Abstract
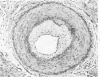
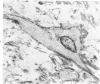
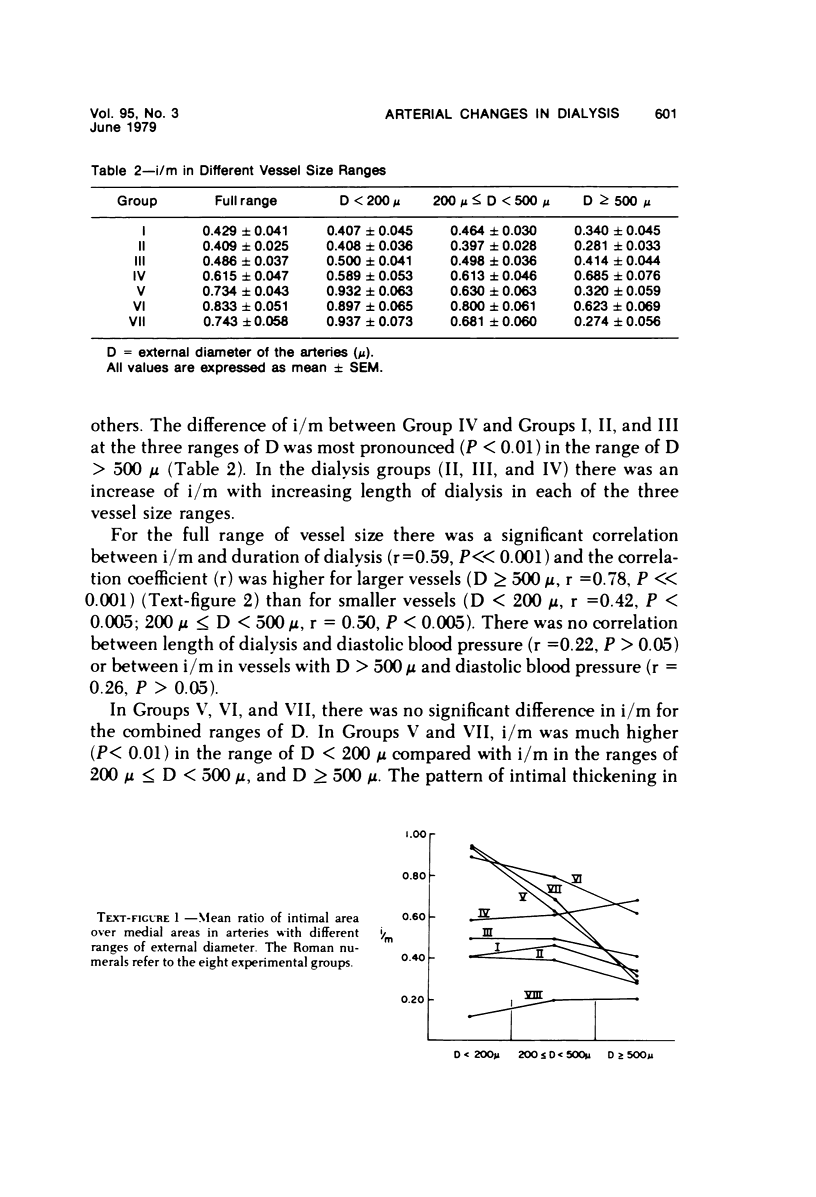
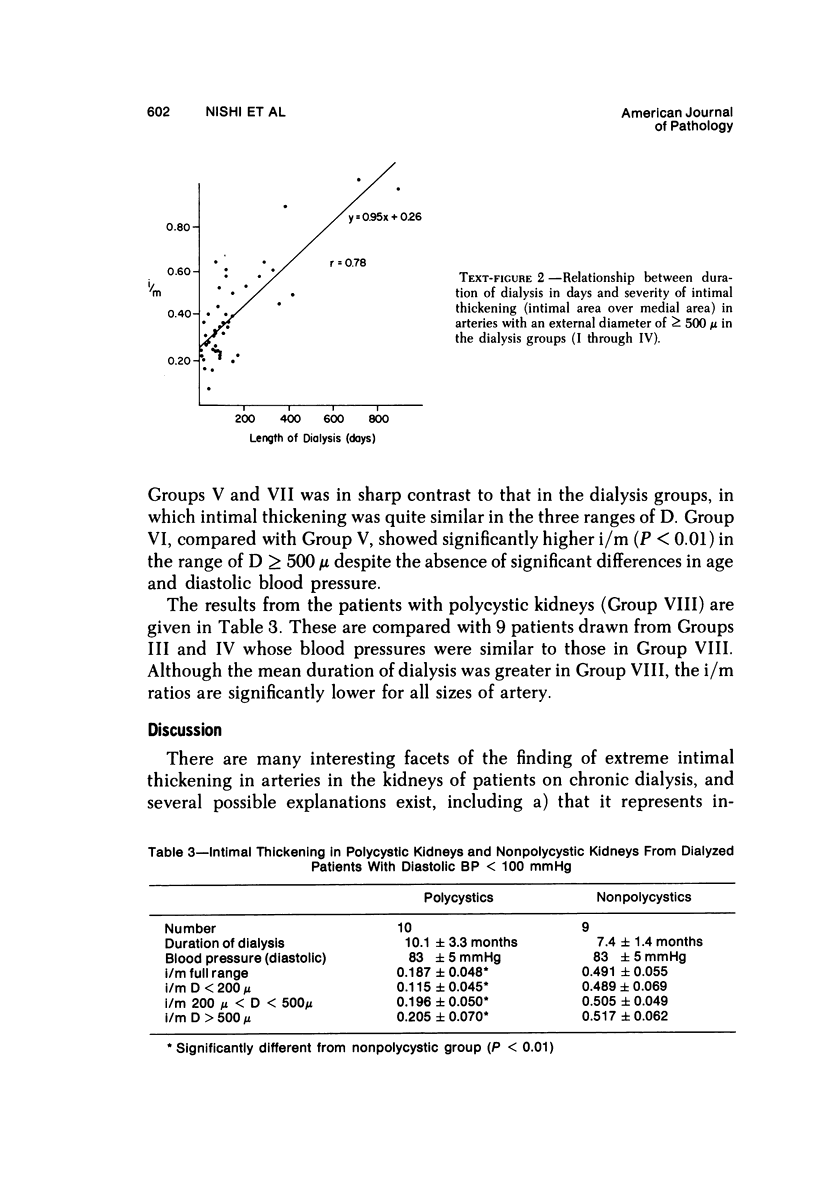
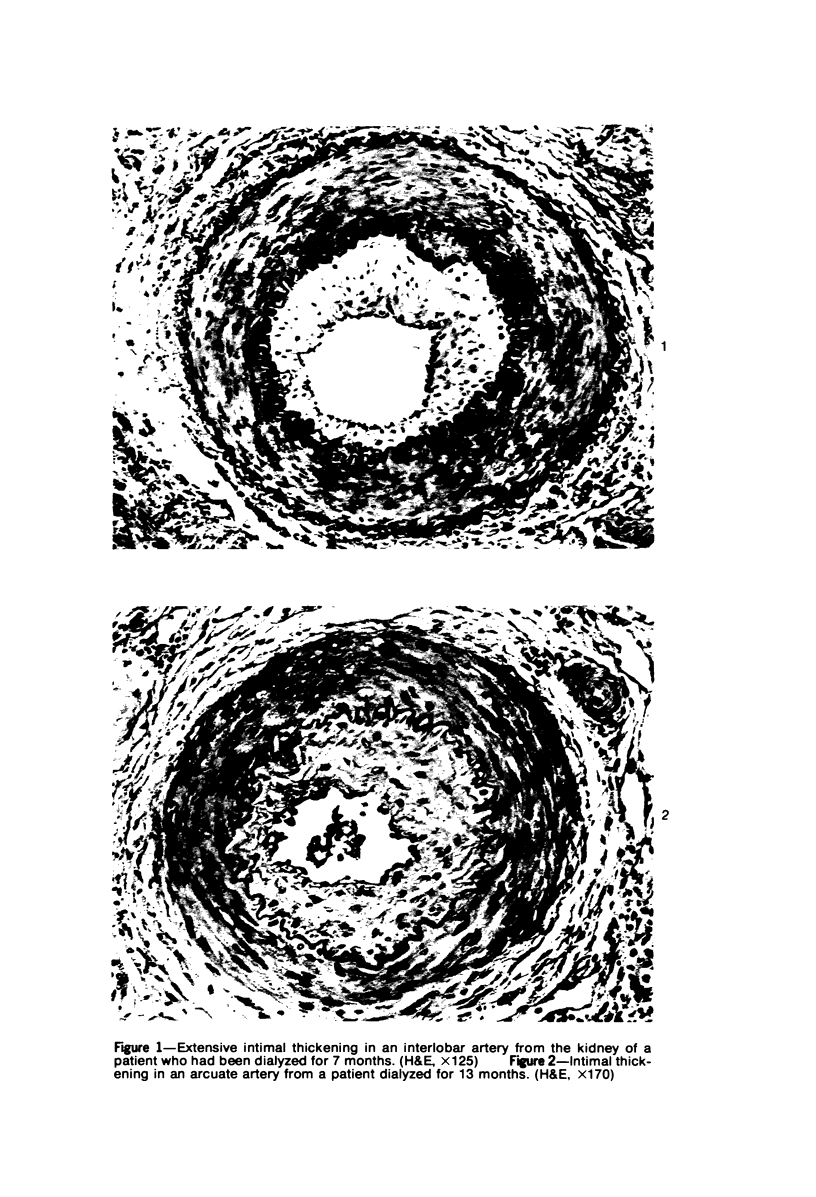
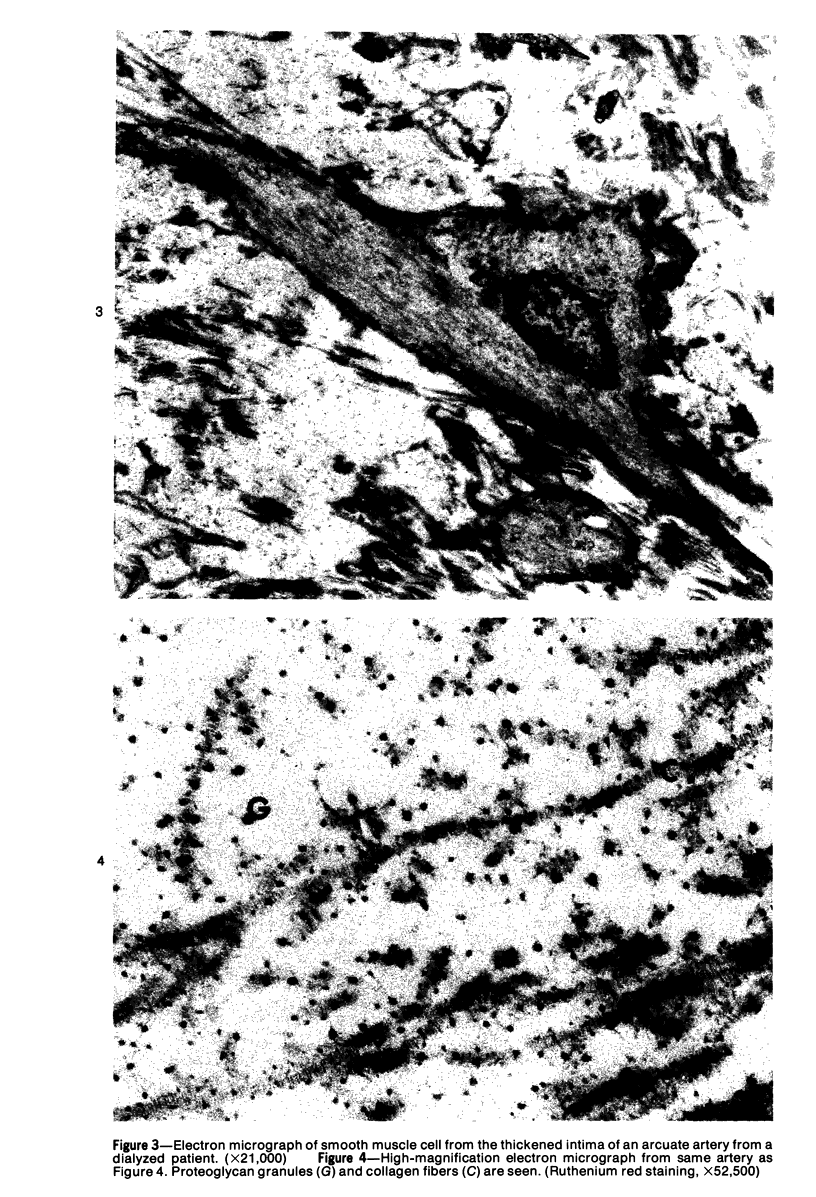
Arterial intimal thickening is common in the end-stage kidneys of patients maintained on hemodialysis. We measured the intimal thickening in patients dialyzed for varying periods and in patients with the malignant phase of essential hypertension and with scleroderma-associated renal failure. The ratio of intimal area to medical area in intrarenal arteries was used as a measure of intimal thickening. In the dialysis groups, intimal thickening was relatively constant in arteries of all sizes and correlated with duration of dialysis, particularly in larger arteries. In the malignant hypertension and scleroderma groups, the intimal thickening was greatest in arteries less than 200 mu in diameter and least in those over 500 mu in diameter. There was much less intimal thickening in arteries of all sizes in kidneys of patients with end-stage polycystic disease than in other end-stage kidneys from patients with a similar diastolic blood pressure and similar duration of dialysis. We believe that the intimal thickening in dialyzed patients is probably a disuse type of change and may be related to reduction in the area of the renal microvascular bed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALIS J. U., HAUST M. D., MORE R. H. ELECTRON-MICROSCOPIC STUDIES IN HUMAN ATHEROSCLEROSIS; CELLULAR ELEMENTS IN AORTIC FATTY STREAKS. Exp Mol Pathol. 1964 Oct;90:511–525. doi: 10.1016/0014-4800(64)90031-0. [DOI] [PubMed] [Google Scholar]
- Bagdade J. D., Albers J. J. Plasma high-density lipoprotein concentrations in chronic-hemodialysis and renal-transplant patients. N Engl J Med. 1977 Jun 23;296(25):1436–1439. doi: 10.1056/NEJM197706232962504. [DOI] [PubMed] [Google Scholar]
- Burke J. F., Jr, Francos G. C., Moore L. L., Cho S. Y., Lasker N. Accelerated atherosclerosis in chronic-dialysis patients--another look. Nephron. 1978;21(4):181–185. doi: 10.1159/000181391. [DOI] [PubMed] [Google Scholar]
- Cook T. A., Yates P. O. A critical survey of techniques for arterial mensuration. J Pathol. 1972 Oct;108(2):119–127. doi: 10.1002/path.1711080205. [DOI] [PubMed] [Google Scholar]
- FURUYAMA M. Histometrical investigations of arteries in reference to arterial hypertension. Tohoku J Exp Med. 1962 May 25;76:388–414. doi: 10.1620/tjem.76.388. [DOI] [PubMed] [Google Scholar]
- Geer J. C., Haust M. D. Smooth muscle cells in atherosclerosis. Monogr Atheroscler. 1972;2(0):1–140. [PubMed] [Google Scholar]
- Heptinstall R. H., Hill G. S. Steroid-induced hypertension in the rat. A study of the effects of renal artery constriction on hypertension caused by deoxycorticosterone. Lab Invest. 1967 May;16(5):751–767. [PubMed] [Google Scholar]
- Heptinstall R. H. Pathology of end-stage kidney disease. Am J Med. 1968 May;44(5):656–663. doi: 10.1016/0002-9343(68)90250-7. [DOI] [PubMed] [Google Scholar]
- Ibels L. S., Simons L. A., King J. O., Williams P. F., Neale F. C., Stewart J. H. Studies on the nature and causes of hyperlipidaemia in uraemia, maintenance dialysis and renal transplantation. Q J Med. 1975 Oct;44(176):601–614. [PubMed] [Google Scholar]
- LEVINE R. J., BOSHELL B. R. Renal involvement in progressive systemic sclerosis (scleroderma). Ann Intern Med. 1960 Mar;52:517–529. doi: 10.7326/0003-4819-52-3-517. [DOI] [PubMed] [Google Scholar]
- Lindner A., Charra B., Sherrard D. J., Scribner B. H. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974 Mar 28;290(13):697–701. doi: 10.1056/NEJM197403282901301. [DOI] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- Lundin A. P., Friedman E. A. Vascular consequences of maintenance hemodialysis--an uproven case. Nephron. 1978;21(4):177–180. doi: 10.1159/000181390. [DOI] [PubMed] [Google Scholar]
- MCQUEEN E. G., HODGE J. V. Modification of secondary lesions in renal hypertensive rats by control of the blood-pressure with reserpine. Q J Med. 1961 Apr;30:213–230. [PubMed] [Google Scholar]
- McManus J. F., Hughson M. D., Fitts C. T., Williams A. V. Studies on "end-stage" kidneys. Nodule formation in intrarenal arteries and arterioles. Lab Invest. 1977 Oct;37(4):339–349. [PubMed] [Google Scholar]
- Pesonen E., Martimo P., Rapola J. Histometry of the arterial wall. A new technique with the aid of automatic data processing. Lab Invest. 1974 Apr;30(4):550–555. [PubMed] [Google Scholar]
- Stemerman M. B., Spaet T. H., Pitlick F., Cintron J., Lejnieks I., Tiell M. L. Intimal healing. The pattern of reendothelialization and intimal thickening. Am J Pathol. 1977 Apr;87(1):125–142. [PMC free article] [PubMed] [Google Scholar]
- VAN CITTERS R. L., WAGNER B. M., RUSHMER R. F. Architecture of small arteries during vasoconstriction. Circ Res. 1962 Apr;10:668–675. doi: 10.1161/01.res.10.4.668. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Ross R. Proteoglycans in primate arteries. I. Ultrastructural localization and distribution in the intima. J Cell Biol. 1975 Dec;67(3):660–674. doi: 10.1083/jcb.67.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]