying anti-Fas induced caspase 3 activation results in kinase activation and redistribution of MEKK1 from an insoluble to a soluble cytoplasmic compartment.
MATERIALS AND METHODS
Plasmid Expression and Amino-Terminal Sequencing of Proteolysed MEKK1 Proteins.
Full-length MEKK1 was expressed from the first methionine codon in the sequence of Lange-Carter et al. (4) by using a dual T7 and EF1-driven expression vector (pUna3) constructed in our laboratory. MEKK1 was epitope-tagged at the C terminus by using either the EE epitope (lerg-EEEEYMPME-term) or the glutathione S-transferase (GST) protein. MEKK1 fragments were isolated from 109 293 cells transfected with MEKK1-GST-pUna3 by affinity chromatography using 250 μl of glutathione agarose beads. SDS-eluted proteins were separated by using SDS/PAGE, and electroblotted onto Immobilon-P (Millipore) and stained with Amido black. Edman sequencing was performed by W. Lane (Harvard) and resulted in sequences that match the murine MEKK predicted protein sequence at the sites shown in Fig. 1.
Figure 1.
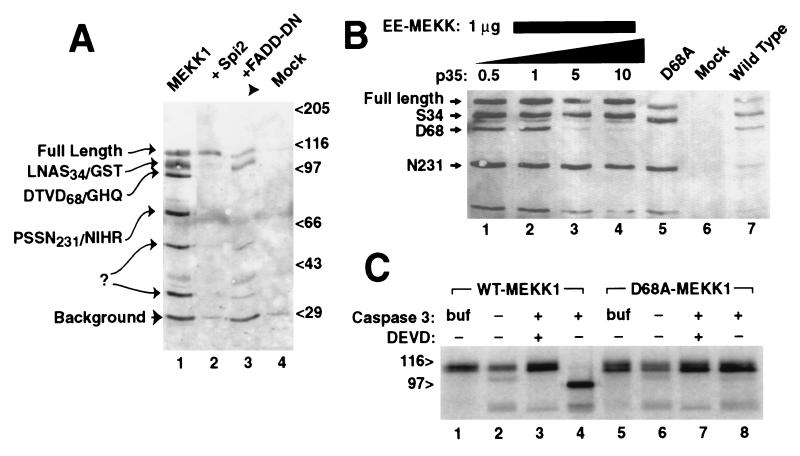
MEKK1 is cleaved at amino acid D68 by caspase 3/CPP32. (A) C-terminally GST-tagged MEKK1 expressed in 293 cells demonstrated six plasmid derived bands (lane 1) detected here by glutathione precipitation and anti-GST immunoblotting. Amino terminal sequencing of these fragments confirmed proteolysis at the indicated sites, following amino acids S34, D68, and N231; two other cleavage sites were not determined. Cotransfection of either the vaccinia virus protease inhibitor Spi-2 (lane 2) or dominant negative Δ1–79 FADD (lane 3) prevented cleavage at D68 and smaller bands. (B) Cleavage of MEKK at D68 was blocked by cotransfection of increasing molar ratios of plasmid encoding baculoviral caspase inhibitor p35 (0.5–10 μg), with minimal effects on other cleavage fragments (lanes 1–4). Mutation of codon D68 of MEKK1 to alanine prevented cleavage at codon 68 (lane 5). (C) Cleavage of MEKK1 by caspase 3 in vitro. Full-length murine MEKK1, either wild-type (lanes 1–4) or mutant D68A (lanes 5–8) expressed by using in vitro transcription/translation was treated with buffer alone (lanes 1 and 5) or with extracts of untransformed bacterial cells (lanes 2 and 6), or bacterial extracts expressing active caspase 3 (lanes 3, 4, 7, and 8). Caspase 3 cleaved MEKK1, yielding a 100-kDa band. The caspase inhibitor z-DEVD-fmk blocked the cleavage (lane 3), and the D68A mutant was resistant to cleavage (lane 8).
Full-length (200 kDa) rat MEKK1 clone was obtained from Melanie Cobb (Univ. of Texas Southwestern) and transferred to our EE-epitope tagged vaccinia virus vector (EE-TM1). Mouse MEKK1 expressed using this vector has been described previously (6). The complete coding sequence of Spi-2 was cloned into pUna3 by using PCR primers derived from the GenBank sequence. Dominant negative Δ79 FADD was from D. Goeddel (Genetech), and its use in our lab has been described before (21).
In Vitro Cleavage of MEKK1 with Caspase 3.
The complete coding region of caspase 3 (CPP32) was amplified by using PCR and cloned into a pGEX KG vector. Partial (>50%) proteolytic activation of caspase 3 in bacterial extracts was confirmed by immunoblotting against caspase 3 to detect the active cleaved fragment. Unpurified bacterial extracts containing caspase 3 were used to cleave in vitro-translated MEKK1 in reticulocyte lysate, and compared with reactions by using identical lysates of untransformed bacteria, or to lysis buffer alone.
Kinase Assays.
SAPK activity using GST-Jun (5–73) substrate (from James Woodgett, Ontario Cancer Institute, Toronto) was determined as described in ref. 6. Precipitations of cells extracted in lysis buffer (250 mM NaCl/50 mM Mops, pH 7.5/5 mM EDTA/1 mM DTT/2.0 μg/ml aprotinin/2.0 μg/ml leupeptin/50 μg/ml phenylmethylsulfonyl fluoride/10 mM NaF/5 mM Na pyrophosphate/1 mM Na vanadate) were performed by using either polyclonal antibodies against SAPK β1 that recognize both 46-kDa and 54-kDa forms of SAPK (available from the Antibody Cooperative; http://www.antibodycoop.com) or αEE. MEKK1 assays were performed similarly by using polyclonal anti-N-terminal peptide antibody recognizing MEKK1 (43-Y, Santa Cruz Biotechnology), and GST-SEK 1 protein as substrate.
Immunoblotting.
Anti-EE antibodies were used to recognize C-terminal EE-tagged MEKK proteins from transfected cells, as described previously (13). EE antibodies were from Gernot Walter (Univ. of California, San Diego) but are now available commercially from Babco (Richmond, CA). Endogenous MEKK1 was detected from 108 Jurkat cells by immunoprecipitation using antibody 43-Y (Santa Cruz Biotechnology), followed by immunoblotting using a polyclonal anti-MEKK1 (catalytic domain peptide) and chemiluminescent detection. IκB-α was detected by using antibody C-21 (Santa Cruz Biotechnology). In some experiments, Triton X-100 soluble and insoluble fractions were prepared by lysis of cultures in lysis buffer (above) and recovery of insoluble proteins by centrifugation at 15,000 × g for 5 min. Pellets were washed by resuspension in lysis buffer and resedimentation. Triton-insoluble proteins were dissolved in 2% SDS sample buffer with 10 mM DTT before electrophoresis.
Immunofluorescence.
MEKK1 proteins were expressed in CV1 cells by using the vaccinia virus system (14) as described (13) except early time points (4 hr postinfection) were chosen to minimize viral effects. Cells were fixed with paraformaldehyde and permeablized, and EE-tagged MEKK1 was detected by using anti-EE mAb and fluoresceinated anti-mouse antibody. Confocal imaging (Bio-Rad) was performed at identical section heights.
Site-Directed Mutagenesis.
D68A MEKK1 was engineered by using a PCR-based four-primer mutagenesis protocol as described (13).
Reagents.
z-VAD-fmk and z-DEVD-fmk were purchased from Enzyme Systems Products (Livermore, CA). αCPP32 (caspase 3) mAbs were purchased from Transduction Laboratories (Lexington, KY).
Additional protocols and vector sequences are available on our lab Web server (http://Templeton.CWRU.edu).
RESULTS AND DISCUSSION
To identify the origin of MEKK1 fragments within the cell we expressed MEKK1 containing a C-terminal GST fusion protein and purified C-terminal fragments by glutathione affinity. Purified protein bands were isolated and sequenced via amino terminal Edman degradation. Three fragments were successfully sequenced, representing cleavage after codons S34, D68, and N231 (Fig. 1A, lane 1).
The D68 cleavage site in MEKK1 (DTVD68/G) is similar to that at which caspase 3 cleaves (poly)ADP ribose polymerase (DEVD/G) (15). We hypothesized that this fragment results from proteolysis by caspase 3 or similar proteases within the transfected cells. We tested this by coexpression of molecules that block activation of the caspase cascade, specifically the vaccinia-virus homolog of CrmA termed Spi-2 (16, 17) (Fig. 1A, lane 2) and the dominant negative mutant ΔN79-FADD, (18, 19). Both of these proteins prevented appearance of the D68-cleaved band. MEKK1 expressed using the vaccinia virus expression system does not display the proteolytic bands of MEKK1 (J.C.D., unpublished work, and see Fig. 3), probably because of Spi-2 and other viral protease inhibitors.
Figure 3.
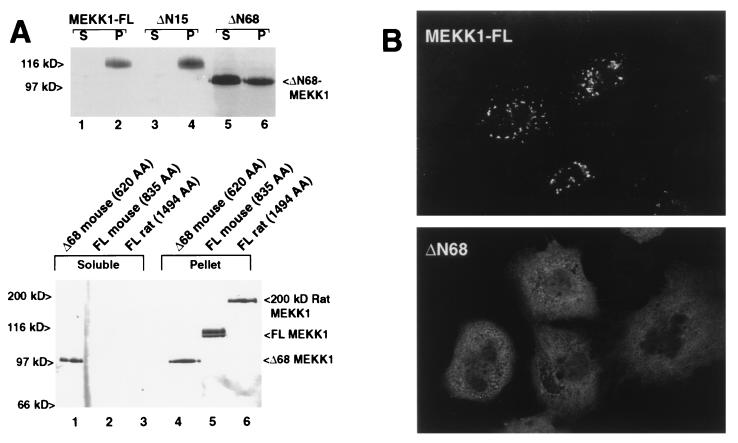
(A) (Upper) MEKK is tethered to a Triton-insoluble compartment by amino acids between 15 and 68. MEKK1 was expressed in CV1 cells by using the T7-vaccinia virus protocol to eliminate proteolytic cleavage. Both full-length (lanes 1 and 2) and ΔN15 MEKK (lanes 3 and 4) were found exclusively in the particulate (P) fraction, and not in the Triton soluble (S) fraction. Mutant ΔN68 that mimics cleavage of MEKK at D68 was primarily found in the soluble fraction. (Lower) Full-length mouse MEKK1 (120 kDa) or Δ68 MEKK1 as in the upper panel were compared with the 200-kDa MEKK1 form isolated from rat. The 200-kDa rat full-length MEKK1 also was found predominantly in the Triton-insoluble compartment. (B) Redistribution of truncated MEKK1 detected by immunofluorescence. Wild-type MEKK1 expressed by using the T7-vaccinia virus system was observed 4 hr after infection in coarse cytoplasmic particles (Upper). Deletion mutant ΔN68 MEKK (Lower) was detected in the cytoplasm and demonstrated a fine reticular pattern. EE-epitope tagged MEKK1 was detected by using anti-EE mAb and confocal imaging.
Inhibition of D68 cleavage by the baculovirus caspase inhibitor p35 (20) was dose-dependent (Fig. 1B, lanes 1–4). Mutation of codon D68 to an alanine residue (Fig. 1B, lane 5) also prevented cleavage after codon 68. MEKK1 translated in vitro was cleaved by bacterially expressed caspase 3 (Fig. 1C, lane 4). Cleavage was blocked by the chemical caspase inhibitor z-DEVD-fmk (Fig. 1C, lane 3). Mutant D68A MEKK1 was not cleaved (Fig. 1C, lanes 5–8). Although caspase 3 cleaved MEKK1 in this in vitro experiment, it is not certain that this protease is responsible for MEKK1 cleavage in vivo.
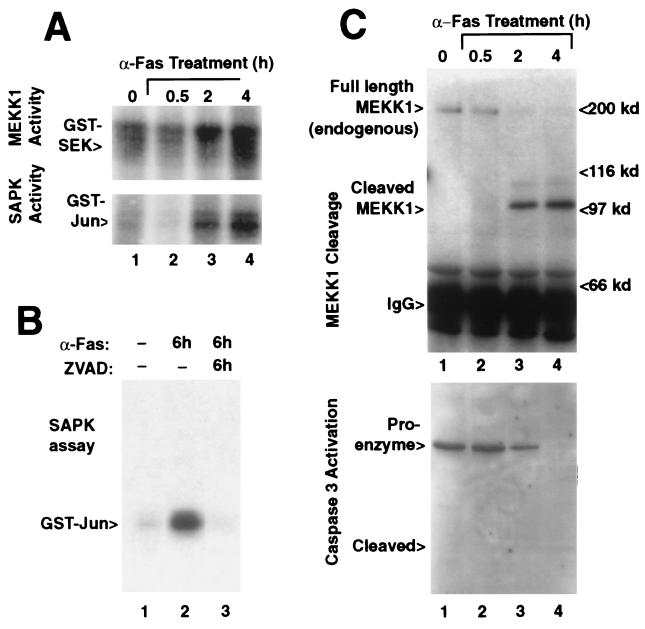
MEKK1 activation in Jurkat lymphoma cells treated with anti-Fas antibody (Fig. 2A, Upper), paralleled SAPK activation (Fig. 2A, Lower). SAPK activation by anti-Fas was inhibited by the caspase inhibitor z-VAD-fmk (Fig. 2B). Anti-Fas treatment of Jurkat cells resulted in a unique 100-kDa form of endogenous MEKK1, consistent with the D68 cleavage product (Fig. 2C, Upper), which coincided with the proteolytic activation of caspase 3 (Fig. 2C, Lower). Endogenous MEKK1 migrating around 200 kDa was seen in extracts of untreated Jurkat cell extracts and was absent from extracts of the anti-Fas treated cells after 2 hr of treatment.
Figure 2.
SAPK and MEKK1 activity correlate with the onset of caspase 3 activation after anti-Fas-induced apoptosis. (A) MEKK1 (Upper) and SAPK (Lower) activity in anti-Fas-treated Jurkat cells gradually increased over a time course of 4 hr (Upper). (B) The activation of SAPK during 6-hr anti-Fas-treatment (lane 2) was blocked by z-VAD-fmk beginning at 15 min before addition of the antibody (lane 3). (C) Cleavage of endogenous MEKK1 in anti-Fas-treated Jurkat cells (Upper) was evidenced by a 100-kDa MEKK1 form detected after 2 hr that was absent in the untreated cells (lane 1). MEKK was detected by immunoprecipitation followed by immunoblotting against MEKK1. Caspase 3 (CPP32) proteolytic activation (Lower) from the same cell lysates correlate with appearance of the proteolysed MEKK1 fragment. Caspase 3 was detected by immunoblotting.
We previously have used the vaccinia virus-T7 polymerase system (14) to express MEKK1 and have found that MEKK1 thus expressed is not proteolysed, likely owing to virus-encoded serpins (16). Full-length murine MEKK1 thus expressed was insoluble in Triton X-100 buffer (Fig. 3A, Upper, lanes 1 and 2), as was ΔN15 MEKK1, lacking 15 N-terminal codons (Fig. 3A, Upper, lanes 3 and 4). Mutant MEKK ΔN68, lacking 68 N-terminal codons (mimicking the D68 cleaved protein) was largely soluble in Triton buffer (Fig. 3A, Upper, lanes 5 and 6). Thus, sequences between codons 15 and 68 retain MEKK1 within the Triton insoluble fraction. The 200-kDa form of MEKK1 isolated from rat (12), which presumably represents the actual full-length protein, also was found in the Triton-insoluble compartment (Fig. 3A, Lower).
Full-length wild-type MEKK protein was detected by anti-epitope tag immunofluorescence in a particulate, cytoplasmic distribution (Fig. 3B). In contrast, ΔN68 MEKK1 showed a diffuse cytoplasmic staining with a faint reticular pattern. Thus, amino acids proximal to D68 are required for inclusion in this particulate, Triton-insoluble compartment of the cell. Full-length rat MEKK1 also was reported to be Triton-insoluble (12), although those authors concluded that MEKK1 had a membrane association and did not visualize MEKK1 by immunofluorescence. The D68 cleavage site is also found within the rat MEKK1 (DTLDG, residues 866–870).
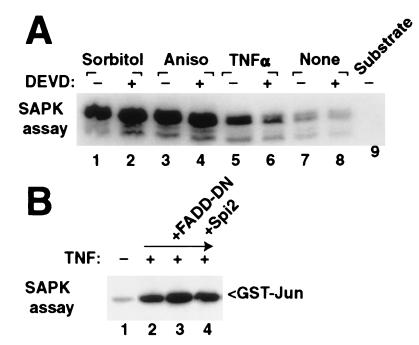
Although caspase inhibitors block apoptosis-associated activation of SAPK, chemical caspase inhibitors z-DEVD-fmk (Fig. 4) or z-VAD-fmk (not shown) do not block rapid SAPK activation by hyperosmotic shock, anisomycin, or tumor necrosis factor α (TNFα). Similarly, a dominant negative deletion of FADD and the viral caspase inhibitor Spi-2 did not disrupt rapid stress signaling in response to TNFα (Fig. 4B). FADD-DN previously has been shown to not block SAPK activation (21, 22) and actually may increase response of SAPK to cell stress.
Figure 4.
Conventional stress agents activate SAPK through protease independent pathways. (A) Activation of endogenous SAPK by hypertonic sorbitol (lanes 1 and 2), anisomycin (lanes 3 and 4), or TNFα (lanes 5 and 6) after 30 min was not inhibited by preincubation with the caspase inhibitor z-DEVD-fmk (500 nM, even lanes). (B) Epitope-tagged SAPK expressed in 293 cells was stimulated by TNFα (lane 2) and was not suppressed by coexpression of dominant negative FADD or Spi-2 (lanes 3 and 4). SAPK activity was determined by immunoprecipitation of endogenous SAPK (A) or epitope-tagged p54 SAPK (B) and in vitro kinase reaction by using GST-Jun as substrate.
Our results suggest that during apoptosis, caspase 3 or related proteases cleave MEKK1 at the peptide bond after D68 and liberate it from a particulate, Triton-insoluble cytoplasmic complex. This mechanism is distinct from rapid activation of SAPK by chemical and cytokine cell stresses that do not require caspase activity. MEKK1 liberated from the insoluble compartment thus may have substrates distinct from those substrates used during normal, nonapoptotic stress signaling.
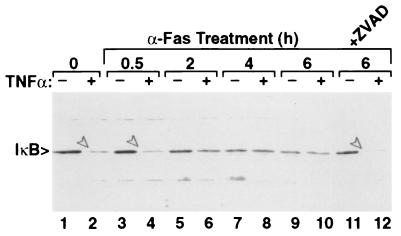
The role of MEKK1 and SAPK activation in apoptosis is unclear. Expression of a catalytically active fragment of MEKK1 induced apoptosis (23), whereas disruption of the SEK1 gene in mice rendered thymocytes resistant to Fas-mediated killing (24). Other studies using dominant negative SEK1 and SAPK also have concluded that SAPK does not play a role in Fas-mediated apoptosis (8). Fas-mediated apoptosis in Jurkat cells has been shown to involve activation of MKK6 (25); MKK6 is a substrate of MEKK1 (M. Yan and D.J.T., unpublished work). In contrast to its possible role in inducing apoptosis, MEKK1 activates the transcription factor NFκB (26), which is an anti-apoptotic event (22, 27). Caspase activation and subsequent cleavage of MEKK1 or other targets may prevent the typical activation of NFκB in an attempt to prevent the NFκB “survival response.” In support of this, anti-Fas treated Jurkat cells fail to degrade IκB in response to TNFα stimulation (Fig. 5), suggesting that a Fas-mediated signaling process (perhaps cleavage of MEKK1) prevents subsequent activation of the pathway leading to IκB degradation.
Figure 5.
Fas-stimulated Jurkat cells are insensitive to TNFα-induced IκB degradation. Jurkat cells were stimulated with anti-Fas for the indicated times. Some cultures (even lanes) were treated with 30 ng/ml TNFα for 30 min before harvest. IκB was detected by immunoblotting. Arrows in lanes 2, 4, and 12 denote IκB degraded in response to TNFα. Degradation was hindered by 2 or more hours of anti-Fas treatment. z-VAD inhibition of caspases (lanes 11 and 12) allowed IκB degradation in response to TNFα.
It is likely that activation of SAPK and MEKK1 during Fas-induced apoptosis mediates only some aspects of the apoptotic phenotype. For example, induction of active Δ367 MEKK1 in 3T3 cells does not result in apoptotic cell death (6), but does produce morphologic cell rounding and the initiation of unscheduled DNA synthesis (M. Yan and D.J.T., unpublished work). We propose the model shown in Fig. 6. Normal stress-induced signaling through MEKK1 involves activation of SAPK and parallel activation of NFκB, which results in an antiapoptotic signal. Fas-induced activation of caspase 3 or similar proteases cleave and activate MEKK1, but alter the localization of MEKK1 within the cell. Alternate substrates of MEKK1 or SAPK that result in apoptotic signaling then may be recognized. TNFα-induced activation of NFκB via degradation of IκB is prevented, because of either cleavage of MEKK1 or another uncharacterized mechanism.
Figure 6.
Model of caspase-dependent and independent activation of kinase signaling through MEKK1-SAPK. (Upper) MEKK1 is anchored in a Triton-insoluble compartment within the cell. Cell stressors acting acutely stimulate MEKK1 and SAPK without disrupting this compartment and recognize downstream substrates. Parallel activation of NFκB results in an anti-apoptotic signal. (Lower) Fas-induced activation of caspase 3 or related proteases cleave MEKK1 at codon D68 and release it from the Triton-insoluble compartment. Substrates recognized by the soluble kinase may be distinct from stress-induced substrates and may mediate some aspects of the apoptotic phenotype. TNFα induced activation of NFκB via degradation of IκB is prevented, because of either cleavage of MEKK1 or another uncharacterized mechanism.
Recently, the protein kinase PAK2 has been shown to be cleaved by caspases (28), an event that is thought to impart membrane alterations in apoptosis. Thus, in addition to activation of the MEKK1-SAPK cascade, activation of caspases during apoptosis likely promotes aspects of the apoptotic phenotype through several mechanisms.
After these studies were concluded, another group identified caspase cleavage and activation of MEKK1 after disruption of adhesion to the cell substratum (29). This group concluded that MEKK1 cleavage was an obligate activator of the apoptotic response. Their result suggests that MEKK1 cleavage may result from activation of the caspase cascade resulting from substrate detachment in addition to Fas ligation, and potentially many other stimuli.
Acknowledgments
We thank Minhong Yan and Alan Levine for helpful discussions. This work was supported by National Institutes of Health Grants CA-68738 (to J.D.) and CA-66134 (to D.J.T.) and American Cancer Society Award BE240 (to D.J.T.).
ABBREVIATIONS
- SAPK
stress-activated protein kinase
- MEKK1
mitogen-activated protein kinase/Erk kinase kinase-1
- GST
glutathione S-transferase
- TNFα
tumor necrosis factor α
References
- 1.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. Nature (London) 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 2.Kyriakis J M, Avruch J. J Biol Chem. 1996;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- 3.Waskiewicz A J, Cooper J A. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 4.Lange-Carter C A, Pleiman C M, Gardner A M, Blumer K J, Johnson G L. Science. 1993;260:315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez I, Hughes R T, Mayer B, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Nature (London) 1994;372:795–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 6.Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Nature (London) 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 7.Han J, Lee J-D, Bibbs L, Ulevitch R J. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 8.Lenczowski J M, Dominguez L, Eder A M, King L B, Zacharchuk C M, Ashwell J D. Mol Cell Biol. 1997;17:170–181. doi: 10.1128/mcb.17.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juo P, Kuo C J, Reynolds S E, Konz R F, Raingeaud J, Davis R J, Biemann H P, Blenis J. Mol Cell Biol. 1997;17:24–35. doi: 10.1128/mcb.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alnemri E S. J Cell Biochem. 1997;64:33–42. doi: 10.1002/(sici)1097-4644(199701)64:1<33::aid-jcb6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Cahill M A, Peter M E, Kischkel F C, Chinnaiyan A M, Dixit V M, Krammer P H, Nordheim A. Oncogene. 1996;13:2087–2096. [PubMed] [Google Scholar]
- 12.Xu S, Robbins D J, Christerson L B, English J M, Vanderbilt C A, Cobb M H. Proc Natl Acad Sci USA. 1996;93:5291–5295. doi: 10.1073/pnas.93.11.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan M, Templeton D J. J Biol Chem. 1994;269:19067–19073. [PubMed] [Google Scholar]
- 14.Moss B, Elroy-Stein O, Mizukami T, Alexander W A, Fuerst T R. Nature (London) 1990;348:91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, et al. Nature (London) 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 16.Kotwal G J, Moss B. J Virol. 1989;63:600–606. doi: 10.1128/jvi.63.2.600-606.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobbelstein M, Shenk T. J Virol. 1996;70:6479–6485. doi: 10.1128/jvi.70.9.6479-6485.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinnaiyan A M, O’Rourke K, Yu G L, Lyons R H, Garg M, Duan D R, Xing L, Gentz R, Ni J, Dixit V M. Science. 1996;274:990–992. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 19.Hsu H, Shu H B, Pan M G, Goeddel D V. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 20.Xue D, Horvitz H R. Nature (London) 1995;377:248–251. doi: 10.1038/377248a0. [DOI] [PubMed] [Google Scholar]
- 21.Natoli G, Costanzo A, Ianni A, Templeton D, Woodgett J, Balsano C, Levrero M. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z G, Hsu H, Goeddel D V, Karin M. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 23.Johnson N L, Gardner A M, Diener K M, Lange-Carter C A, Gleavy J, Jarpe M B, Minden A, Karin M, Zon L I, Johnson G L. J Biol Chem. 1996;271:3229–3237. doi: 10.1074/jbc.271.6.3229. [DOI] [PubMed] [Google Scholar]
- 24.Nishina H, Fischer K D, Radvanyi L, Shahinian A, Hakem R, Rubie E A, Bernstein A, Mak T W, Woodgett J R, Penninger J M. Nature (London) 1997;385:350–353. doi: 10.1038/385350a0. [DOI] [PubMed] [Google Scholar]
- 25.Huang S, Jiang Y, Li Z, Nishida E, Mathias P, Lin S, Ulevitch R J, Nemerow G R, Han J. Immunity. 1997;6:739–749. doi: 10.1016/s1074-7613(00)80449-5. [DOI] [PubMed] [Google Scholar]
- 26.Lee F S, Hagler J, Chen Z J, Maniatis T. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 27.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 28.Rudel T, Bokoch G M. Science. 1997;276:1571–1574. doi: 10.1126/science.276.5318.1571. [DOI] [PubMed] [Google Scholar]
- 29.Cardone M H, Salvesen G S, Widmann C, Johnson G, Frisch S M. Cell. 1997;90:315–323. doi: 10.1016/s0092-8674(00)80339-6. [DOI] [PubMed] [Google Scholar]