Abstract
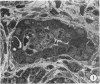
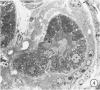
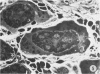
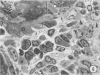
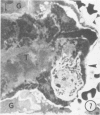
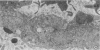
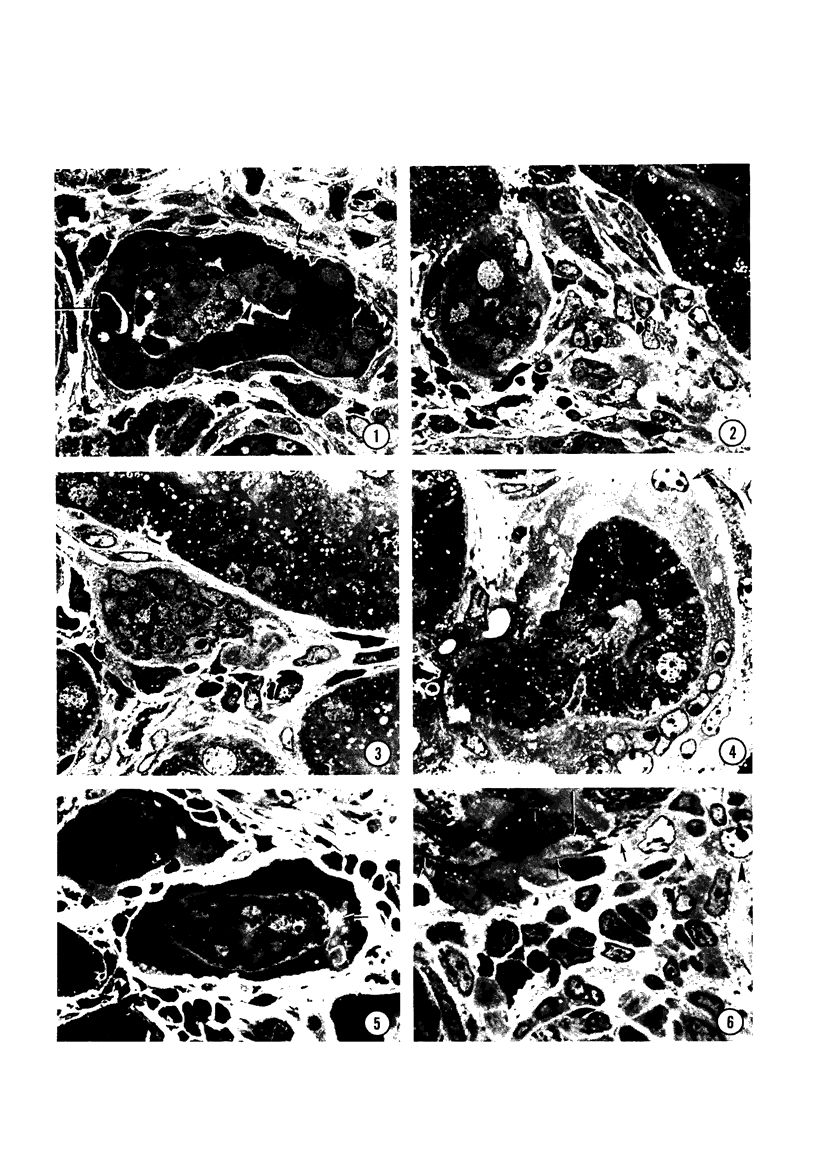
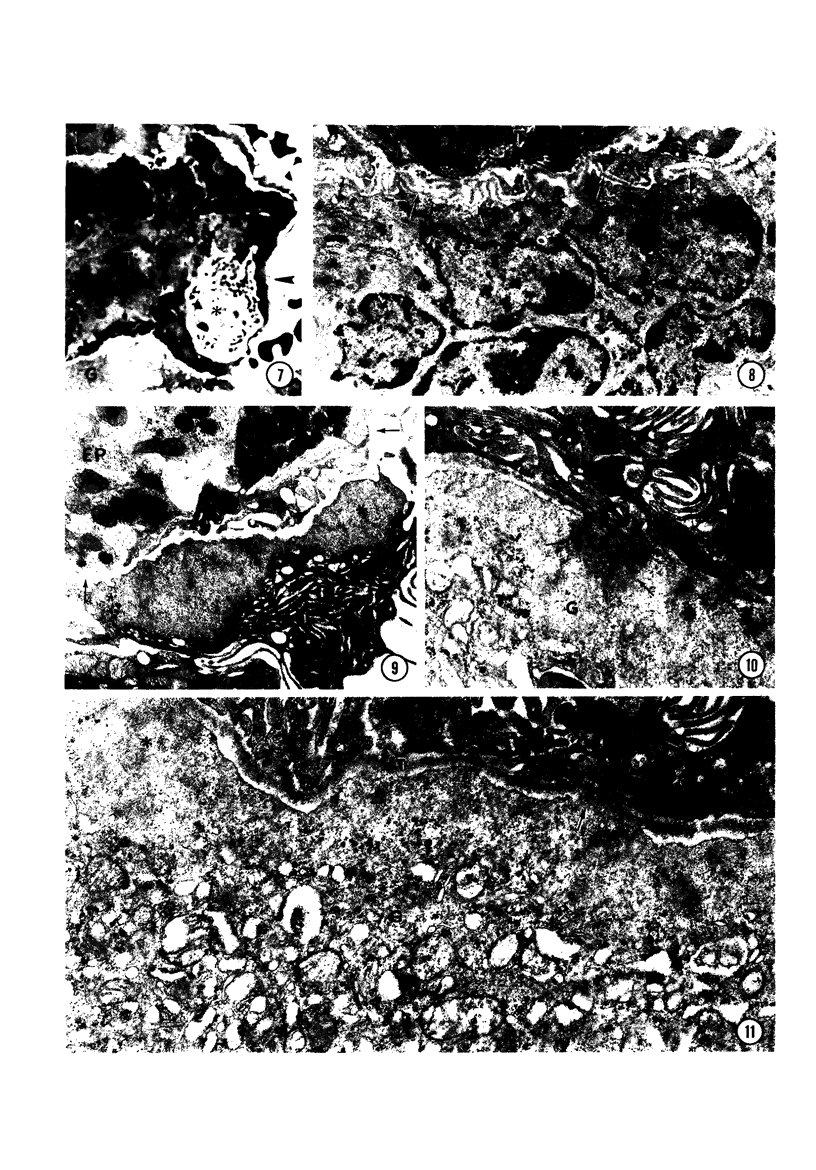
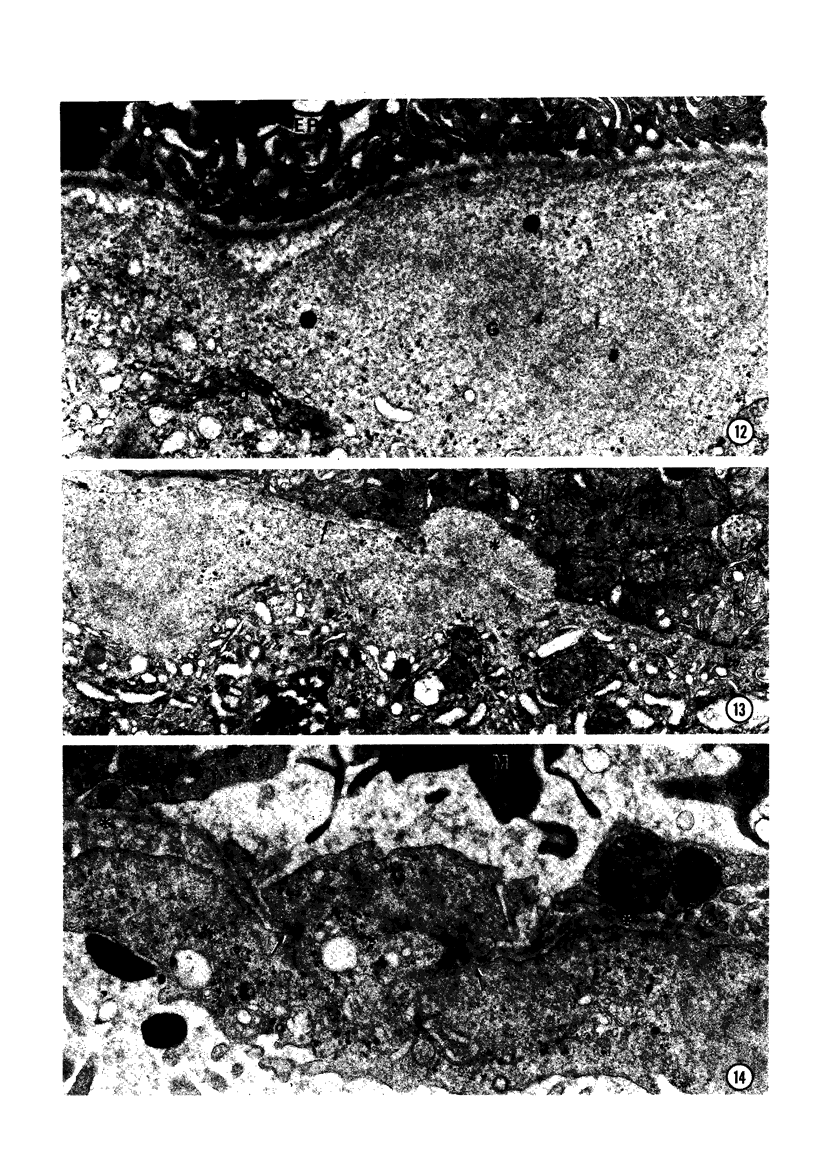
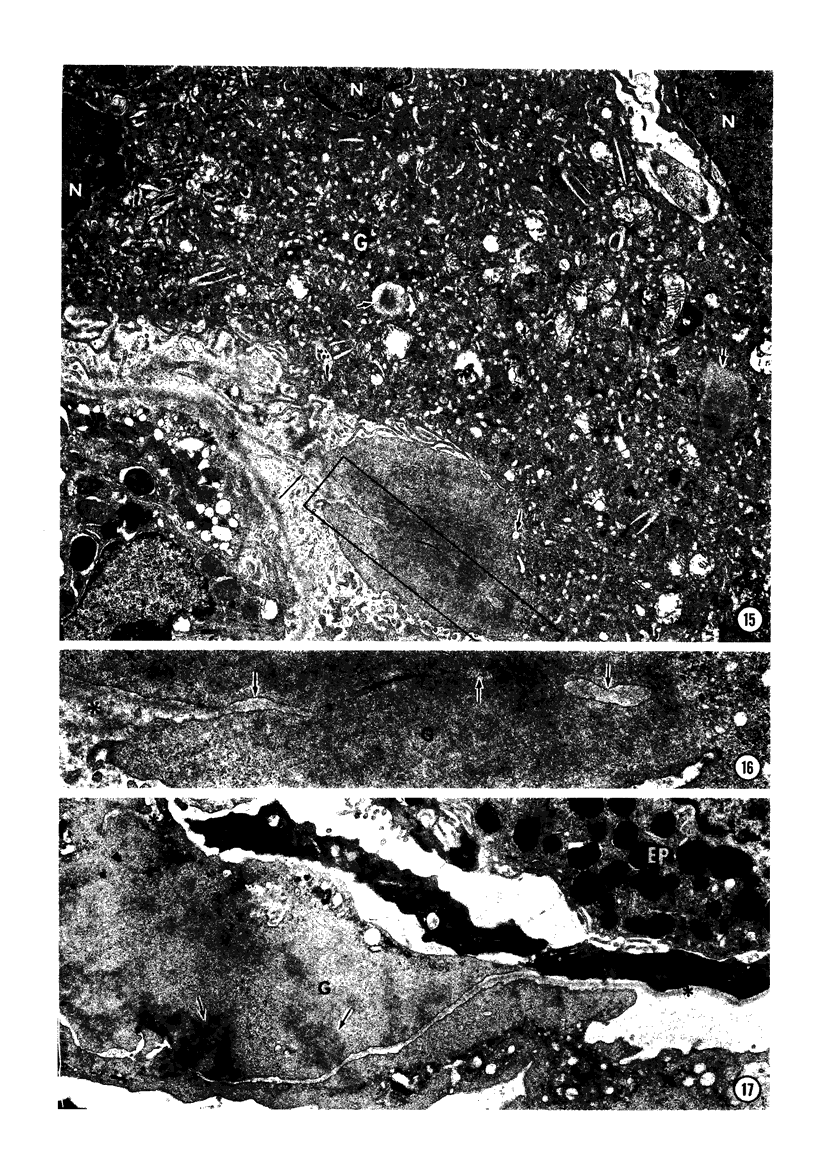
In order to analyze the role of phagocytic cells in experimental antitubular basement membrane (TBM) antibody-mediated nephritis, Hartley guinea pigs (GP) were immunized with rabbit tubular basement membrane (TBM) in complete Freund's adjuvant and pertussis vaccine. Renal tissue was obtained 10 to 15, 15 to 25, and 25 to 35 days after the start of immunization. Severe renal tubulointerstitial (RTI) nephritis developed in 95% of the animals. Linear deposits of IgG and C3 along TBM were seen 10 days after initial immunization. A few days later, monocytes and macrophages infiltrated the interstitium and subsequently differentiated into epithelioid and foreign body-type giant cells (GC). The GC were most actively involved in the destruction of the TBM: Cytoplasmic pseudopodia of the GC adhered to the TBM; the areas of membrane apposition were several microns in length; no evidence of specialization was found in the plasma membrane adjoining the TBM; no cellular organelles, except for abundant microfilaments, were seen in the contact regions. The initial contact was followed by lysis of plasma membrane of the GC and TBM, perforation of TBM, and phagocytosis of TBM fragments. Concomitantly, fluorescent staining for IgG along the TBM became discontinuous or disappeared. Destruction of TBM was accompanied by degeneration of tubular epithelial cells and collapse of tubular architecture. The morphologic observations are consistent with the hypothesis that, in GP, autoimmune RTI nephritis damage of TBM results from the cooperation of humoral and cellular mechanisms, probably akin to those of antibody-mediated lymphocytotoxicity.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Andres G. A., Accinni L., Beiser S. M., Christian C. L., Cinotti G. A., Erlanger B. F., Hsu K. C., Seegal B. C. Localization of fluorescein-labeled antinucleoside antibodies in glomeruli of patients with active systemic lupus erythematosus nephritis. J Clin Invest. 1970 Nov;49(11):2106–2118. doi: 10.1172/JCI106428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins R. C., Holdsworth S. R., Glasgow E. F., Matthews F. E. The macrophagen in human rapidly progressive glomerulonephritis. Lancet. 1976 Apr 17;1(7964):830–832. doi: 10.1016/s0140-6736(76)90480-3. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Schneeberger E. E., Collins A. B., McCluskey R. T. Evidence for a pathogenic role of a cell-mediated immune mechanism in experimental glomerulonephritis. J Exp Med. 1978 Jul 1;148(1):246–260. doi: 10.1084/jem.148.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biberfeld P. Cytotoxic interaction of phytohemagglutinin-stimulated blood lymphocytes with monolayer cells: a study by light and electron microscopy. Cell Immunol. 1971 Feb;2(1):54–72. doi: 10.1016/0008-8749(71)90025-6. [DOI] [PubMed] [Google Scholar]
- Biberfeld P., Johansson A. Contact areas of cytotoxic lymphocytes and target cells. An electron microscopic study. Exp Cell Res. 1975 Aug;94(1):79–87. doi: 10.1016/0014-4827(75)90533-9. [DOI] [PubMed] [Google Scholar]
- COCHRANE C. G., UNANUE E. R., DIXON F. J. A ROLE OF POLYMORPHONUCLEAR LEUKOCYTES AND COMPLEMENT IN NEPHROTOXIC NEPHRITIS. J Exp Med. 1965 Jul 1;122:99–116. doi: 10.1084/jem.122.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerottini J. C., Brunner K. T. Cell-mediated cytotoxicity, allograft rejection, and tumor immunity. Adv Immunol. 1974;18:67–132. doi: 10.1016/s0065-2776(08)60308-9. [DOI] [PubMed] [Google Scholar]
- Cohn Z. A. Macrophage physiology. Fed Proc. 1975 Jul;34(8):1725–1729. [PubMed] [Google Scholar]
- Cohn Z. A. The structure and function of monocytes and macrophages. Adv Immunol. 1968;9:163–214. doi: 10.1016/s0065-2776(08)60443-5. [DOI] [PubMed] [Google Scholar]
- Galindo B., Lazdins J., Castillo R. Fusion of normal rabbit alveolar macrophages induced by supernatant fluids from BCG-sensitized lymph node cells after elicitation by antigen. Infect Immun. 1974 Feb;9(2):212–216. doi: 10.1128/iai.9.2.212-216.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth S. R., Thomson N. M., Glasgow E. F., Dowling J. P., Atkins R. C. Tissue culture of isolated glomeruli in experimental crescentic glomerulonephritis. J Exp Med. 1978 Jan 1;147(1):98–109. doi: 10.1084/jem.147.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka Y., Koshi Y. Electron microscopic study of cell fusion by HVJ virions. Virology. 1968 Mar;34(3):419–434. doi: 10.1016/0042-6822(68)90062-7. [DOI] [PubMed] [Google Scholar]
- Kalina M., Berke G. Contact regions of cytotoxic T lymphocyte-target cell conjugates. Cell Immunol. 1976 Jul;25(1):41–51. doi: 10.1016/0008-8749(76)90095-2. [DOI] [PubMed] [Google Scholar]
- Kalina M., Hollander N. The effect of cytochalasin B on effector--target cell interaction. Quantitative and ultrastructural study. Immunology. 1975 Oct;29(4):709–717. [PMC free article] [PubMed] [Google Scholar]
- Kalowski S., McKay D. G., Howes E. L., Jr, Csavossy I., Wolfson M. Multinucleated giant cells in antiglomerular basement membrane antibody-induced glomerulonephritis. Nephron. 1976;16(6):415–426. doi: 10.1159/000180665. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Shigematsu H., Kobayashi Y. Cellular aspects of rabbit Masugi nephritis. II. Progressive glomerular injuries with crescent formation. Lab Invest. 1972 Dec;27(6):620–631. [PubMed] [Google Scholar]
- Lehman D. H., Wilson C. B., Dixon F. J. Interstitial nephritis in rats immunized with heterologous tubular basement membrane. Kidney Int. 1974 Mar;5(3):187–195. doi: 10.1038/ki.1974.23. [DOI] [PubMed] [Google Scholar]
- Mahieu P., Winand R. J. Chemical structure of tubular and glomerular basement membranes of human kidney. Isolation, purification, carbohydrate and amino acid composition. Eur J Biochem. 1970 Feb;12(3):410–418. doi: 10.1111/j.1432-1033.1970.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Mariano M., Spector W. G. The formation and properties of macrophage polykaryons (inflammatory giant cells). J Pathol. 1974 May;113(1):1–19. doi: 10.1002/path.1711130102. [DOI] [PubMed] [Google Scholar]
- McKeever P. E., Garvin A. J., Hardin D. H., Spicer S. S. Immune complex receptors on cell surfaces. II. Cytochemical evaluation of their abundance on different immune cells: distribution, uptake, and regeneration. Am J Pathol. 1976 Sep;84(3):437–456. [PMC free article] [PubMed] [Google Scholar]
- Plaut M., Lichtenstein L. M., Henney C. S. Studies on the mechanism of lymphocyte-mediated cytolysis. 3. The role of microfilaments and microtubules. J Immunol. 1973 Mar;110(3):771–780. [PubMed] [Google Scholar]
- Rudofsky U. H., Pollara B. Studies on the pathogenesis of experimental autoimmune renal tubulointerstitial disease in guinea pigs. 1. Inhibition of tissue injury in leukocyte-depleted passive transfer recipients. Clin Immunol Immunopathol. 1975 Sep;4(3):425–439. doi: 10.1016/0090-1229(75)90011-2. [DOI] [PubMed] [Google Scholar]
- Rudofsky U. H., Steblay R. W., Pollara B. Inhibition of experimental autoimmune renal tubulointerstitial disease in guinea pigs by depletion of complement with cobra venom factor. Clin Immunol Immunopathol. 1975 Jan;3(3):396–407. doi: 10.1016/0090-1229(75)90027-6. [DOI] [PubMed] [Google Scholar]
- Ryser J. E., Sordat B., Cerottini J. C., Brunner K. T. Mechanism of target cell lysis of cytolytic T lymphocytes. I. Characterization of specific lymphocyte-target cell conjugates separated by velocity sedimentation. Eur J Immunol. 1977 Feb;7(2):110–117. doi: 10.1002/eji.1830070211. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Cotran R. S., Pardo V., Unanue E. R. A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med. 1978 Feb 1;147(2):369–384. doi: 10.1084/jem.147.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu H. Glomerular events during the initial phase of rat Masugi nephritis. Virchows Arch B Cell Pathol. 1970;5(3):187–200. [PubMed] [Google Scholar]
- Spector W. G., Heesom N. The production of granulomata by antigen-antibody complexes. J Pathol. 1969 May;98(1):31–39. doi: 10.1002/path.1710980105. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Studies on the renal glomerular basement membrane. Preparation and chemical composition. J Biol Chem. 1967 Apr 25;242(8):1915–1922. [PubMed] [Google Scholar]
- Steblay R. W., Rudofsky U. Renal tubular disease and autoantibodies against tubular basement membrane induced in guinea pigs. J Immunol. 1971 Aug;107(2):589–594. [PubMed] [Google Scholar]
- Steblay R. W., Rudofsky U. Transfer of experimental autoimmune renal cortical tubular and interstitial disease in guinea pigs by serum. Science. 1973 Jun 1;180(4089):966–968. doi: 10.1126/science.180.4089.966. [DOI] [PubMed] [Google Scholar]
- Sugisaki T., Klassen J., Milgrom F., Andres G. A., McCluskey R. T. Immunopathologic study of an autoimmune tubular and interstitial renal disease in brown Norway rats. Lab Invest. 1973 Jun;28(6):658–671. [PubMed] [Google Scholar]
- Sutton J. S., Weiss L. Transformation of monocytes in tissue culture into macrophages, epithelioid cells, and multinucleated giant cells. An electron microscope study. J Cell Biol. 1966 Feb;28(2):303–332. doi: 10.1083/jcb.28.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Benacerraf B. Immunologic events in experimental hypersensitivity granulomas. Am J Pathol. 1973 Jun;71(3):349–364. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Dixon F. J. Experimental glomerulonephritis: immunological events and pathogenetic mechanisms. Adv Immunol. 1967;6:1–90. doi: 10.1016/s0065-2776(08)60521-0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Secretory function of mononuclear phagocytes: a review. Am J Pathol. 1976 May;83(2):396–418. [PMC free article] [PubMed] [Google Scholar]
- Van Zwieten M. J., Leber P. D., Bhan A. K., McCluskey R. T. Experimental cell-mediated interstitial nephritis induced with exogenous antigens. J Immunol. 1977 Feb;118(2):589–593. [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- Warfel A. H. Macrophage fusion and multinucleated giant cell formation, surface morphology. Exp Mol Pathol. 1978 Apr;28(2):163–176. doi: 10.1016/0014-4800(78)90049-7. [DOI] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]