Abstract
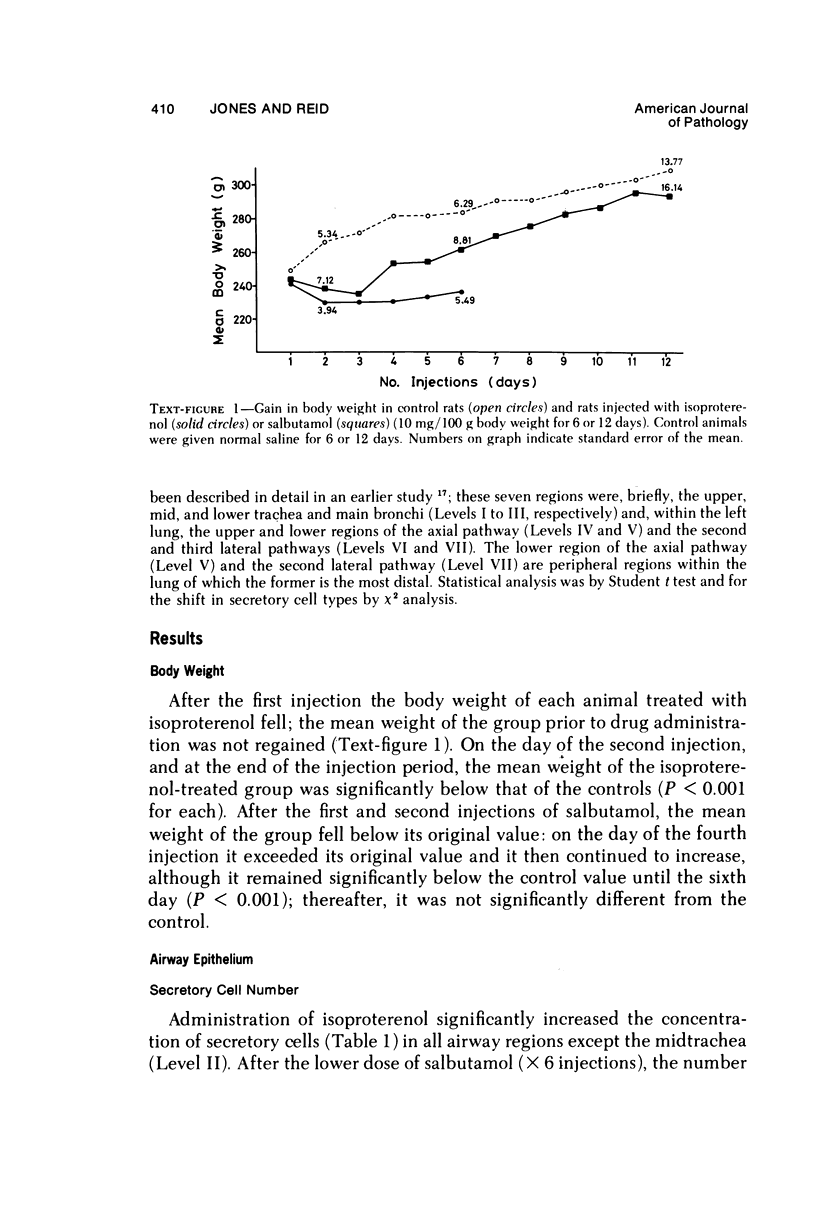
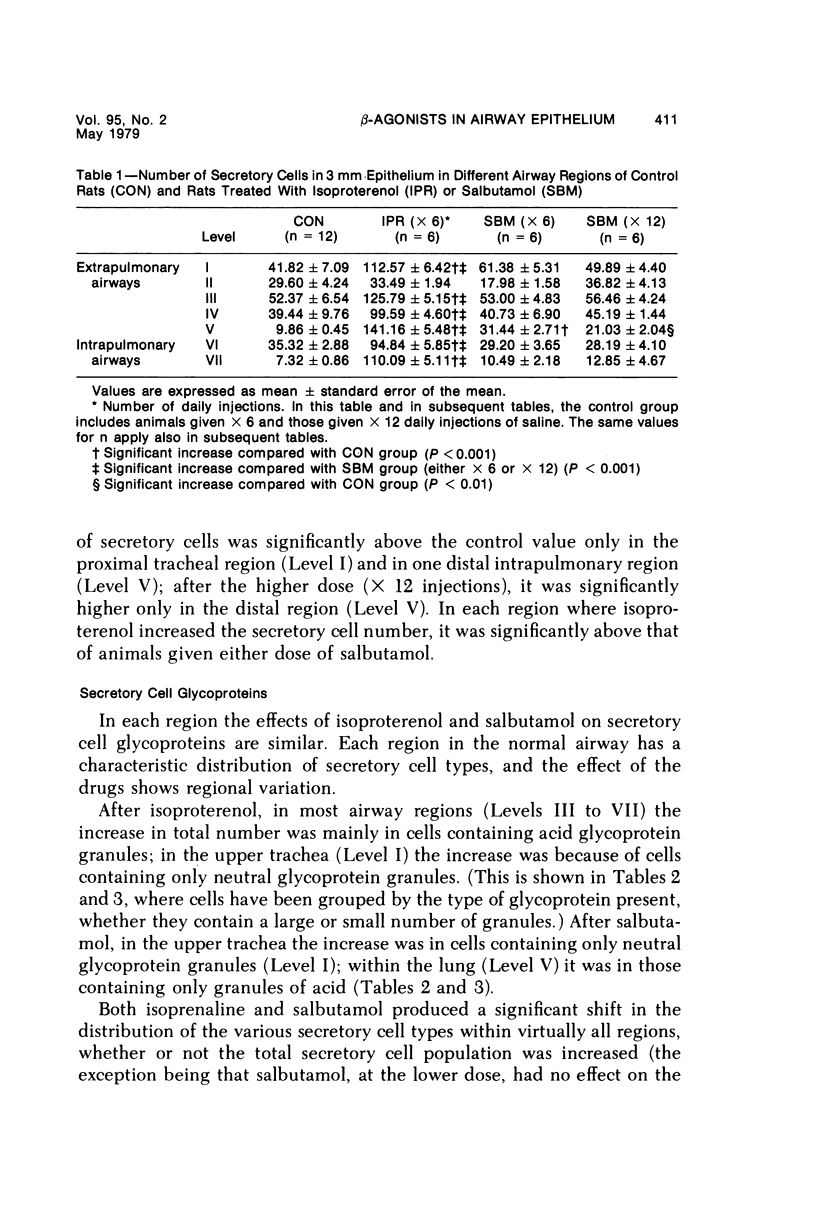
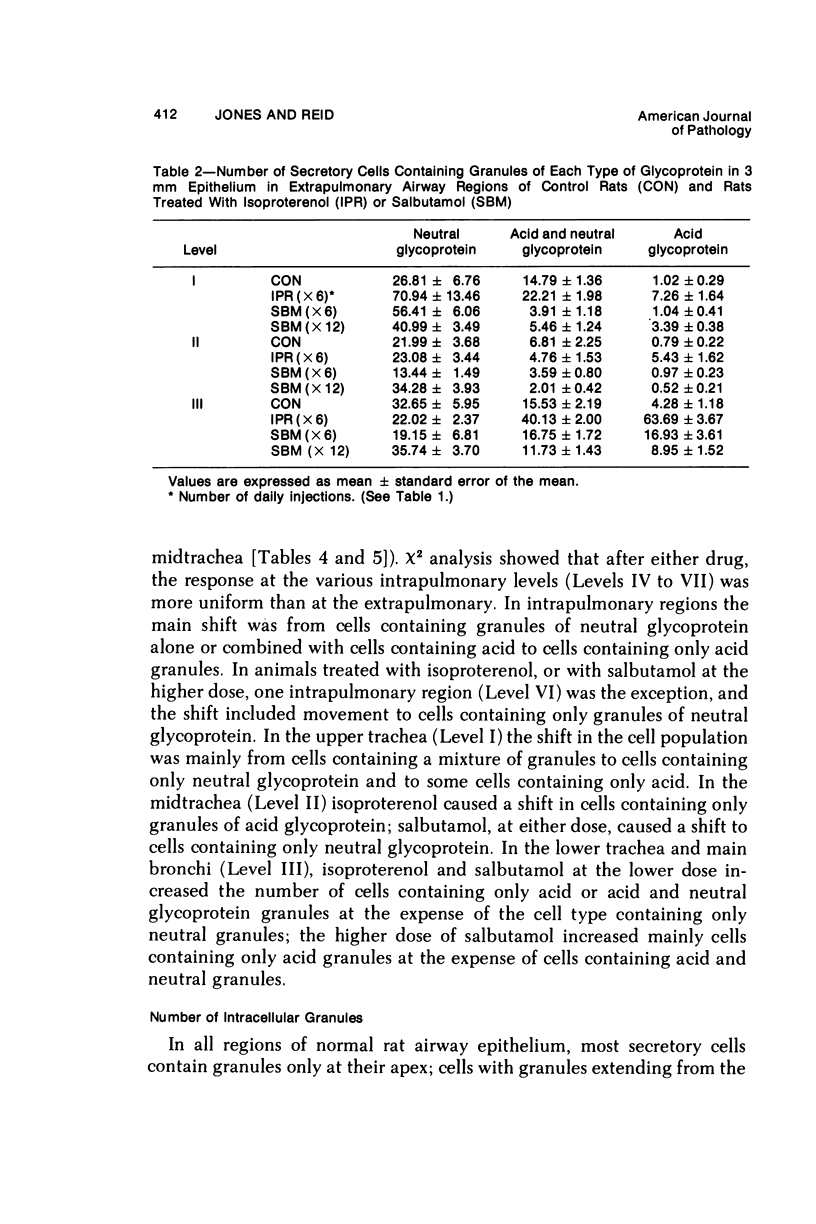
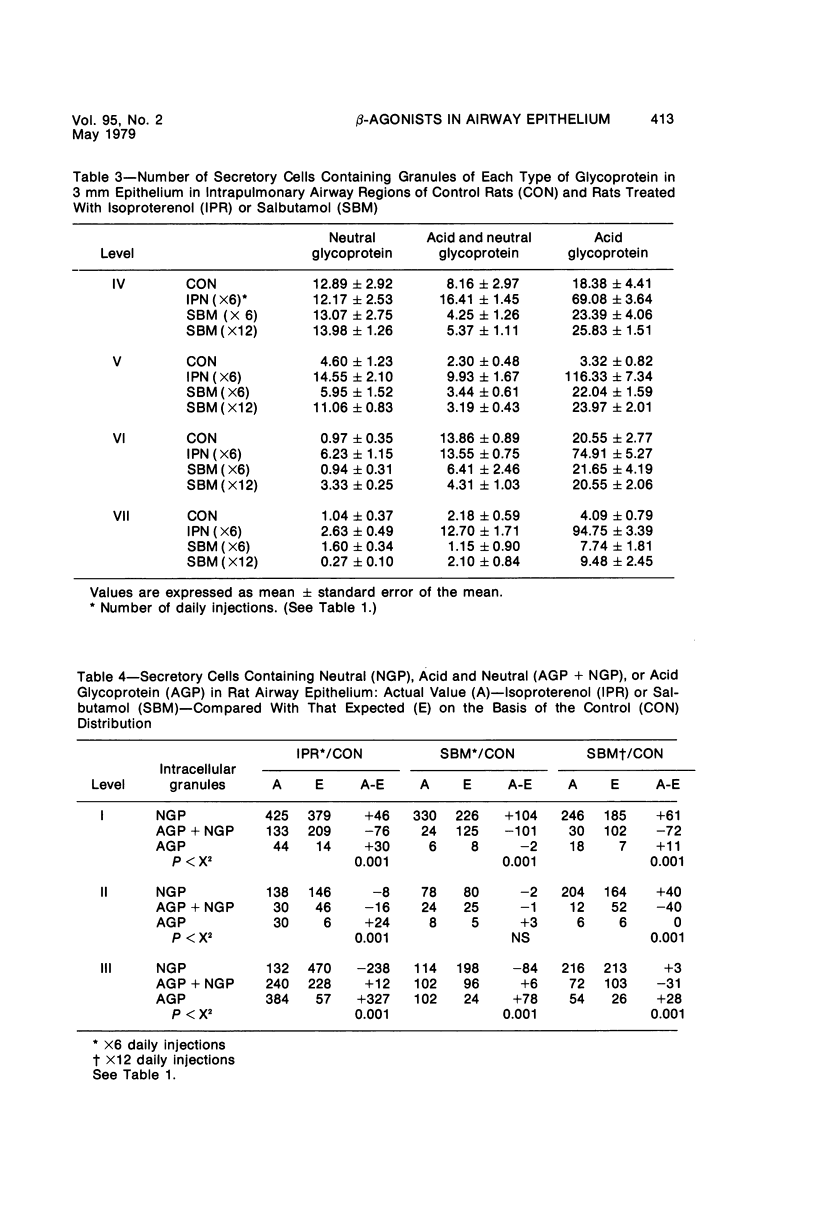
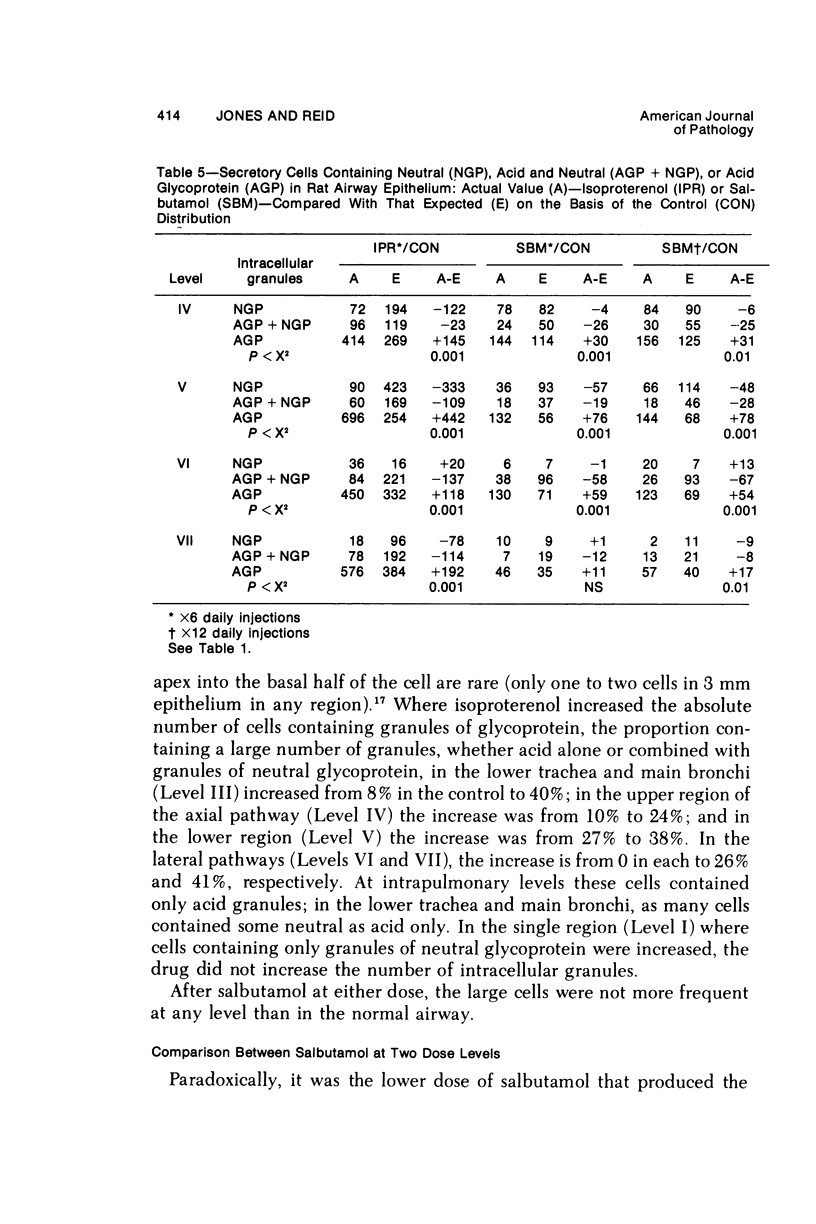
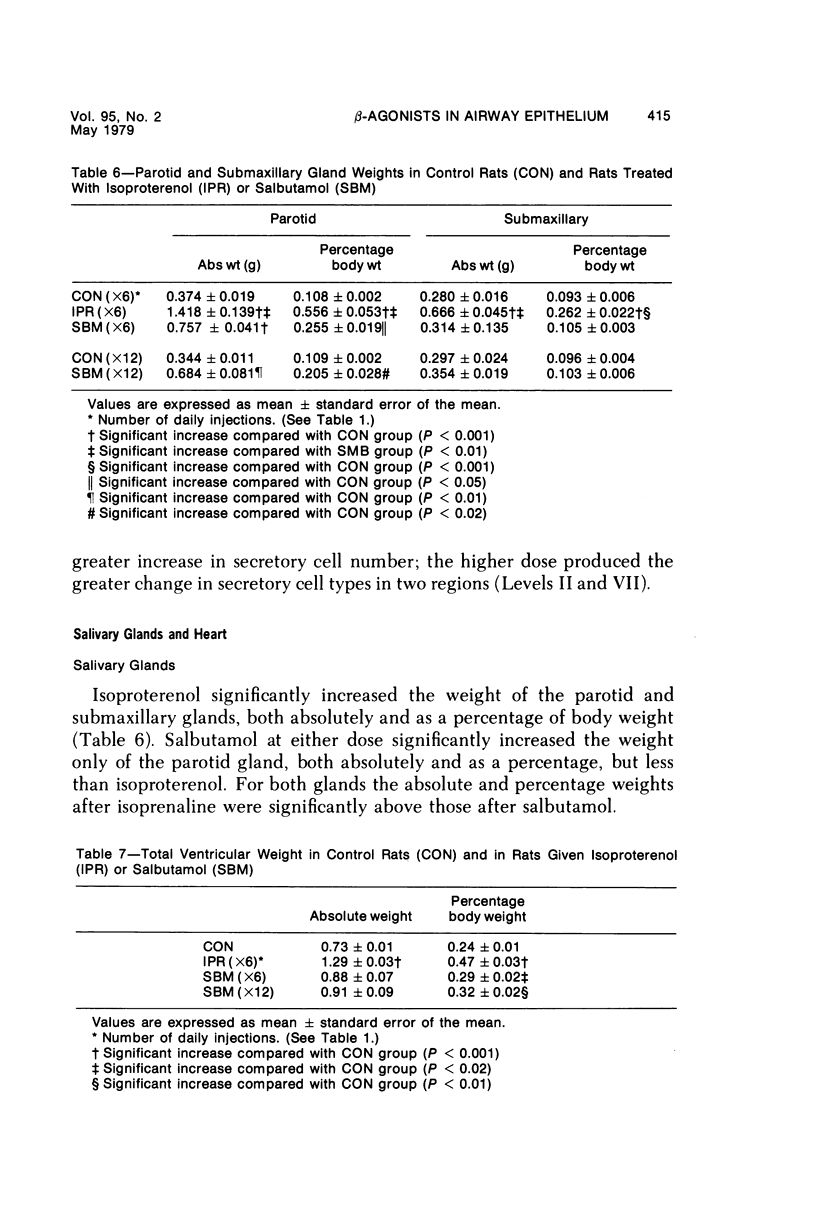
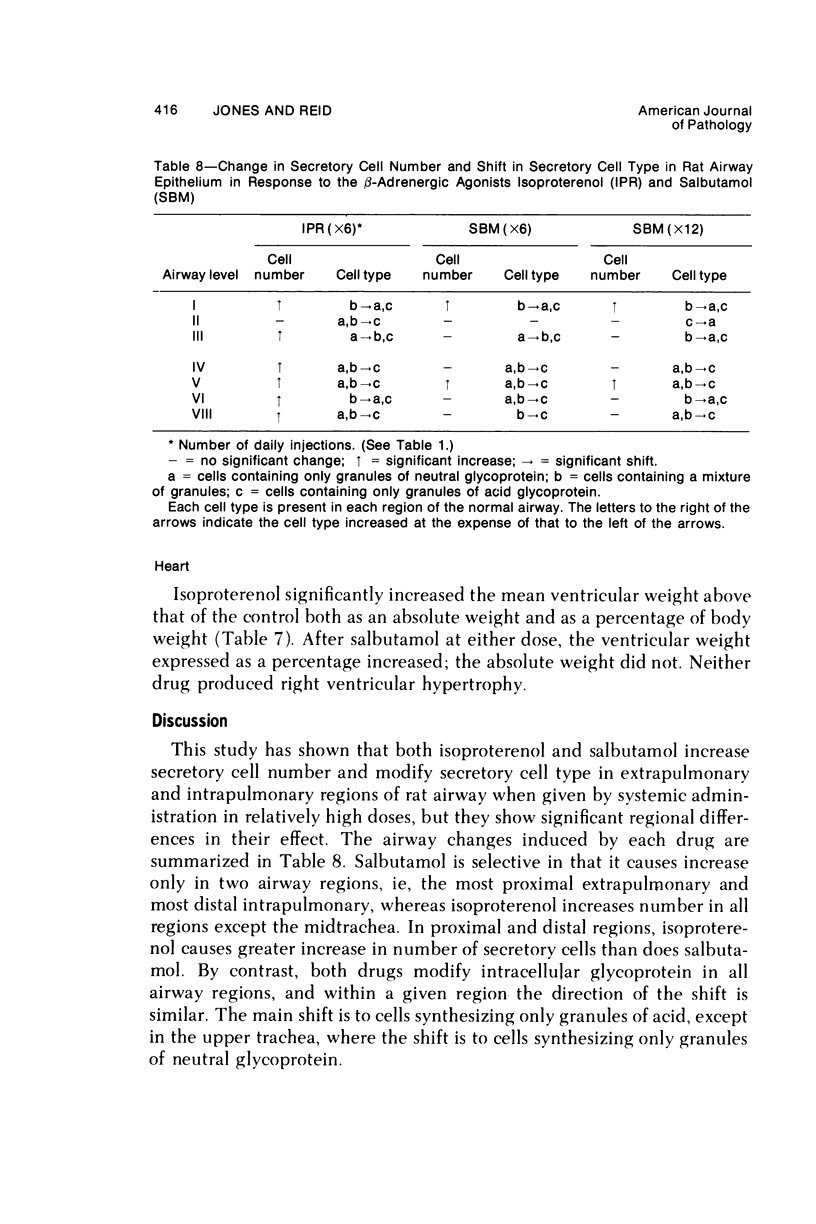
This study describes the effect of systemic administration of the beta-adrenergic agonists isoproterenol and salbutamol on the secretory cell populations in seven regions of rat airway epithelium (three extrapulmonary and four intrapulmonary) and on the size of salivary glands and heart. Isoproterenol (a nonselective beta-adrenergic agonist) significantly increases secretory cell number in all airway regions except the midtrachea; salbutamol (a selective beta 2 agonist) increases secretory cell number only in proximal and peripheral regions. The absolute number of secretory cells is greatest in the most peripheral region after isoproterenol administration and in the most proximal region after salbutamol, although both drugs produce the greatest relative increase at the periphery. In proximal and, particularly, peripheral regions, the increase by isoproterenol (less than 3- and 14-fold, respectively) is greater than by salbutamol (less than 2- and less than 3-fold, respectively). In all airway regions, both drugs modify intracellular glycoprotein in the secretory cell population; within a given region, modification is much the same. In the most proximal region, the population of cells synthesizing only granules of neutral glycoprotein significantly increases while in other regions increase is in cells synthesizing only granules of acid. A significant shift in glycoprotein synthesis occurs whether or not the secretory cell population is increased, which suggests that existing as well as newly appearing cells modify their product. Isoproterenol significantly increases the size of the parotid and submaxillary glands; salbutamol increases the size of the parotid only. Isoproterenol significantly increases the weight of both ventricles of the heart; salbutamol has no such effect.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansel D. G. Rat salivary glands after long-term isoproterenol administration. Arch Otolaryngol. 1974 Oct;100(4):256–261. doi: 10.1001/archotol.1974.00780040266003. [DOI] [PubMed] [Google Scholar]
- BARKA T. INDUCED CELL PROLIFERATION: THE EFFECT OF ISOPROTERENOL. Exp Cell Res. 1965 Mar;37:662–679. doi: 10.1016/0014-4827(65)90214-4. [DOI] [PubMed] [Google Scholar]
- Baskerville A. The development and persistence of bronchialgland hypertrophy and goblet-cell hyperplasia in the pig after injection of isoprenaline. J Pathol. 1976 May;119(1):35–47. doi: 10.1002/path.1711190107. [DOI] [PubMed] [Google Scholar]
- Bolduc P., Reid L. Mitotic index of the bronchial and alveolar lining of the normal rat lung. Am Rev Respir Dis. 1976 Dec;114(6):1121–1128. doi: 10.1164/arrd.1976.114.6.1121. [DOI] [PubMed] [Google Scholar]
- Bolduc P., Reid L. The effect of isoprenaline and pilocarpine on mitotic index and goblet cell number in rat respiratory epithelium. Br J Exp Pathol. 1978 Jun;59(3):311–318. [PMC free article] [PubMed] [Google Scholar]
- Brenner G. M., Stanton H. C. Adrenergic mechanism responsible for submandibular salivary glandular hypertrophy in the rat. J Pharmacol Exp Ther. 1970 May;173(1):166–175. [PubMed] [Google Scholar]
- Brittain R. T., Dean C. M., Jack D. Sympathomimetic bronchodilator drugs. Pharmacol Ther B. 1976;2(3):423–462. doi: 10.1016/0306-039x(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Burges R. A., Blackburn K. J. Adenyl cyclase and the differentiation of -adrenoreceptors. Nat New Biol. 1972 Feb 23;235(60):249–250. doi: 10.1038/newbio235249a0. [DOI] [PubMed] [Google Scholar]
- Daly M. J., Farmer J. B., Levy G. P. Comparison of the bronchodilator and cardiovascular actions of salbutamol, isoprenaline and orciprenaline in guinea-pigs and dogs. Br J Pharmacol. 1971 Nov;43(3):624–638. doi: 10.1111/j.1476-5381.1971.tb07192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham J. P., Baserga R., Butcher F. R. The effect of isoproterenol and its analogs upon adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate levels in mouse parotid gland in vivo. Relationship to the stimulation of DNA synthesis. Biochim Biophys Acta. 1974 Nov 4;372(1):196–217. doi: 10.1016/0304-4165(74)90087-7. [DOI] [PubMed] [Google Scholar]
- FULTON R. M., HUTCHINSON E. C., JONES A. M. Ventricular weight in cardiac hypertrophy. Br Heart J. 1952 Jul;14(3):413–420. doi: 10.1136/hrt.14.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Huber G. L. Quantitative differences in goblet cells in the tracheal epithelium of male and female rats. Am Rev Respir Dis. 1977 Apr;115(4):595–599. doi: 10.1164/arrd.1977.115.4.595. [DOI] [PubMed] [Google Scholar]
- Iravani J. Das Flimmerepithel. Beitr Klin Erforsch Tuberk Lungenkr. 1968;138(4):313–324. [PubMed] [Google Scholar]
- Jeffery P. K., Reid L. New observations of rat airway epithelium: a quantitative and electron microscopic study. J Anat. 1975 Nov;120(Pt 2):295–320. [PMC free article] [PubMed] [Google Scholar]
- Jones R., Bolduc P., Reid L. Goblet cell glycoprotein and tracheal gland hypertrophy in rat airways: the effect of tobacco smoke with or without the anti-inflammatory agent phenylmethyloxadiazole. Br J Exp Pathol. 1973 Apr;54(2):229–239. [PMC free article] [PubMed] [Google Scholar]
- Jones R., Reid L. Secretory cell hyperplasia and modification of intracellular glycoprotein in rat airways induced by short periods of exposure to tobacco smoke, and the effect of the antiinflammatory agent phenylmethyloxadiazole. Lab Invest. 1978 Jul;39(1):41–49. [PubMed] [Google Scholar]
- Lamb D., Reid L. Mitotic rates, goblet cell increase and histochemical changes in mucus in rat bronchial epithelium during exposure to sulphur dioxide. J Pathol Bacteriol. 1968 Jul;96(1):97–111. doi: 10.1002/path.1700960111. [DOI] [PubMed] [Google Scholar]
- Legge J. S., Gaddie J., Palmer K. N. Comparison of two oral selective beta2-adrenergic stimulant drugs in bronchial asthma. Br Med J. 1971 Mar 20;1(5750):637–639. doi: 10.1136/bmj.1.5750.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamud D. Adenyl cyclase: relationship to stimulated DNA synthesis in parotid glands. Biochem Biophys Res Commun. 1969 Jun 6;35(5):754–758. doi: 10.1016/0006-291x(69)90470-7. [DOI] [PubMed] [Google Scholar]
- SELYE H., VEILLEUX R., CANTIN M. Excessive stimulation of salivary gland growth by isoproterenol. Science. 1961 Jan 6;133(3445):44–45. doi: 10.1126/science.133.3445.44. [DOI] [PubMed] [Google Scholar]
- Sturgess J., Reid L. An organ culture study of the effect of drugs on the secretory activity of the human bronchial submucosal gland. Clin Sci. 1972 Oct;43(4):533–543. doi: 10.1042/cs0430533. [DOI] [PubMed] [Google Scholar]
- Sturgess J., Reid L. The effect of isoprenaline and pilocarpine on (a) bronchial mucus-secreting tissue and (b) pancreas, salivary glands, heart, thymus, liver and spleen. Br J Exp Pathol. 1973 Aug;54(4):388–403. [PMC free article] [PubMed] [Google Scholar]
- U'Prichard D. C., Bylund D. B., Snyder S. H. (+/-)-[3H]Epinephrine and (-)[3H]dihydroalprenolol binding to beta1- and beta2-noradrenergic receptors in brain, heart, and lung membranes. J Biol Chem. 1978 Jul 25;253(14):5090–5102. [PubMed] [Google Scholar]
- WELLS H. Submandibular salivary gland weight increase by administration of isoproterenol to rats. Am J Physiol. 1962 Mar;202:425–428. doi: 10.1152/ajplegacy.1962.202.3.425. [DOI] [PubMed] [Google Scholar]
- Yoneda K. Pilocarpine stimulation of the bronchiolar Clara cell secretion. Lab Invest. 1977 Nov;37(5):447–452. [PubMed] [Google Scholar]
- van As A. The role of selective beta2-adrenoceptor stimulants in the control of ciliary activity. Respiration. 1974;31(2):146–151. doi: 10.1159/000193106. [DOI] [PubMed] [Google Scholar]


