Abstract
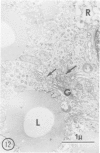
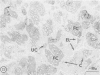
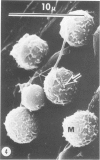
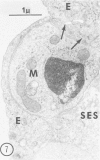
Hypercholesterolemia was induced in pigs by feeding a chow diet supplemented with 1.5% cholesterol and 19.5% lard for periods up to 12 weeks. The aortic intima from areas of spontaneously differing permeability to proteins, as demarcated by their uptake of Evans blue dye, was examined using light microscopy and both scanning and transmission electron microscopy to describe the earliest detectable changes in intimal morphology induced by the diet. After 2, 4, and 6 weeks of feeding, cholesterol/lardfed pigs demonstrated monocyte adherence to the endothelium in areas of enhanced permeability (blue areas) in 86% of samples examined, as compared to 52% in areas of lesser permeability (white areas) and 17% in control animals. Similarly, the number of monocytes in the intima was higher in blue areas than in adjacent white areas or blue areas from control animals. After 12 weeks of feeding, all blue areas showed intimal monocytes, with fewer seen in white areas. Aortic endothelial cells in hypercholesterolemic pigs were normal in ultrastructural appearance, except they contained more lysosomes and cytoplasmic filaments than those from control animals. No lesions were observed at 2, 4, and 6 weeks, although plasma cholesterol levels were substantially elevated (200-400 mg/dl) at these times. A marked hyper-β-lipoproteinemia was evident from 4 weeks onward, but no elevation of serum triglycerides was evident at any stage. Plasma phospholipid concentrations increased but not in direct proportion to cholesterol levels. At 12 weeks, foam cell lesions were observed in areas of enhanced permeability but not in adjacent areas of normal permeability. Lesion foam cells appeared to be derived from the monocytes which adhered to and penetrated the endothelium at earlier stages, since no intimal involvement, or lipid engorgement, by medial smooth muscle cells was observed.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BESSIS M., THIERY J. P. Electron microscopy of human white blood cells and their stem cells. Int Rev Cytol. 1961;12:199–241. doi: 10.1016/s0074-7696(08)60541-0. [DOI] [PubMed] [Google Scholar]
- BUCK R. C. The fine structure of the aortic endothelial lesions in experimental cholesterol atherosclerosis of rabbits. Am J Pathol. 1958 Sep-Oct;34(5):897–909. [PMC free article] [PubMed] [Google Scholar]
- Becker C. G., Murphy G. E. Demonstration of contractile protein in endothelium and cells of the heart valves, endocardium, intima, arteriosclerotic plaques, and Aschoff bodies of rheumatic heart disease. Am J Pathol. 1969 Apr;55(1):1–37. [PMC free article] [PubMed] [Google Scholar]
- Bell F. P., Adamson I. L., Schwartz C. J. Aortic endothelial permeability to albumin: focal and regional patterns of uptake and transmural distribution of 131I-albumin in the young pig. Exp Mol Pathol. 1974 Feb;20(1):57–68. doi: 10.1016/0014-4800(74)90043-4. [DOI] [PubMed] [Google Scholar]
- Bell F. P., Gallus A. S., Schwartz C. J. Focal and regional patterns of uptake and the transmural distribution of 131-I-fibrinogen in the pig aorta in vivo. Exp Mol Pathol. 1974 Apr;20(2):281–292. doi: 10.1016/0014-4800(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Bálint A., Veress B., Jellinek H. Modifications of surface coat of aortic endothelial cells in hyperlipemic rats. Pathol Eur. 1974;9(2):105–108. [PubMed] [Google Scholar]
- Caplan B. A., Gerrity R. G., Schwartz C. J. Endothelial cell morphology in focal areas of in vivo Evans blue uptake in the young pig aorta. I. Quantitative light microscopic findings. Exp Mol Pathol. 1974 Aug;21(1):102–117. doi: 10.1016/0014-4800(74)90082-3. [DOI] [PubMed] [Google Scholar]
- Caplan B. A., Schwartz C. J. Increased endothelial cell turnover in areas of in vivo Evans Blue uptake in the pig aorta. Atherosclerosis. 1973 May-Jun;17(3):401–417. doi: 10.1016/0021-9150(73)90031-2. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Florentin R. A., Nam S. C., Daoud A. S., Jones R., Scott R. F., Morrison E. S., Kim D. N., Lee K. T., Thomas W. A., Dodds W. J. Dietary-induced atherosclerosis in miniature swine. Exp Mol Pathol. 1968 Jun;8(3):263–301. doi: 10.1016/s0014-4800(68)80001-2. [DOI] [PubMed] [Google Scholar]
- GEER J. C., McGILL H. C., Jr, STRONG J. P. The fine structure of human atherosclerotic lesions. Am J Pathol. 1961 Mar;38:263–287. [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Badonnel M. C., Rona G. Cytoplasmic contractile apparatus in aortic endothelial cells of hypertensive rats. Lab Invest. 1975 Feb;32(2):227–234. [PubMed] [Google Scholar]
- Gabbiani G., Elemer G., Badonnel M. C., Hüttner I. Morphological functional changes of aortic endothelium during different types of hypertension. Prog Biochem Pharmacol. 1977;13:1–8. [PubMed] [Google Scholar]
- Gerrity R. G., Richardson M., Caplan B. A., Cade J. F., Hirsh J., Schwartz C. J. Endotoxin-induced vascular endothelial injury and repair. II. Focal injury, en face morphology, (3H)thymidine uptake and circulating endothelial cells in the dog. Exp Mol Pathol. 1976 Feb;24(1):59–69. doi: 10.1016/0014-4800(76)90057-5. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G., Richardson M., Somer J. B., Bell F. P., Schwartz C. J. Endothelial cell morphology in areas of in vivo Evans blue uptake in the aorta of young pigs. II. Ultrastructure of the intima in areas of differing permeability to proteins. Am J Pathol. 1977 Nov;89(2):313–334. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G., Schwartz C. J. Endothelial cell injury in early mild hypercholesterolemia. Prog Biochem Pharmacol. 1977;13:213–219. [PubMed] [Google Scholar]
- Goldfischer S., Schiller B., Wolinsky H. Lipid accumulation in smooth muscle cell lysosomes im primate atherosclerosis. Am J Pathol. 1975 Mar;78(3):497–504. [PMC free article] [PubMed] [Google Scholar]
- Hammersen F. Endothelial contractility - an undecided problem in vascular research. Beitr Pathol. 1976 May;157(4):327–348. doi: 10.1016/s0005-8165(76)80049-2. [DOI] [PubMed] [Google Scholar]
- Humphrey C. D., Pittman F. E. A simple methylene blue-azure II-basic fuchsin stain for epoxy-embedded tissue sections. Stain Technol. 1974 Jan;49(1):9–14. doi: 10.3109/10520297409116929. [DOI] [PubMed] [Google Scholar]
- Joris I., Majno G., Ryan G. B. Endothelial contraction in vivo: a study of the rat mesentery. Virchows Arch B Cell Pathol. 1972;12(1):73–83. doi: 10.1007/BF02893987. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., Castelli W. P., Gordon T., McNamara P. M. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann Intern Med. 1971 Jan;74(1):1–12. doi: 10.7326/0003-4819-74-1-1. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Suzuki M., O'Neal R. M. Leukocyte lipids of human blood. Am J Clin Pathol. 1967 Sep;48(3):314–319. doi: 10.1093/ajcp/48.3.314. [DOI] [PubMed] [Google Scholar]
- Lee K. T., Lee K. J., Lee S. K., Imai H., O'Neal R. M. Poorly differentiated subendothelial cells in swine aortas. Exp Mol Pathol. 1970 Aug;13(1):118–129. doi: 10.1016/0014-4800(70)90089-4. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- Marshall J. R., O'Neal R. M. The lipophage in hyperlipemic rats: an electron microscopic study. Exp Mol Pathol. 1966 Feb;5(1):1–11. doi: 10.1016/0014-4800(66)90002-5. [DOI] [PubMed] [Google Scholar]
- Naito H. K., Holzbach R. T., Corbusier C. Characterization of serum lipids and lipoproteins of prairie dogs fed a chow diet or cholesterol-supplemented diet. Exp Mol Pathol. 1977 Aug;27(1):81–92. doi: 10.1016/0014-4800(77)90021-1. [DOI] [PubMed] [Google Scholar]
- Naito H. K. Modification of the Fiske and SubbaRow method for total phospholipid in serum. Clin Chem. 1975 Sep;21(10):1454–1456. [PubMed] [Google Scholar]
- Naito H. K., Wada M., Ehrhart L. A., Lewis L. A. Polyacrylamide-gel disc-electrophoresis as a screening procedure for serum lipoprotein abnormalities. Clin Chem. 1973 Feb;19(2):228–234. [PubMed] [Google Scholar]
- POOLE J. C., FLOREY H. W. Changes in the endothelium of the aorta and the behaviour of macrophages in experimental atheroma of rabbits. J Pathol Bacteriol. 1958 Apr;75(2):245–251. doi: 10.1002/path.1700750202. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- STILL W. J., O'NEAL R. M. Electron microscopic study of experimental atherosclerosis in the rat. Am J Pathol. 1962 Jan;40:21–35. [PMC free article] [PubMed] [Google Scholar]
- SUZUKI M., GREENBERG S. D., ADAMS J. G., O'NEAL R. M. EXPERIMENTAL ATHEROSCLEROSIS IN THE DOG; A MORPHOLOGIC STUDY. Exp Mol Pathol. 1964 Oct;90:455–467. doi: 10.1016/0014-4800(64)90026-7. [DOI] [PubMed] [Google Scholar]
- SUZUKI M., O'NEAL R. M. ACCUMULATION OF LIPIDS IN THE LEUKOCYTES OF RATS FED ATHEROGENIC DIETS. J Lipid Res. 1964 Oct;5:624–627. [PubMed] [Google Scholar]
- Somer J. B., Schwartz C. J. Focal 3 H-cholesterol uptake in the pig aorta. Atherosclerosis. 1971 May-Jun;13(3):293–304. doi: 10.1016/0021-9150(71)90073-6. [DOI] [PubMed] [Google Scholar]
- Stary H. C., Strong J. P. The fine structure of nonatherosclerotic intimal thickening, of developing, and of regressing atherosclerotic lesions at the bifurcation of the left coronary artery. Adv Exp Med Biol. 1976;67(00):89–108. doi: 10.1007/978-1-4614-4618-7_5. [DOI] [PubMed] [Google Scholar]
- Stefanovich V., Gore I. Cholesterol diet nd permeability of rabbit aorta. Exp Mol Pathol. 1971 Feb;14(1):20–29. doi: 10.1016/0014-4800(71)90049-9. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B., Spaet T. H., Pitlick F., Cintron J., Lejnieks I., Tiell M. L. Intimal healing. The pattern of reendothelialization and intimal thickening. Am J Pathol. 1977 Apr;87(1):125–142. [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., O'Neal R. M. Circulating lipophages, serum lipids, and atherosclerosis in rats. Arch Pathol. 1967 Feb;83(2):169–174. [PubMed] [Google Scholar]
- Thomas W. A., Florentin R. A., Nam S. C., Kim D. N., Jones R. M., Lee K. T. Preproliferative phase of atherosclerosis in swine fed cholesterol. Arch Pathol. 1968 Dec;86(6):621–643. [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Fabbrini P., Resi L. On the presence of a concanavalin-A reactive coat over the endothelial aortic surface and its modifications during early experimental cholesterol atherogenesis in rabbits. Virchows Arch A Pathol Pathol Anat. 1973 Jun 29;359(4):299–307. doi: 10.1007/BF00548601. [DOI] [PubMed] [Google Scholar]
- Wolinsky H., Goldfischer S., Schiller B., Kasak L. E. Lysosomes in aortic smooth muscle cells. Effects of hypertension. Am J Pathol. 1973 Dec;73(3):727–734. [PMC free article] [PubMed] [Google Scholar]
- Zemplenyi T., Rosenstein A. J. Arterial enzymes and their relation to atherosclerosis in pigeons. Exp Mol Pathol. 1975 Apr;22(2):225–241. doi: 10.1016/0014-4800(75)90066-0. [DOI] [PubMed] [Google Scholar]
- Zemplényi T. Vascular enzymes and the relevance of their study to problems of atherogenesis. Med Clin North Am. 1974 Mar;58(2):293–321. doi: 10.1016/s0025-7125(16)32160-5. [DOI] [PubMed] [Google Scholar]
- de Duve C. The participation of lysosomes in the transformation of smooth muscle cells to foamy cells in the aorta of cholesterol-fed rabbits. Acta Cardiol. 1974;Suppl 20:9–25. [PubMed] [Google Scholar]