Abstract
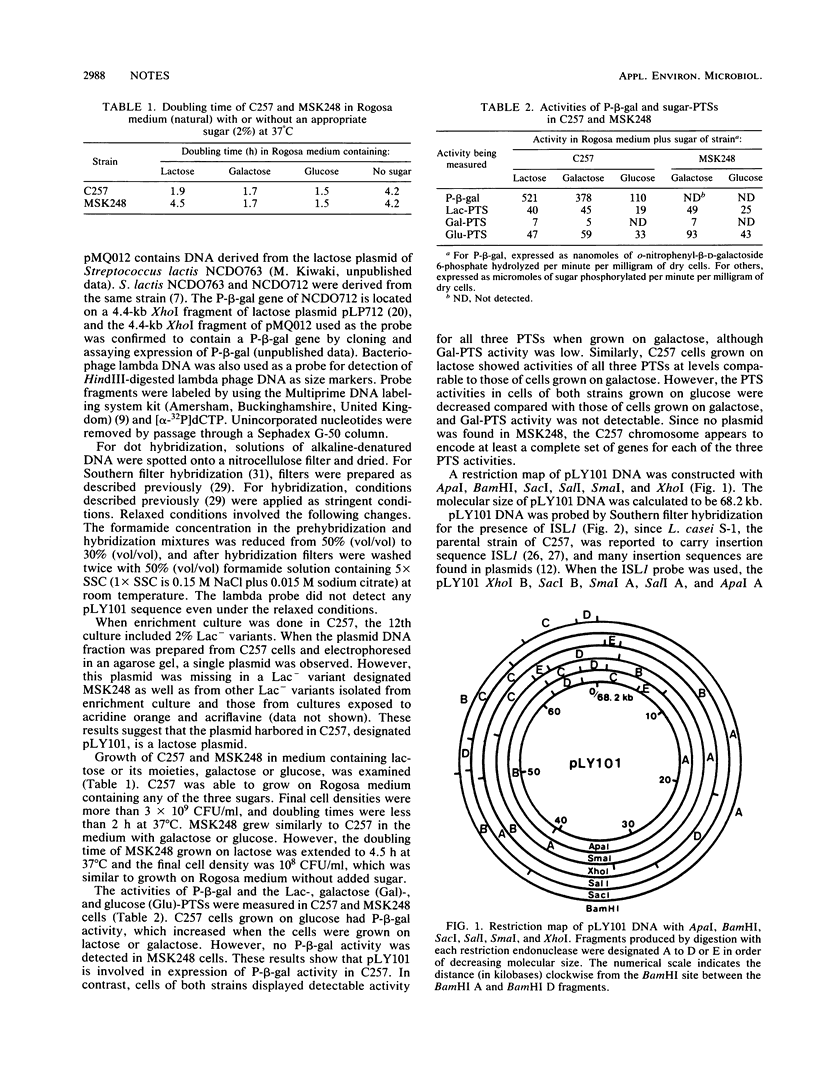
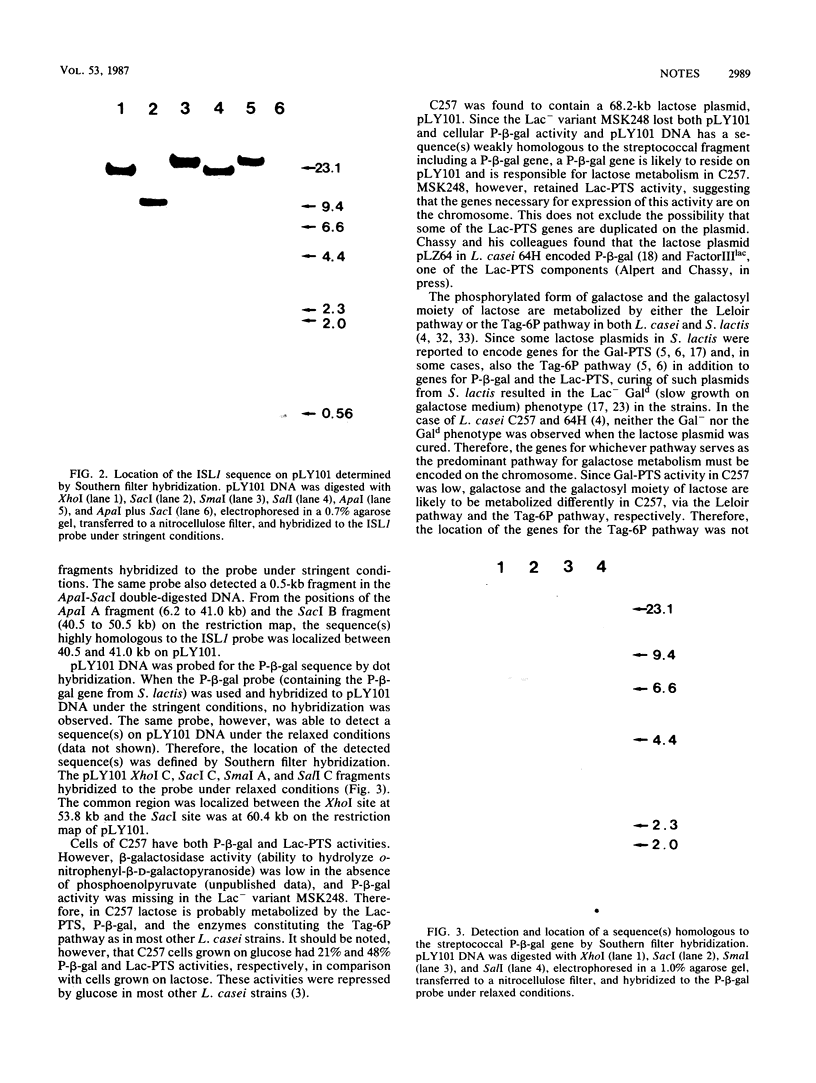
A starter strain, Lactobacillus casei C257, was found to carry a lactose plasmid, pLY101. Restriction mapping showed that pLY101 DNA was 68.2 kilobases long. Since a non-lactose-utilizing variant of C257, MSK248, lost phospho-β-galactosidase (P-β-gal) activity and pLY101 DNA had a sequence(s) homologous to the streptococcal fragment including a P-β-gal gene, pLY101 is likely to encode a P-β-gal gene required for lactose metabolism in C257. MSK248 grew in galactose medium at a rate identical to that of C257 and retained phosphoenolpyruvate-dependent phosphotransferase system activity for lactose similar to that of C257. Therefore, the C257 chromosome appears to encode a complete set of genes for the lactose-phosphotransferase system and the predominant galactose metabolic pathway in C257. pLY101 DNA had a sequence homologous to a lactobacillus insertion sequence, ISL1, which mapped more than 12 kilobases from the sequence homologous to the streptococcal P-β-gal fragment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Thompson J. Regulation and characterization of the galactose-phosphoenolpyruvate-dependent phosphotransferase system in Lactobacillus casei. J Bacteriol. 1983 Jun;154(3):1204–1214. doi: 10.1128/jb.154.3.1204-1214.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M., Thompson J. Regulation of lactose-phosphoenolpyruvate-dependent phosphotransferase system and beta-D-phosphogalactoside galactohydrolase activities in Lactobacillus casei. J Bacteriol. 1983 Jun;154(3):1195–1203. doi: 10.1128/jb.154.3.1195-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow V. L., Davey G. P., Pearce L. E., Thomas T. D. Plasmid linkage of the D-tagatose 6-phosphate pathway in Streptococcus lactis: effect on lactose and galactose metabolism. J Bacteriol. 1983 Jan;153(1):76–83. doi: 10.1128/jb.153.1.76-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFTHYMIOU C., HANSEN P. A. An antigenic analysis of Lactobacillus acidophilus. J Infect Dis. 1962 May-Jun;110:258–267. doi: 10.1093/infdis/110.3.258. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Harlander S. K., McKay L. L., Schachtele C. F. Molecular cloning of the lactose-metabolizing genes from Streptococcus lactis. Appl Environ Microbiol. 1984 Aug;48(2):347–351. doi: 10.1128/aem.48.2.347-351.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine J. M., Lee L. N., LeBlanc D. J. Molecular and genetic characterization of lactose-metabolic genes of Streptococcus cremoris. J Bacteriol. 1986 Sep;167(3):855–862. doi: 10.1128/jb.167.3.855-862.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M., Mada M., Ishiwa H. Protoplast fusion of Lactobacillus fermentum. Appl Environ Microbiol. 1986 Aug;52(2):392–393. doi: 10.1128/aem.52.2.392-393.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek. 1983 Sep;49(3):209–224. doi: 10.1007/BF00399499. [DOI] [PubMed] [Google Scholar]
- LeBlanc D. J., Crow V. L., Lee L. N., Garon C. F. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J Bacteriol. 1979 Feb;137(2):878–884. doi: 10.1128/jb.137.2.878-884.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. J., Hansen J. B., Jagusztyn-Krynicka E. K., Chassy B. M. Cloning and expression of the beta-D-phosphogalactoside galactohydrolase gene of Lactobacillus casei in Escherichia coli K-12. J Bacteriol. 1982 Dec;152(3):1138–1146. doi: 10.1128/jb.152.3.1138-1146.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S., Gasson M. J. Cloning, expression and location of the Streptococcus lactis gene for phospho-beta-D-galactosidase. J Gen Microbiol. 1986 Feb;132(2):331–340. doi: 10.1099/00221287-132-2-331. [DOI] [PubMed] [Google Scholar]
- McKay L. L. Functional properties of plasmids in lactic streptococci. Antonie Van Leeuwenhoek. 1983 Sep;49(3):259–274. doi: 10.1007/BF00399502. [DOI] [PubMed] [Google Scholar]
- Park Y. H., McKay L. L. Distinct galactose phosphoenolpyruvate-dependent phosphotransferase system in Streptococcus lactis. J Bacteriol. 1982 Feb;149(2):420–425. doi: 10.1128/jb.149.2.420-425.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premi L., Sandine W. E., Elliker P. R. Lactose-hydrolyzing enzymes of Lactobacillus species. Appl Microbiol. 1972 Jul;24(1):51–57. doi: 10.1128/am.24.1.51-57.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Kadota M., Kiwaki M., Hirokawa H., Tsuchida N. ISL1: a new transposable element in Lactobacillus casei. Mol Gen Genet. 1985;200(2):193–198. doi: 10.1007/BF00425423. [DOI] [PubMed] [Google Scholar]
- Shimizu-Kadota M., Sakurai T. Prophage Curing in Lactobacillus casei by Isolation of a Thermoinducible Mutant. Appl Environ Microbiol. 1982 Jun;43(6):1284–1287. doi: 10.1128/aem.43.6.1284-1287.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Kadota M., Sakurai T., Tsuchida N. Prophage Origin of a Virulent Phage Appearing on Fermentations of Lactobacillus casei S-1. Appl Environ Microbiol. 1983 Feb;45(2):669–674. doi: 10.1128/aem.45.2.669-674.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Kadota M., Tsuchida N. Physical mapping of the virion and the prophage DNAs of a temperate Lactobacillus phage phi FSW. J Gen Microbiol. 1984 Feb;130(2):423–430. doi: 10.1099/00221287-130-2-423. [DOI] [PubMed] [Google Scholar]
- Thompson J. Galactose transport systems in Streptococcus lactis. J Bacteriol. 1980 Nov;144(2):683–691. doi: 10.1128/jb.144.2.683-691.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]