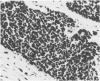
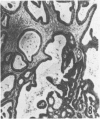
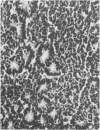
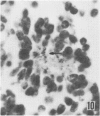
Abstract
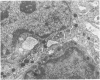
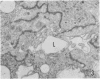
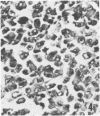
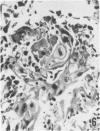
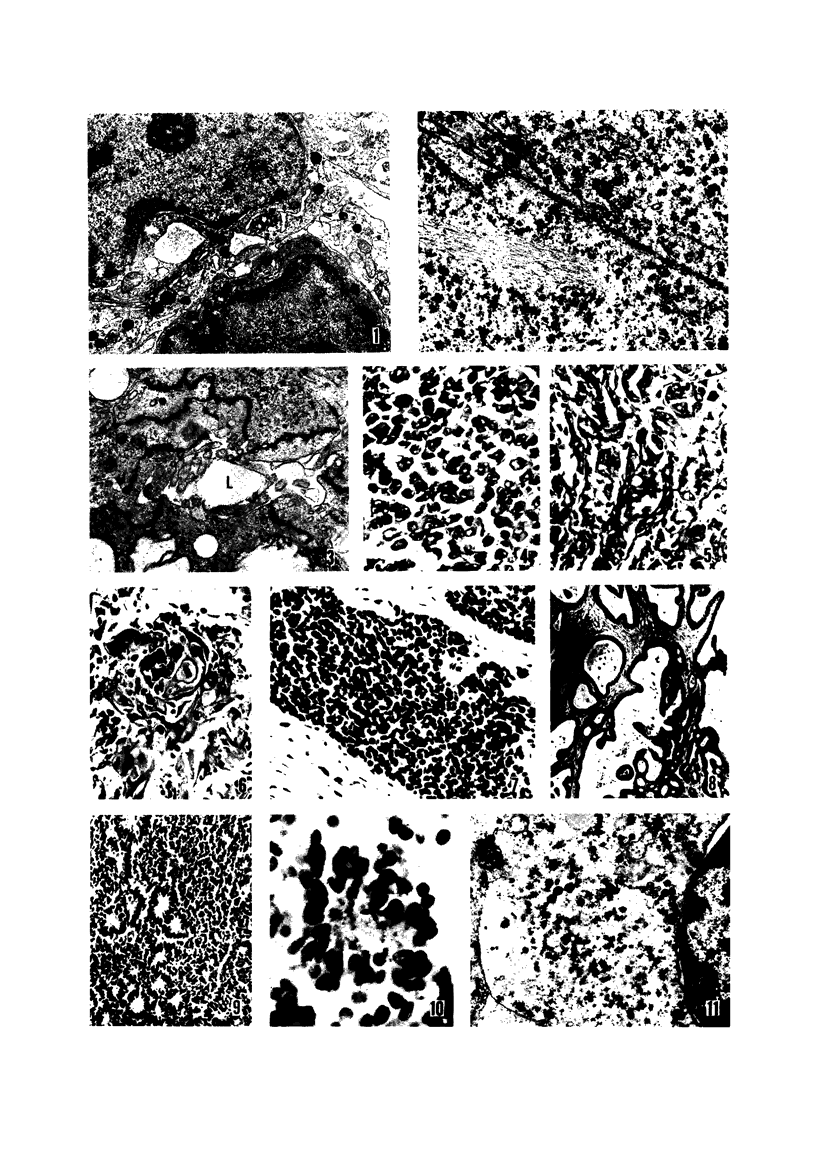
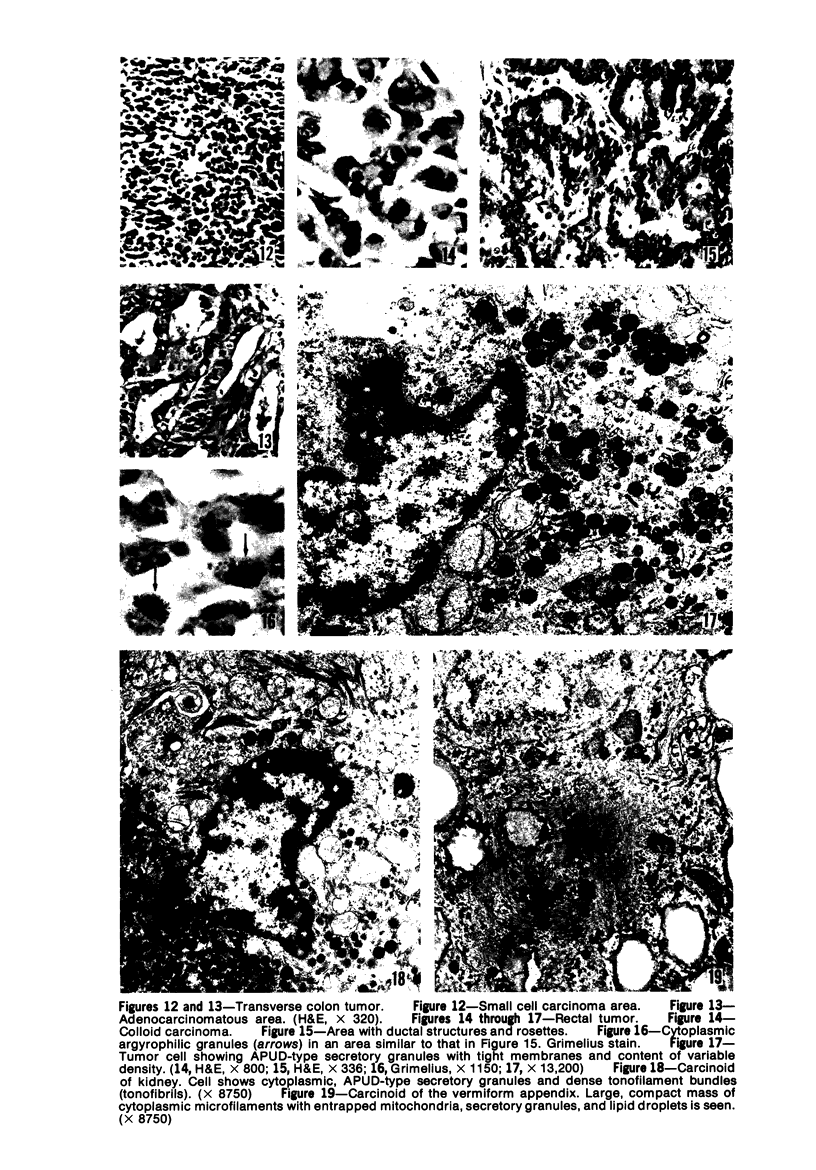
Twenty-seven small cell carcinomas of the lung and three tumors of the large intestine with combined adenocarcinomatous and small cell and/or anaplastic carcinoid-type histologic features were studied by light and electron microscopy. It was shown that the small cells have morphologic characteristics of APUD cells. Also presented are the histologic features of a carcinoma of the lung with large cell undifferentiated carcinoma, adenocarcinoma, squamous cell carcinoma, and giant cell carcinoma areas in the primary site and in several metastatic foci. Two of the renal metastases showed small cell carcinoma. The combined tumors and the numerous other similar neoplasms described in the literature and reviewed here suggest an endodermal origin for digestive and respiratory tract APUD cells based on the hypothesis that cancer is a clonal proliferation, and mucous and squamous cell differentiation is an endodermal rather than neural crest characteristic. The ultrastructural features of tumors of cells of known neural crest origin, including a medullary carcinoma of the thyroid, three carotid body tumors, a pheochromocytoma, and two cutaneous melanomas were compared with those of other APUD cell tumors including small cell carcinomas of the lung, two bronchial carcinoids, a carcinoid of the appendix, and a carcinoid of the kidney. Cells of the latter group sometimes possessed cytoplasmic tonofibrils, round compact masses of cytoplasmic microfilaments, and ductal lumina. These features were lacking in the former group and may signify a different embryologic origin. The histologic, histopathologic, and embryologic evidence regarding the origin of digestive and respiratory tract APUD cells is reviewed, showing that the former are, and the latter probably are, of endodermal and not neuroectodermal origin.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZZOPARDI J. G., POLLOCK D. J. ARGENTAFFIN AND ARGYROPHIL CELLS IN GASTRIC CARCINOMA. J Pathol Bacteriol. 1963 Oct;86:443–451. doi: 10.1002/path.1700860219. [DOI] [PubMed] [Google Scholar]
- Alpert L. I., Bochetto J. F., Jr Carotid body tumor: ultrastructural observations. Cancer. 1974 Sep;34(3):564–573. doi: 10.1002/1097-0142(197409)34:3<564::aid-cncr2820340314>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- An T. Cytoplasmic inclusions in bronchial carcinoid. Hum Pathol. 1978 Mar;9(2):241–242. doi: 10.1016/s0046-8177(78)80121-x. [DOI] [PubMed] [Google Scholar]
- Andrew A. APUD cells, apudomas and the neural crest. S Afr Med J. 1976 May 29;50(23):890–898. [PubMed] [Google Scholar]
- Andrew A. An experimental investigation into the possible neural crest origin of pancreatic APUD (islet) cells. J Embryol Exp Morphol. 1976 Jun;35(3):577–593. [PubMed] [Google Scholar]
- Andrew A. Further evidence that enterochromaffin cells are not derived from the neural crest. J Embryol Exp Morphol. 1974 Jun;31(3):589–598. [PubMed] [Google Scholar]
- Balsam A., Bernstein G., Goldman J., Sachs B. A., Rifkin H. Ectopic adrenocorticotropin syndrome associated with carcinoma of the colon. Gastroenterology. 1972 Apr;62(4):636–641. [PubMed] [Google Scholar]
- Bates H. R., Jr, Belter L. F. Composite carcinoid tumor (argentaffinoma-adenocarcinoma) of the colon: report of 2 cases. Dis Colon Rectum. 1967 Nov-Dec;10(6):467–470. doi: 10.1007/BF02616821. [DOI] [PubMed] [Google Scholar]
- Bensch K. G., Corrin B., Pariente R., Spencer H. Oat-cell carcinoma of the lung. Its origin and relationship to bronchial carcinoid. Cancer. 1968 Dec;22(6):1163–1172. doi: 10.1002/1097-0142(196811)22:6<1163::aid-cncr2820220612>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Bordi C., Anversa P., Vitali-Mazza L. Ultrastructural study of a calcitonin-secreting tumor. Typhology of the tumor cells and origin of amyloid. Virchows Arch A Pathol Pathol Anat. 1972;357(2):145–161. doi: 10.1007/BF00548361. [DOI] [PubMed] [Google Scholar]
- Braunstein H., Stephens C. L., Gibson R. L. Secretory granules in medullary carcinoma of the thyroid. Electron microscopic demonstration. Arch Pathol. 1968 Mar;85(3):306–313. [PubMed] [Google Scholar]
- Brown R. E., Schweisthal M. R., Still W. J. Ultrastructural observations on the embryogenesis of islet cells in the rat pancreas in vitro. Proc Soc Exp Biol Med. 1971 Feb;136(2):441–445. doi: 10.3181/00379727-136-35283. [DOI] [PubMed] [Google Scholar]
- Chang W. W., Leblond C. P. Renewal of the epithelium in the descending colon of the mouse. I. Presence of three cell populations: vacuolated-columnar, mucous and argentaffin. Am J Anat. 1971 May;131(1):73–99. doi: 10.1002/aja.1001310105. [DOI] [PubMed] [Google Scholar]
- Chang W. W., Leblond C. P. Renewal of the epithelium in the descending colon of the mouse. II. Renewal of argentaffin cells. Am J Anat. 1971 May;131(1):101–109. doi: 10.1002/aja.1001310106. [DOI] [PubMed] [Google Scholar]
- Chejfec G., Gould V. E. Malignant gastric neuroendogrinomas. Ultrastructural and biochemical characterization of their secretory activity. Hum Pathol. 1977 Jul;8(4):433–440. doi: 10.1016/s0046-8177(77)80007-5. [DOI] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am J Anat. 1974 Dec;141(4):503–519. doi: 10.1002/aja.1001410405. [DOI] [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974 Dec;141(4):537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Dische F. E. Argentaffin and non-argentaffin carcinoid tumorurs of the appendix. J Clin Pathol. 1968 Jan;21(1):60–66. doi: 10.1136/jcp.21.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine J., Le Douarin N. M. Analysis of endoderm formation in the avian blastoderm by the use of quail-chick chimaeras. The problem of the neurectodermal origin of the cells of the APUD series. J Embryol Exp Morphol. 1977 Oct;41:209–222. [PubMed] [Google Scholar]
- GIBBS N. M. The histogenesis of carcinoid tumours of the rectum. J Clin Pathol. 1963 May;16:206–214. doi: 10.1136/jcp.16.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallager H. S. View from the giant's shoulder. The fifth annual Wendell G. Scott Lecture. Cancer. 1977 Jul;40(1):185–194. doi: 10.1002/1097-0142(197707)40:1<185::aid-cncr2820400130>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Gibbs N. M. Incidence and significance of argentaffin and paneth cells in some tumours of the large intestine. J Clin Pathol. 1967 Nov;20(6):826–831. doi: 10.1136/jcp.20.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S. L., Toker C. Composite tumor of small intestine. Mt Sinai J Med. 1976 Mar-Apr;43(2):153–156. [PubMed] [Google Scholar]
- Goldenberg D. M., Fisher E. R. Histogenetic relationship between carcinoids and mucin-secreting carcinomas of colon as revealed by heterotransplantation. Br J Cancer. 1970 Sep;24(3):610–614. doi: 10.1038/bjc.1970.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Licea A., Hartmann W. H., Yardley J. H. Medullary carcinoma of the thyroid. Ultrastructural evidence of its origin from the parafollicular cell and its possible relation to carcinoid tumors. Am J Clin Pathol. 1968 Apr;49(4):512–520. doi: 10.1093/ajcp/49.4.512. [DOI] [PubMed] [Google Scholar]
- Hage E. Endocrine cells in the bronchial mucosa of human foetuses. Acta Pathol Microbiol Scand A. 1972;80(2):225–234. doi: 10.1111/j.1699-0463.1972.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Hammar S., Sale G. Multiple hormone producing islet cell carcinomas of the pancreas. A morphological and biochemical investigation. Hum Pathol. 1975 May;6(3):349–362. doi: 10.1016/s0046-8177(75)80097-9. [DOI] [PubMed] [Google Scholar]
- Haqqani M. T., Williams G. Mucin producing carcinoid tumours of the vermiform appendix. J Clin Pathol. 1977 May;30(5):473–480. doi: 10.1136/jcp.30.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez F. J., Reid J. D. Mixed carcinoid and mucus-secreting intestinal tumors. Arch Pathol. 1969 Nov;88(5):489–496. [PubMed] [Google Scholar]
- Holmes E. J. Morphogenesis of gastric adenomatous polyps. Transformation to invasive carcinoma of intestinal type. Cancer. 1966 Jun;19(6):794–802. doi: 10.1002/1097-0142(196606)19:6<794::aid-cncr2820190608>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Ibanez M. L. Medullary carcinoma of the thyroid gland. Pathol Annu. 1974;9(0):263–290. [PubMed] [Google Scholar]
- Jones R. A., MacFarlane A. Carcinomas and carcinoid tumours of the appendix in a district general hospital. J Clin Pathol. 1976 Aug;29(8):687–692. doi: 10.1136/jcp.29.8.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepes J. J. Transitional cell tumor of the pituitary gland developing from a Rathke's cleft cyst. Cancer. 1978 Jan;41(1):337–343. doi: 10.1002/1097-0142(197801)41:1<337::aid-cncr2820410145>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Klein H. Z. Mucinous carcinoid tumor of the vermiform appendix. Cancer. 1974 Mar;33(3):770–777. doi: 10.1002/1097-0142(197403)33:3<770::aid-cncr2820330324>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Kubo T., Watanabe H. Neoplastic argentaffin cells in gastric and intestinal carcinomas. Cancer. 1971 Feb;27(2):447–454. doi: 10.1002/1097-0142(197102)27:2<447::aid-cncr2820270232>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- LAUREN P. THE TWO HISTOLOGICAL MAIN TYPES OF GASTRIC CARCINOMA: DIFFUSE AND SO-CALLED INTESTINAL-TYPE CARCINOMA. AN ATTEMPT AT A HISTO-CLINICAL CLASSIFICATION. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- LAUREN P. The cell structure and secretion in intestinal cancer, with reference to benign epithelial tumours of the bowel. Acta Pathol Microbiol Scand Suppl. 1961;Suppl 152:1–151. [PubMed] [Google Scholar]
- Le Douarin N. M., Teillet M. A. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973 Aug;30(1):31–48. [PubMed] [Google Scholar]
- Le Douarin N., Le Lièvre C., Fontaine J. Recherches expérimentales sur l'origine embryologique du corps carotidien chez ls Oiseaux. C R Acad Sci Hebd Seances Acad Sci D. 1972 Jul 24;275(4):583–586. [PubMed] [Google Scholar]
- Le Douarin N., Teillet M. A. Localisation, par la méthode des greffes interspécifiques du territoire neural dont dérivent les cellules adrénales surrénaliennes chez l'embryon d'oiseau. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jan 18;272(3):481–484. [PubMed] [Google Scholar]
- Le Douarin N., Teillet M. A. Sur quelques aspects de la migration des cellules neurales chez l'embryon de Poulet étudiée par la méthode des greffes hétérospécifiques de tube nerveux. C R Seances Soc Biol Fil. 1970 Sep 25;164(2):390–397. [PubMed] [Google Scholar]
- Like A. A., Orci L. Embryogenesis of the human pancreatic islets: a light and electron microscopic study. Diabetes. 1972;21(2 Suppl):511–534. doi: 10.2337/diab.21.2.s511. [DOI] [PubMed] [Google Scholar]
- Marks L. J., Berde B., Klein L. A., Roth J., Goonan S. R., Blumen D., Nabseth D. C. Inappropriate vasopressin secretion and carcinoma of the pancreas. Am J Med. 1968 Dec;45(6):967–974. doi: 10.1016/0002-9343(68)90196-4. [DOI] [PubMed] [Google Scholar]
- Matsuyama M., Suzuki H. Differentiation of immature mucous cells into parietal, argyrophil, and chief cells in stomach grafts. Science. 1970 Jul 24;169(3943):385–387. doi: 10.1126/science.169.3943.385. [DOI] [PubMed] [Google Scholar]
- McDonald G. S., Hourihane D. O. Mucinous carcinoid tumour of the appendix containing Paneth cells. Ir J Med Sci. 1977 Nov;146(11):386–389. doi: 10.1007/BF03030994. [DOI] [PubMed] [Google Scholar]
- Melmed R. N., Benitez C. J., Holt S. J. Intermediate cells of the pancreas. I. Ultrastructural characterization. J Cell Sci. 1972 Sep;11(2):449–475. doi: 10.1242/jcs.11.2.449. [DOI] [PubMed] [Google Scholar]
- Meyer J. S. Fine structure of two amyloid-forming medullary carcinomas of thyroid. Cancer. 1968 Mar;21(3):406–425. doi: 10.1002/1097-0142(196803)21:3<406::aid-cncr2820210310>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- POEL W. E., YERGANIAN G. Adenocarcinoma of the pancreas in diabetes-prone Chinese hamsters. Am J Med. 1961 Dec;31:861–863. doi: 10.1016/0002-9343(61)90026-2. [DOI] [PubMed] [Google Scholar]
- PREHN R. T. A CLONAL SELECTION THEORY OF CHEMICAL CARCINOGENESIS. J Natl Cancer Inst. 1964 Jan;32:1–17. [PubMed] [Google Scholar]
- Parks T. G. Malignant carcinoid and adenocarcinoma of the stomach. Br J Surg. 1970 May;57(5):377–379. doi: 10.1002/bjs.1800570514. [DOI] [PubMed] [Google Scholar]
- Pearse A. G. Common cytochemical properties of cells producing polypeptide hormones, with particular reference to calcitonin and the thyroid C cells. Vet Rec. 1966 Nov 19;79(21):587–590. doi: 10.1136/vr.79.21.587. [DOI] [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M., Heath C. M. Development, differentiation and derivation of the endocrine polypeptide cells of the mouse pancreas. Immunofluorescence, cytochemical and ultrastructural studies. Diabetologia. 1973 Apr;9(2):120–129. doi: 10.1007/BF01230691. [DOI] [PubMed] [Google Scholar]
- Pearse A. G., Polak J. M. Neural crest origin of the endocrine polypeptide (APUD) cells of the gastrointestinal tract and pancreas. Gut. 1971 Oct;12(10):783–788. doi: 10.1136/gut.12.10.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse A. G. The cytochemistry and ultrastructure of polypeptide hormone-producing cells of the APUD series and the embryologic, physiologic and pathologic implications of the concept. J Histochem Cytochem. 1969 May;17(5):303–313. doi: 10.1177/17.5.303. [DOI] [PubMed] [Google Scholar]
- Pearse A. G. The diffuse neuroendocrine system and the apud concept: related "endocrine" peptides in brain, intestine, pituitary, placenta, and anuran cutaneous glands. Med Biol. 1977 Jun;55(3):115–125. [PubMed] [Google Scholar]
- Pictet R. L., Rall L. B., Phelps P., Rutter W. J. The neural crest and the origin of the insulin-producing and other gastrointestinal hormone-producing cells. Science. 1976 Jan 16;191(4223):191–192. doi: 10.1126/science.1108195. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Pearse A. G., Le Lièvre C., Fontaine J., Le Douarin N. M. Immunocytochemical confirmation of the neural crest origin of avian calcitonin-producing cells. Histochemistry. 1974;40(3):209–214. doi: 10.1007/BF00501955. [DOI] [PubMed] [Google Scholar]
- Pour P. Islet cells as a component of pancreatic ductal neoplasms. I. Experimental study: ductular cells, including islet cell precursors, as primary progenitor cells of tumors. Am J Pathol. 1978 Feb;90(2):295–316. [PMC free article] [PubMed] [Google Scholar]
- Prehn R. T., Prehn L. M. Pathobiology of neoplasia. A teaching monograph. Am J Pathol. 1975 Sep;80(3):529–550. [PMC free article] [PubMed] [Google Scholar]
- Rosai J., Rodriguez H. A. Application of electron microscopy to the differential diagnosis of tumors. Am J Clin Pathol. 1968 Nov;50(5):555–562. doi: 10.1093/ajcp/50.5.555. [DOI] [PubMed] [Google Scholar]
- Schochet S. S., Jr, McCormick W. F., Halmi N. S. Acidophil adenomas with intracytoplasmic filamentous aggregates. A light and electron microscopic study. Arch Pathol. 1972 Jul;94(1):16–22. [PubMed] [Google Scholar]
- Skrabanek P., Powell D. Unifying concept of non-pituitary ACTH-secreting tumors: evidence of common origin of neural-crest tumors, carcinoids, and oat-cell carcinomas. Cancer. 1978 Sep;42(3):1263–1269. doi: 10.1002/1097-0142(197809)42:3<1263::aid-cncr2820420335>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Soga J., Tazawa K., Aizawa O., Wada K., Tuto T. Argentaffin cell adenocarcinoma of the stomach: an atypical carcinoid? Cancer. 1971 Oct;28(4):999–1003. doi: 10.1002/1097-0142(1971)28:4<999::aid-cncr2820280425>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Steinberg M., Cohn I., Jr Primary adenocarcinoma of the appendix. Surgery. 1967 Apr;61(4):644–660. [PubMed] [Google Scholar]
- Subbuswamy S. G., Gibbs N. M., Ross C. F., Morson B. C. Goblet cell carcinoid of the appendix. Cancer. 1974 Aug;34(2):338–344. doi: 10.1002/1097-0142(197408)34:2<338::aid-cncr2820340218>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Tahara E., Haizuka S., Kodama T., Yamada A. The relationship of gastrointestinal endocrine cells to gastric epithelial changes with special reference to gastric cancer. Acta Pathol Jpn. 1975 Mar;25(2):161–177. doi: 10.1111/j.1440-1827.1975.tb00855.x. [DOI] [PubMed] [Google Scholar]
- Teillet M. A., Le Douarin N. La migration des cellules pigmentaires étudiée par la méthode des greffes hétérospécifiques de tube nerveux chez l'embryon d'oiseau. C R Acad Sci Hebd Seances Acad Sci D. 1970 Jun 22;270(25):3095–3098. [PubMed] [Google Scholar]
- Toker C. Observations on the composition of certain colonic tumors. Cancer. 1969 Aug;24(2):256–260. doi: 10.1002/1097-0142(196908)24:2<256::aid-cncr2820240207>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Toker C. Ultrastructure of a chemodectoma. Cancer. 1967 Feb;20(2):271–280. doi: 10.1002/1097-0142(1967)20:2<271::aid-cncr2820200214>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Watanabe H. Argentaffin cells in adenoma of the stomach. Cancer. 1972 Nov;30(5):1267–1274. doi: 10.1002/1097-0142(197211)30:5<1267::aid-cncr2820300519>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Wisniewski M., Toker C. Composite tumor of the gallbladder exhibiting both carcinomatous and carcinoidal patterns. Am J Gastroenterol. 1972 Dec;58(6):633–637. [PubMed] [Google Scholar]
- Wolff M., Ahmed N. Epithelial neoplasms of the vermiform appendix (exclusive of carcinoid). I. Adenocarcinoma of the appendix. Cancer. 1976 May;37(5):2493–2510. doi: 10.1002/1097-0142(197605)37:5<2493::aid-cncr2820370543>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- YOUNG B. A., LEBLOND C. P. THE LIGHT CELL AS COMPARED TO THE FOLLICULAR CELL IN THE THYROID GLAND OF THE RAT. Endocrinology. 1963 Dec;73:669–686. doi: 10.1210/endo-73-6-669. [DOI] [PubMed] [Google Scholar]
- Zweens J., Bouman P. R. Neoformation of insulin-producing islets following ligation of the pancreatic ducts in normal and alloxan-diabetic rats. Acta Physiol Pharmacol Neerl. 1967 Dec;14(4):529–531. [PubMed] [Google Scholar]