Abstract
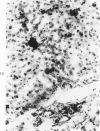
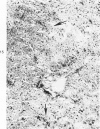
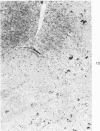
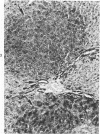
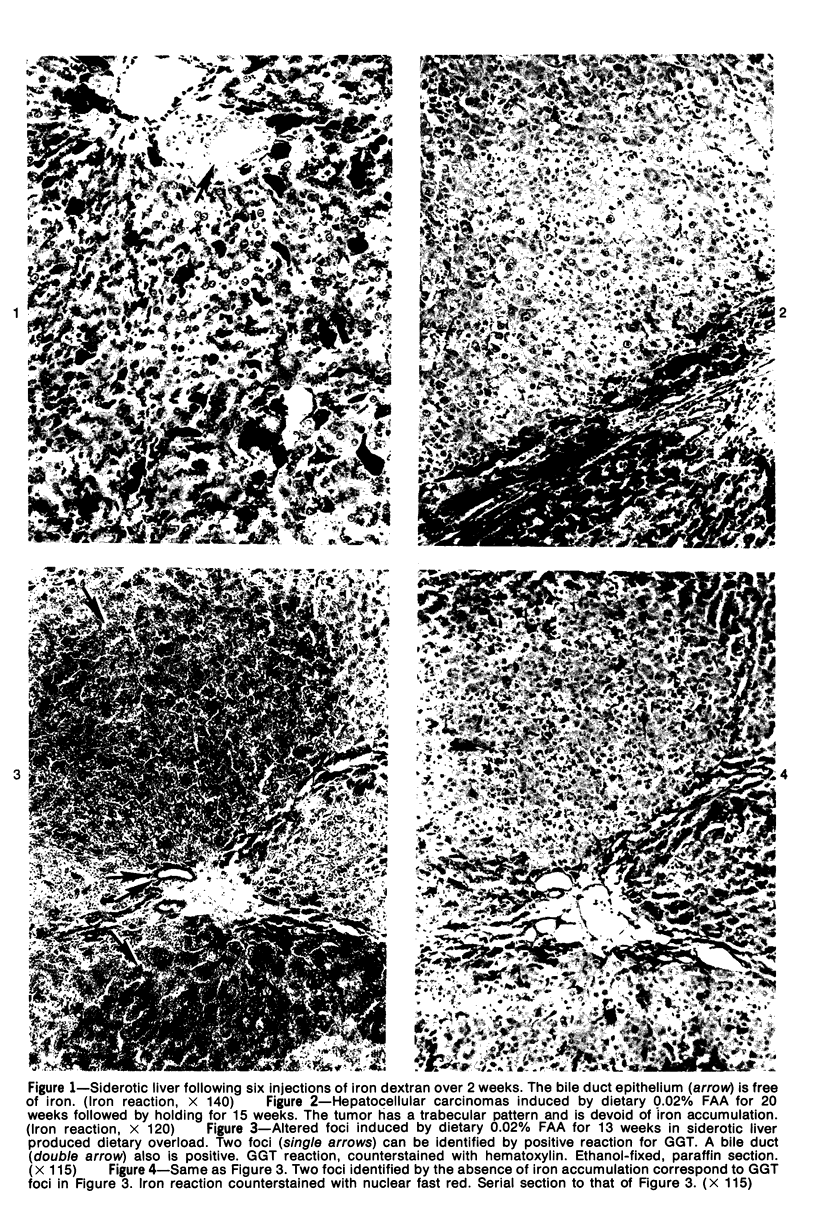
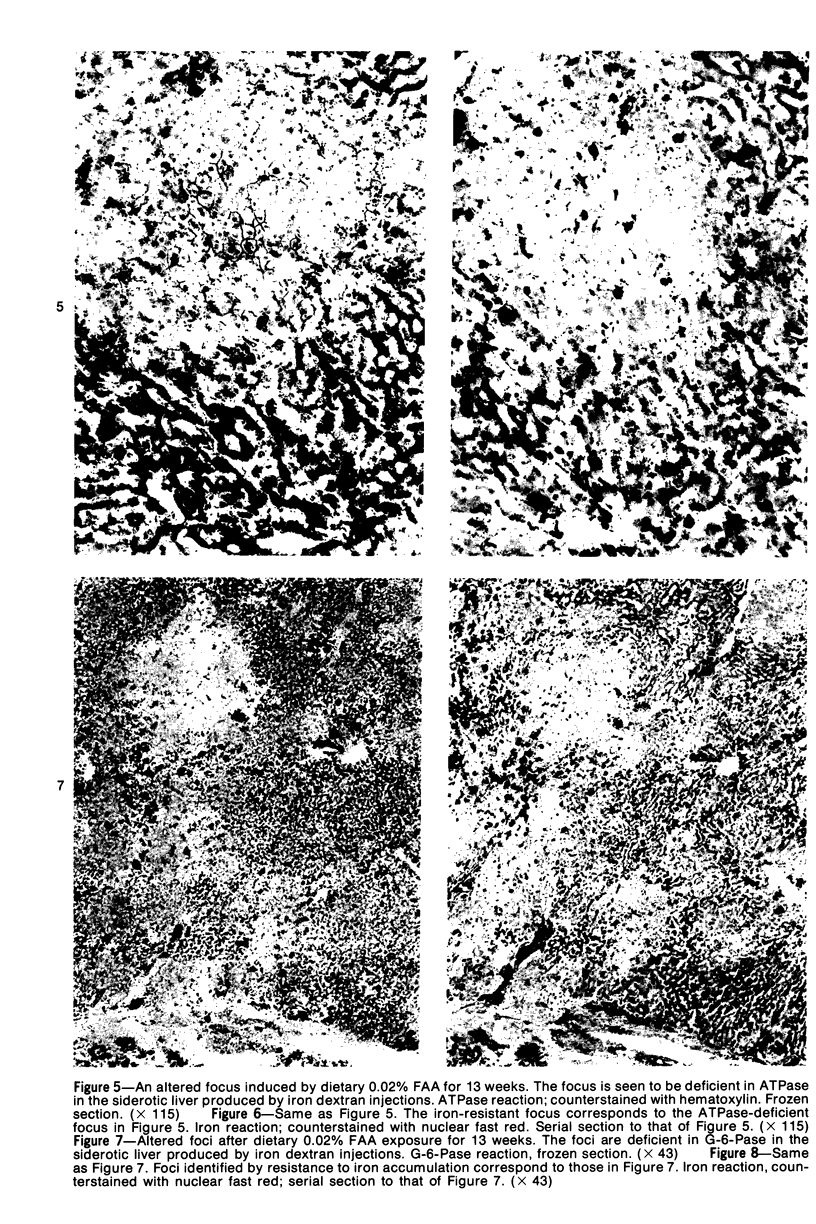
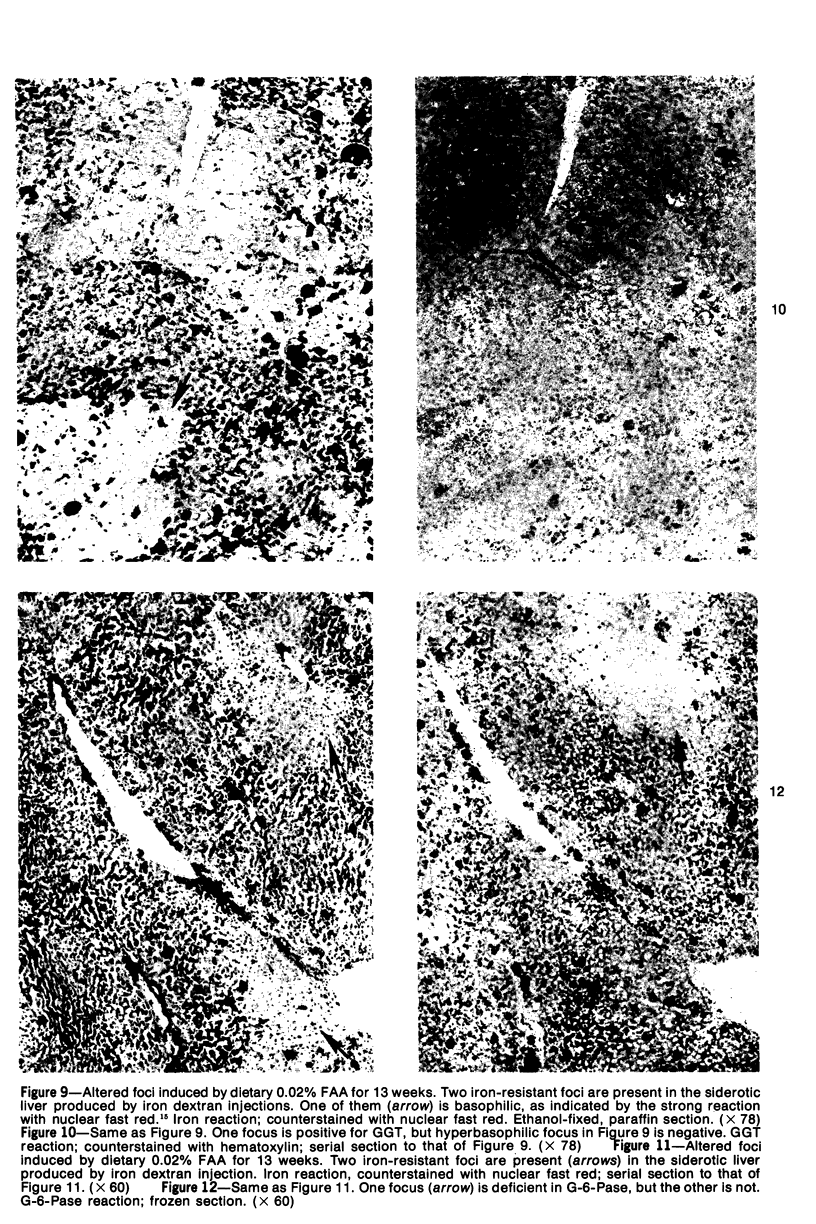
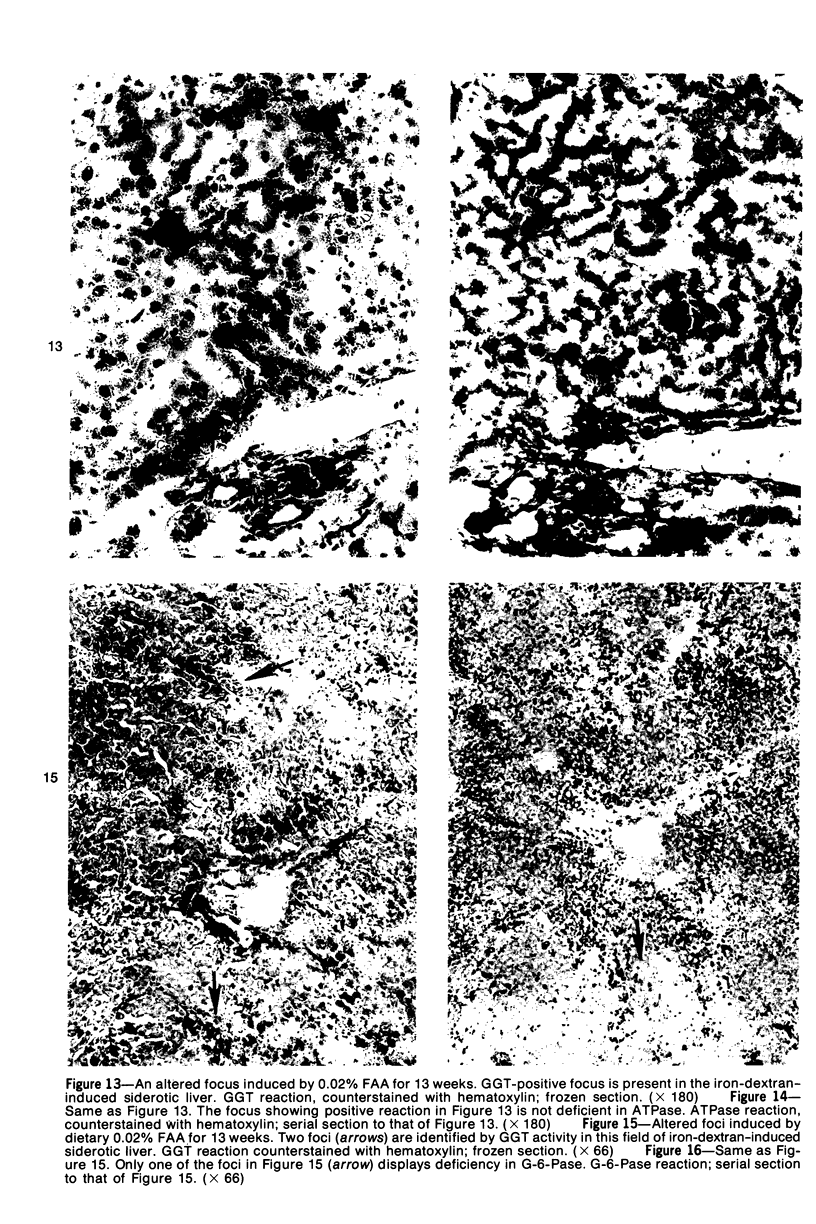
Subcutaneous injection of iron dextran resulted in a hepatic siderosis within 2 weeks in rats, as previously reported for mice. Hepatic carcinomas as well as neoplastic nodules in rats were entirely or mainly free of stainable iron and, thus, could be readily identified histologically. In addition, early carcinogen-induced altered foci were resistant to iron accumulation. In rats fed 0.02% N-2-fluorenylacetamide (FAA) for 13 weeks, the number of iron-resistant foci identified following iron injection was the same as that observed with dietary iron overload. Histochemical investigation of enzymatic markers that have been used to identify foci in rats revealed that foci characterized by enzymatic reactions of positive gamma-glutamyl transpeptidase and decreased adenosine triphosphatase and glucose-6-phosphatase corresponded to those characterized by resistance to iron accumulation. However, in quantitative analysis of the early carcinogen-induced foci in rats given iron dextran following a diet containing 0.02% 2-FAA for 13 weeks, more lesions were detected by resistance to iron accumulation than by any of these other properties. There was considerable phenotypic heterogeneity among foci for the enzyme markers. It is concluded that resistance to iron accumulation is a more sensitive and reliable marker for early carcinogen-induced altered hepatocellular foci than is any other histochemical property.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAOUST R., MOLNAR F. CELLULAR POPULATIONS AND MITOTIC ACTIVITY IN RAT LIVER PARENCHYMA DURING AZO DYE CARCINOGENESIS. Cancer Res. 1964 Dec;24:1898–1909. [PubMed] [Google Scholar]
- Epstein S., Ito N., Merkow L., Farber E. Cellular analysis of liver carcinogenesis: the induction of large hyperplastic nodules in the liver with 2-fluorenylacetamide or ethionine and some aspects of their morphology and glycogen metabolism. Cancer Res. 1967 Sep;27(9):1702–1711. [PubMed] [Google Scholar]
- FARBER E., ICHINOSE H. On the origin of malignant cells in experimental liver cancer. Acta Unio Int Contra Cancrum. 1959;15(1):152–153. [PubMed] [Google Scholar]
- FIRMINGER H. I. Histopathology of carcinogenesis and tumors of the liver in rats. J Natl Cancer Inst. 1955 Apr;15(5 Suppl):1427–1442. [PubMed] [Google Scholar]
- Fiala S., Fiala A. E., Dixon B. -Glutamyl transpeptidase in transplantable, chemically induced rat hepatomas and "spontaneous" mouse hepatomas. J Natl Cancer Inst. 1972 May;48(5):1393–1401. [PubMed] [Google Scholar]
- Fiala S., Fiala E. S. Activation by chemical carcinogens of gamma-glutamyl transpeptidase in rat and mouse liver. J Natl Cancer Inst. 1973 Jul;51(1):151–158. doi: 10.1093/jnci/51.1.151. [DOI] [PubMed] [Google Scholar]
- GOESSNER W., FRIEDRICH-FRESKA H. HISTOCHEMISCHE UNTERSUCHUNGEN UEBER DIE GLUCOSE-6-PHOSPHATASE IN DER RATTENLEBER WAEHREND DER KANZERISIERUNG DURCH NITROSAMINE. Z Naturforsch B. 1964 Sep;19:862–863. [PubMed] [Google Scholar]
- GOLDFARB S., ZAK F. G. Role of injury and hyperplasia in the induction of hepatocellular carcinoma. JAMA. 1961 Nov 18;178:729–731. doi: 10.1001/jama.1961.73040460007007b. [DOI] [PubMed] [Google Scholar]
- Gōmōri G. Microtechnical Demonstration of Iron: A Criticism of its Methods. Am J Pathol. 1936 Sep;12(5):655–664.1. [PMC free article] [PubMed] [Google Scholar]
- Kalengayi M. M., Ronchi G., Desmet V. J. Histochemistry of gamma-glutamyl transpeptidase in rat liver during aflatoxin B1-induced carcinogenesis. J Natl Cancer Inst. 1975 Sep;55(3):579–588. doi: 10.1093/jnci/55.3.579. [DOI] [PubMed] [Google Scholar]
- Kitagawa T. Histochemical analysis of hyperplastic lesions and hepatomas of the liver of rats fed 2-fluorenylacetamide. Gan. 1971 Jun;62(3):207–216. [PubMed] [Google Scholar]
- Newberne P. M., Wogan G. N. Sequential morphologic changes in aflatoxin B carcinogenesis in the rat. Cancer Res. 1968 Apr;28(4):770–781. [PubMed] [Google Scholar]
- POPPER H., STERNBERG S. S., OSER B. L., OSER M. The carcinogenic effect of aramite in rats. A study of hepatic nodules. Cancer. 1960 Sep-Oct;13:1035–1046. doi: 10.1002/1097-0142(196009/10)13:5<1035::aid-cncr2820130526>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Pitot H. C., Barsness L., Goldsworthy T., Kitagawa T. Biochemical characterisation of stages of hepatocarcinogenesis after a single dose of diethylnitrosamine. Nature. 1978 Feb 2;271(5644):456–458. doi: 10.1038/271456a0. [DOI] [PubMed] [Google Scholar]
- Rabes H. M., Scholze P., Jantsch B. Growth kinetics of diethylnitrosamine-induced, enzyme-deficient "preneoplastic" liver cell populations in vivo and in vitro. Cancer Res. 1972 Nov;32(11):2577–2586. [PubMed] [Google Scholar]
- Reuber M. D. Development of preneoplastic and neoplastic lesions of the liver in male rats given 0.025 percent N-2-fluorenyldiacetamide. J Natl Cancer Inst. 1965 Jun;34(6):697–723. [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Scherer E., Emmelot P. Kinetics of induction and growth of precancerous liver-cell foci, and liver tumour formation by diethylnitrosamine in the rat. Eur J Cancer. 1975 Oct;11(10):689–696. doi: 10.1016/0014-2964(75)90042-0. [DOI] [PubMed] [Google Scholar]
- Scherer E., Hoffmann M., Emmelot P., Friedrich-Freksa M. Quantitative study on foci of altered liver cells induced in the rat by a single dose of diethylnitrosamine and partial hepatectomy. J Natl Cancer Inst. 1972 Jul;49(1):93–106. [PubMed] [Google Scholar]
- Scherer E., Hoffmann M. Probable clonal genesis of cellular islands induced in rat liver by diethylnitrosamine. Eur J Cancer. 1971 Aug;7(4):369–371. doi: 10.1016/0014-2964(71)90083-1. [DOI] [PubMed] [Google Scholar]
- Taniguchi N., Tsukada Y., Mukuo K., Hirai H. Effect of hepatocarcinogenic azo dyes on glutathione and related enzymes in rat liver. Gan. 1974 Oct;65(5):381–387. [PubMed] [Google Scholar]
- Watanabe K., Williams G. M. Enhancement of rat hepatocellular-altered foci by the liver tumor promoter phenobarbital: evidence that foci are precursors of neoplasms and that the promoter acts on carcinogen-induced lesions. J Natl Cancer Inst. 1978 Nov;61(5):1311–1314. [PubMed] [Google Scholar]
- Williams G. M., Hirota N., Rice J. M. The resistance of spontaneous mouse hepatocellular neoplasms to iron accumulation during rapid iron loading by parenteral administration and their transplantability. Am J Pathol. 1979 Jan;94(1):65–74. [PMC free article] [PubMed] [Google Scholar]
- Williams G. M., Klaiber M., Parker S. E., Farber E. Nature of early appearing, carcinogen-induced liver lesions to iron accumulation. J Natl Cancer Inst. 1976 Jul;57(1):157–165. doi: 10.1093/jnci/57.1.157. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Yamamoto R. S. Absence of stainable iron from preneoplastic and neoplastic lesions in rat liver with 8-hydroxyquinoline-induced siderosis. J Natl Cancer Inst. 1972 Sep;49(3):685–692. [PubMed] [Google Scholar]