Abstract
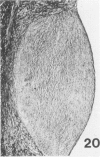
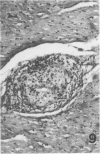
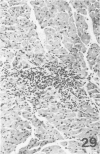
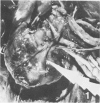
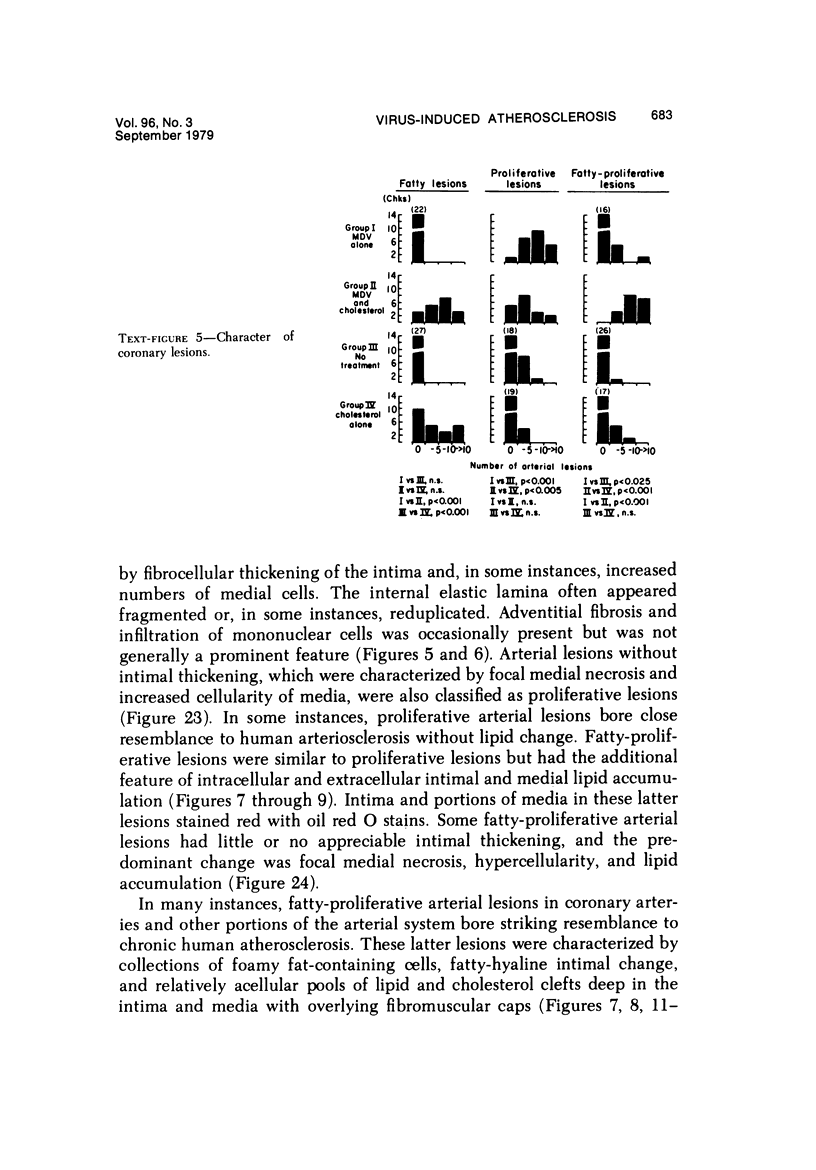
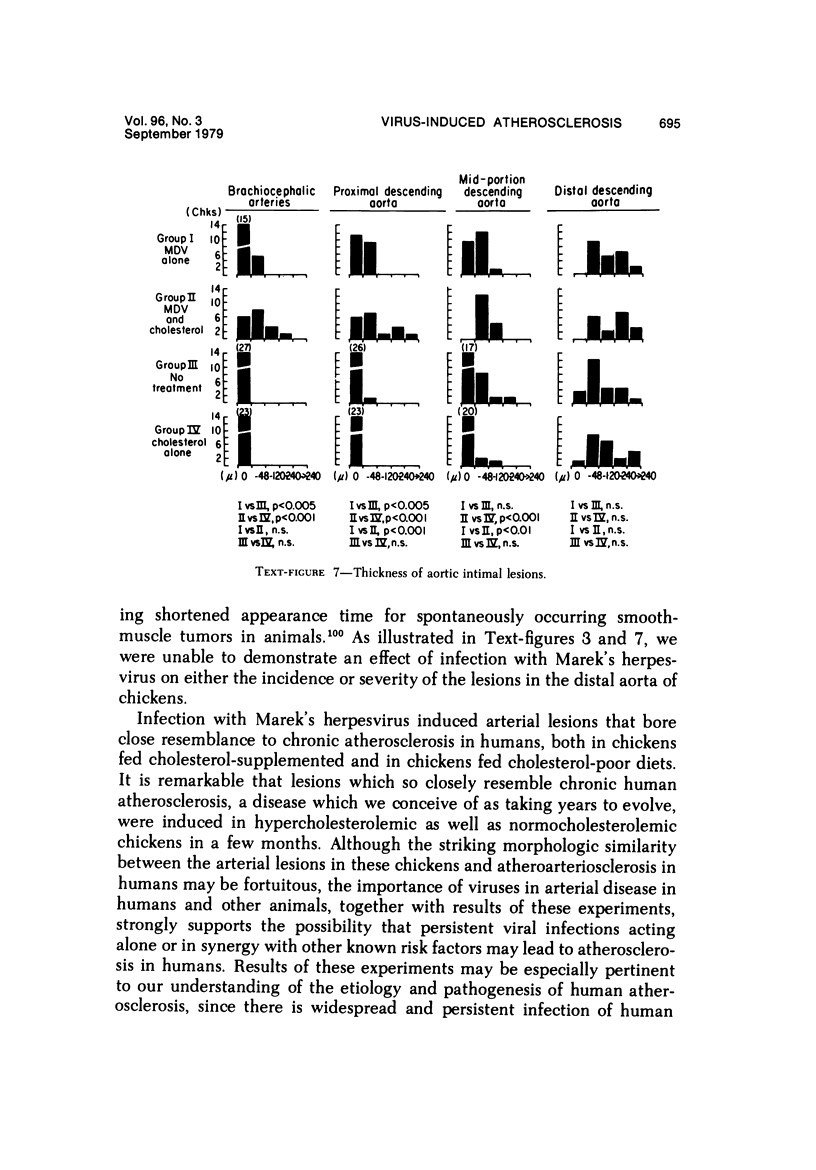
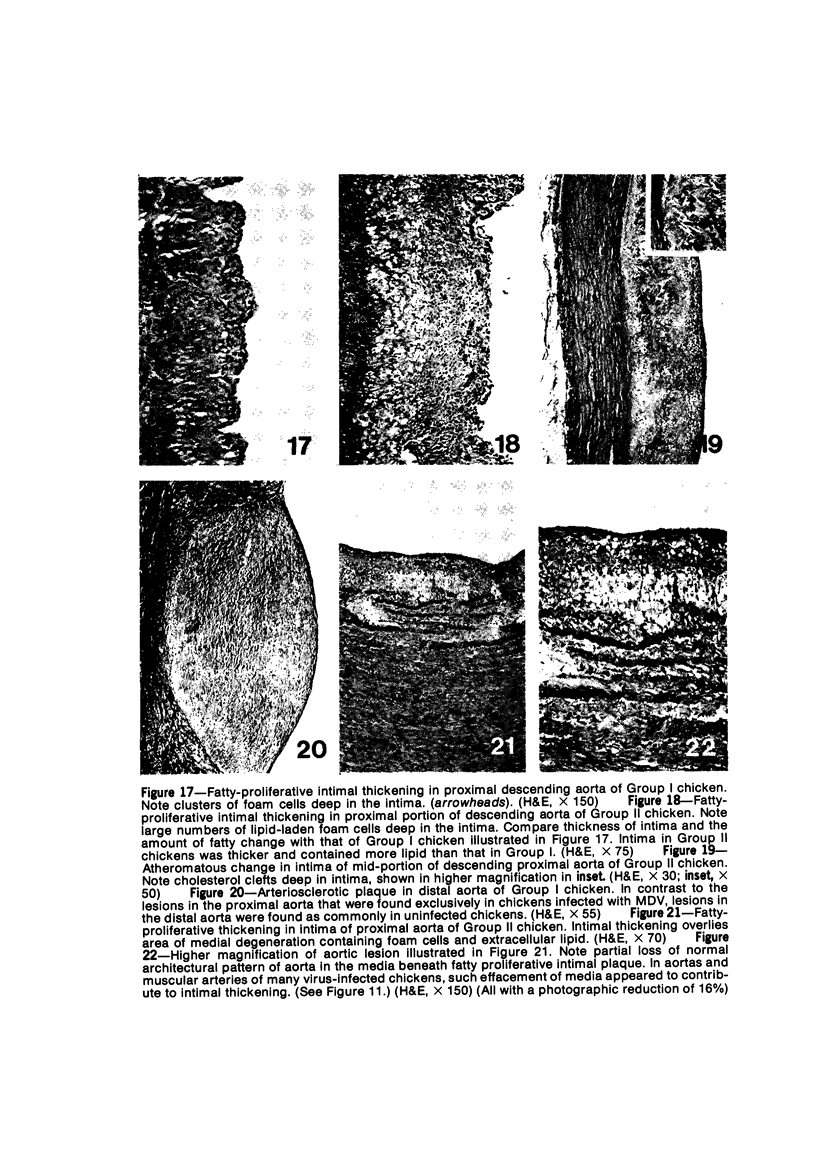
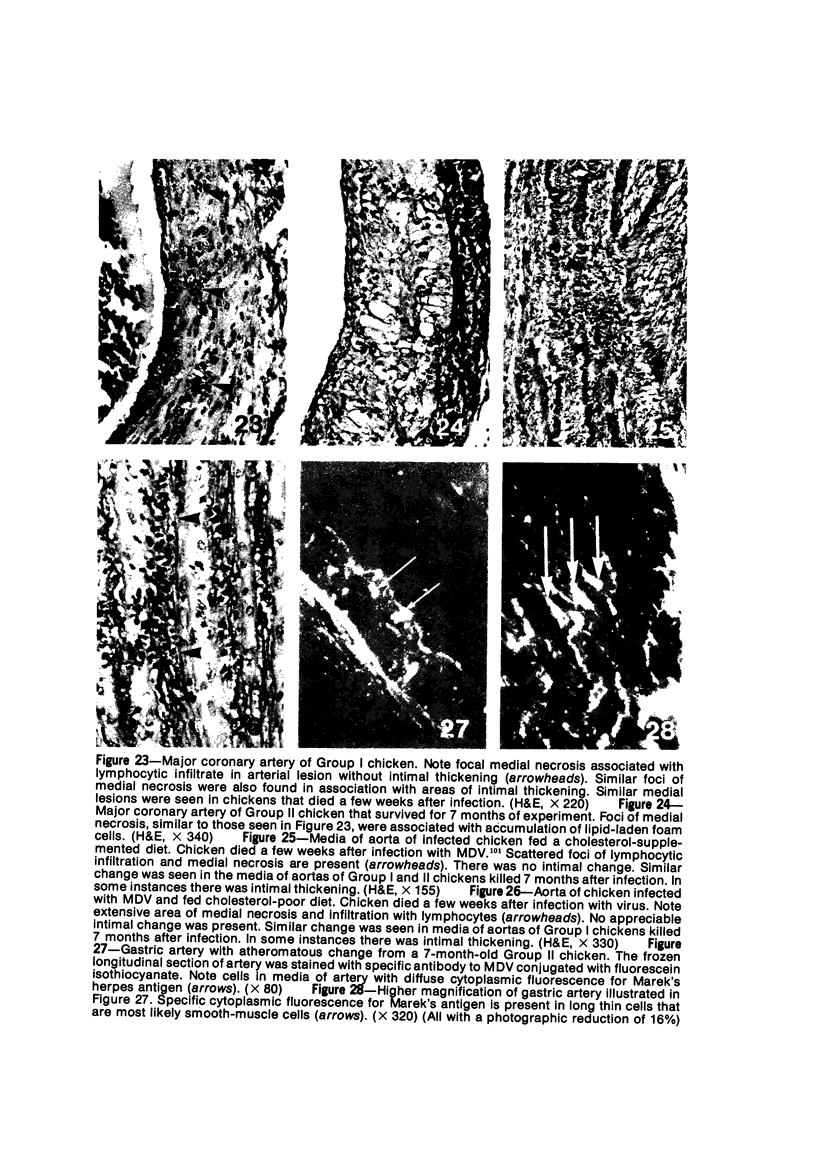
Atheroarteriosclerosis closely resembling that in humans was induced in normocholesterolemic and hypercholesterolemic chickens by infection with Marek's disease herpesvirus (MDV). Four comparably sized groups of chickens were used. Each group was initially fed a diet relatively poor in cholesterol. Group I and II were inoculated intratracheally at 2 days of age with MDV. At 15 weeks, one group of virus-infected chickens (Group II) and one group of uninfected controls (Group IV) were fed a 2% cholesterol supplement for an additional 15 weeks. Group I, infected, and III, uninfected, were continued on a cholesterol-poor diet. All groups were killed at 30 weeks. Striking grossly visible atherosclerotic lesions were seen in large coronary arteries, aortas, and major aortic branches of both Groups I and II but not in those of Groups III and IV. Microscopically, arterial changes in infected animals were characterized by occlusive fibromuscular intimal thickening, which formed fibrous caps overlying areas of atheromatous change. This change closely resembled chronic atherosclerosis in humans. These results may be important to our understanding of human arteriosclerosis, since there is widespread and persistent infection of human populations with as many as five herpesviruses.
Full text
PDF


























Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert R. E., Vanderlaan M., Burns F. J., Nishizumi M. Effect of carcinogens on chicken atherosclerosis. Cancer Res. 1977 Jul;37(7 Pt 1):2232–2235. [PubMed] [Google Scholar]
- Alexander N. J., Clarkson T. B. Vasectomy increases the severity of diet-induced atherosclerosis in Macaca fascicularis. Science. 1978 Aug 11;201(4355):538–541. doi: 10.1126/science.96532. [DOI] [PubMed] [Google Scholar]
- Alonso D. R., Starek P. K., Minick C. R. Studies on the pathogenesis of atheroarteriosclerosis induced in rabbit cardiac allografts by the synergy of graft rejection and hypercholesterolemia. Am J Pathol. 1977 May;87(2):415–442. [PMC free article] [PubMed] [Google Scholar]
- BURKHOLDER P. M., LITTELL A. H., KLEIN P. G. Sectioning at room temperature of unfixed tissues, frozen in a gelatin matrix, for immunohistologic procedures. Stain Technol. 1961 Mar;36:89–91. doi: 10.3109/10520296109113250. [DOI] [PubMed] [Google Scholar]
- Becker C. G., Dubin T., Wiedemann H. P. Hypersensitivity to tobacco antigen. Proc Natl Acad Sci U S A. 1976 May;73(5):1712–1716. doi: 10.1073/pnas.73.5.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P. Implications of the monoclonal character of human atherosclerotic plaques. Ann N Y Acad Sci. 1976;275:96–100. doi: 10.1111/j.1749-6632.1976.tb43342.x. [DOI] [PubMed] [Google Scholar]
- Bulkley B. H., Roberts W. C. The heart in systemic lupus erythematosus and the changes induced in it by corticosteroid therapy. A study of 36 necropsy patients. Am J Med. 1975 Feb;58(2):243–264. doi: 10.1016/0002-9343(75)90575-6. [DOI] [PubMed] [Google Scholar]
- Burch G. E. Editorial: Viruses and arteriosclerosis. Am Heart J. 1974 Apr;87(4):407–412. doi: 10.1016/0002-8703(74)90163-x. [DOI] [PubMed] [Google Scholar]
- Burch G. E., Harb J. M., Hiramoto Y., Shewey L. Viral infection of the aorta of man associated with early atherosclerotic changes. Am Heart J. 1973 Oct;86(4):523–534. doi: 10.1016/0002-8703(73)90150-6. [DOI] [PubMed] [Google Scholar]
- Burch G. E., Rayburn P. EMC viral infection of the coronary blood vessels in newborn mice: viral vasculitis. Br J Exp Pathol. 1977 Oct;58(5):565–571. [PMC free article] [PubMed] [Google Scholar]
- Calnek B. W. Effects of passive antibody on early pathogenesis of Marek's disease. Infect Immun. 1972 Aug;6(2):193–198. doi: 10.1128/iai.6.2.193-198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. E., Loria R. M., Madge G. E. Coxsackievirus B cardiopathy and angiopathy in the hypercholesterolemic host. Atherosclerosis. 1978 Nov;31(3):295–306. doi: 10.1016/0021-9150(78)90065-5. [DOI] [PubMed] [Google Scholar]
- Cole R. K. Studies on genetic resistance to Marek's disease. Avian Dis. 1968 Feb;12(1):9–28. [PubMed] [Google Scholar]
- Curwen K. D., Smith S. C. Aortic glycosaminoglycans in atherosclerosis-susceptible and -resistant pigeons. Exp Mol Pathol. 1977 Aug;27(1):121–133. doi: 10.1016/0014-4800(77)90024-7. [DOI] [PubMed] [Google Scholar]
- Fabricant C. G., Fabricant J., Litrenta M. M., Minick C. R. Virus-induced atherosclerosis. J Exp Med. 1978 Jul 1;148(1):335–340. doi: 10.1084/jem.148.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricant C. G., Krook L., Gillespie J. H. Virus-induced cholesterol crystals. Science. 1973 Aug 10;181(4099):566–567. doi: 10.1126/science.181.4099.566. [DOI] [PubMed] [Google Scholar]
- Fabricant J., Ianconescu M., Calnek B. W. Comparative effects of host and viral factors on early pathogenesis of Marek's disease. Infect Immun. 1977 Apr;16(1):136–144. doi: 10.1128/iai.16.1.136-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher-Dzoga K., Chen R., Wissler R. W. Effects of serum lipoproteins on the morphology, growth, and metabolism of arterial smooth muscle cells. Adv Exp Med Biol. 1974;43(0):299–311. doi: 10.1007/978-1-4684-3243-5_15. [DOI] [PubMed] [Google Scholar]
- Friedman R. J., Moore S., Singal D. P. Repeated endothelial injury and induction of atherosclerosis in normolipemic rabbits by human serum. Lab Invest. 1975 Mar;32(3):404–415. [PubMed] [Google Scholar]
- Gordon T., Garcia-Palmieri M. R., Kagan A., Kannel W. B., Schiffman J. Differences in coronary heart disease in Framingham, Honolulu and Puerto Rico. J Chronic Dis. 1974 Sep;27(7-8):329–344. doi: 10.1016/0021-9681(74)90013-7. [DOI] [PubMed] [Google Scholar]
- HARKAVY J., PERLMAN E. TOBACCO ALLERGY IN CORONARY ARTERY DISEASE. N Y State J Med. 1964 Jun 1;64:1287–1296. [PubMed] [Google Scholar]
- HAYMAKER W., GIRDANY B. R., STEPHENS J., LILLIE R. D., FETTERMAN G. H. Cerebral involvement with advanced periventricular calcification in generalized cytomegalic inclusion disease in the newborn; a clinicopathological report of a case diagnosed during life. J Neuropathol Exp Neurol. 1954 Oct;13(4):562–586. doi: 10.1093/jnen/13.4.562. [DOI] [PubMed] [Google Scholar]
- HINZE H. C., WALKER D. L. Occurrence of focal three-dimensional proliferation in cultured human cells after prolonged infection with herpes simplex virus. J Exp Med. 1961 May 1;113:885–898. doi: 10.1084/jem.113.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas J. E., Yunis E. J. Viral crystalline arrays in human coxsackie myocarditis. Lab Invest. 1970 Oct;23(4):442–446. [PubMed] [Google Scholar]
- Hardin N. J., Minick C. R., Murphy G. E. Experimental induction of atheroarteriosclerosis by the synergy of allergic injury to arteries and lipid-rich diet. 3. The role of earlier acquired fibromuscular intimal thickening in the pathogenesis of later developing atherosclerosis. Am J Pathol. 1973 Nov;73(2):301–326. [PMC free article] [PubMed] [Google Scholar]
- Harker L. A., Slichter S. J., Scott C. R., Ross R. Homocystinemia. Vascular injury and arterial thrombosis. N Engl J Med. 1974 Sep 12;291(11):537–543. doi: 10.1056/NEJM197409122911101. [DOI] [PubMed] [Google Scholar]
- Howard A. N., Patelski J., Bowyer D. E., Gresham G. A. Atherosclerosis induced in hypercholesterolaemic baboons by immunological injury; and the effects of intravenous polyunsaturated phosphatidyl choline. Atherosclerosis. 1971 Jul-Aug;14(1):17–29. doi: 10.1016/0021-9150(71)90035-9. [DOI] [PubMed] [Google Scholar]
- Iverius P. H. The interaction between human plasma lipoproteins and connective tissue glycosaminoglycans. J Biol Chem. 1972 Apr 25;247(8):2607–2613. [PubMed] [Google Scholar]
- Kilham L., Margolis G. Spontaneous hepatitis and cerebellar "hypoplasia" in suckling rats due to congenital infections with rat virus. Am J Pathol. 1966 Sep;49(3):457–475. [PMC free article] [PubMed] [Google Scholar]
- Kim E., Goldberg M. Serum cholesterol assay using a stable Liebermann-Burchard reagent. Clin Chem. 1969 Dec;15(12):1171–1179. [PubMed] [Google Scholar]
- Konno S., Yamamoto H. Pathology of equine infectious anemia. Proposed classification of pathological types of disease. Cornell Vet. 1970 Jul;60(3):393–449. [PubMed] [Google Scholar]
- Liggitt H. D., DeMartini J. C., McChesney A. E., Pierson R. E., Storz J. Experimental transmission of malignant catarrhal fever in cattle: gross and histopathologic changes. Am J Vet Res. 1978 Aug;39(8):1249–1257. [PubMed] [Google Scholar]
- MAURER F. D., GRIESEMER R. A. The pathology of African swine fever; a comparison with hog cholera. Am J Vet Res. 1958 Jul;19(72):517–539. [PubMed] [Google Scholar]
- MURPHY T. O., HAGLIN J. J., FELDER D. A. The progression of experimental atherosclerosis after lumbar sympathectomy. Surg Forum. 1957;7:332–336. [PubMed] [Google Scholar]
- Margolis G., Jacobs L. R., Kilham L. Oxygen tension and the selective tropism of K-virus for mouse pulmonary endothelium. Am Rev Respir Dis. 1976 Jul;114(1):45–51. doi: 10.1164/arrd.1976.114.1.45. [DOI] [PubMed] [Google Scholar]
- Margolis G., Kilham L. Parvovirus infections, vascular endothelium, and hemorrhagic encephalopathy. Lab Invest. 1970 May;22(5):478–488. [PubMed] [Google Scholar]
- Mark-Malchoff D., Marinetti G. V., Hare J. D., Meisler A. Cholesterol content and metabolism in normal and polyoma virus-transformed hamster embryo fibroblasts. Exp Cell Res. 1979 Feb;118(2):377–381. doi: 10.1016/0014-4827(79)90161-7. [DOI] [PubMed] [Google Scholar]
- Mcleod C. G., Stookey J. L., Eddy G. A., Scott K. Pathology of chronic Bolivian hemorrhagic fever in the rhesus monkey. Am J Pathol. 1976 Aug;84(2):211–224. [PMC free article] [PubMed] [Google Scholar]
- Michalak T. Immune complexes of hepatitis B surface antigen in the pathogenesis of periarteritis nodosa. A study of seven necropsy cases. Am J Pathol. 1978 Mar;90(3):619–632. [PMC free article] [PubMed] [Google Scholar]
- Minick C. R. Immunologic arterial injury in atherogenesis. Ann N Y Acad Sci. 1976;275:210–227. doi: 10.1111/j.1749-6632.1976.tb43355.x. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Murphy G. E., Campbell W. G., Jr Experimental induction of athero-arteriosclerosis by the synergy of allergic injury to arteries and lipid-rich diet. I. Effect of repeated injections of horse serum in rabbits fed a dietary cholesterol supplement. J Exp Med. 1966 Oct 1;124(4):635–652. doi: 10.1084/jem.124.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minick C. R., Murphy G. E. Experimental induction of atheroarteriosclerosis by the synergy of allergic injury to arteries and lipid-rich diet. II. Effect of repeatedly injected foreign protein in rabbits fed a lipid-rich, cholesterol-poor diet. Am J Pathol. 1973 Nov;73(2):265–300. [PMC free article] [PubMed] [Google Scholar]
- Minick C. R., Stemerman M. B., Insull W., Jr Role of endothelium and hypercholesterolemia in intimal thickening and lipid accumulation. Am J Pathol. 1979 Apr;95(1):131–158. [PMC free article] [PubMed] [Google Scholar]
- Minick C. R., Stemerman M. G., Insull W., Jr Effect of regenerated endothelium on lipid accumulation in the arterial wall. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1724–1728. doi: 10.1073/pnas.74.4.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Ihnatowycz T. O. Vessel injury and atherosclerosis. Adv Exp Med Biol. 1978;102:145–161. doi: 10.1007/978-1-4757-1217-9_9. [DOI] [PubMed] [Google Scholar]
- Moss N. S., Benditt E. P. The ultrastructure of spontaneous and experimentally induced arterial lesions. II. The spontaneous plaque in the chicken. Lab Invest. 1970 Sep;23(3):231–245. [PubMed] [Google Scholar]
- Murphy F. A., Buchmeier M. J., Rawls W. E. The reticuloendothelium as the target in a virus infection. Pichinde virus pathogenesis in two strains of hamsters. Lab Invest. 1977 Nov;37(5):502–515. [PubMed] [Google Scholar]
- Nahmias A. J., Josey W. E., Naib Z. M., Freeman M. G., Fernandez R. J., Wheeler J. H. Perinatal risk associated with maternal genital herpes simplex virus infection. Am J Obstet Gynecol. 1971 Jul 15;110(6):825–837. doi: 10.1016/0002-9378(71)90580-1. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Immune complex disease in chronic viral infections. J Exp Med. 1971 Sep 1;134(3 Pt 2):32s–40s. [PubMed] [Google Scholar]
- Payne L. N., Frazier J. A., Powell P. C. Pathogenesis of Marek's disease. Int Rev Exp Pathol. 1976;16:59–154. [PubMed] [Google Scholar]
- Pearson T. A., Dillman J., Solez K., Heptinstall R. H. Monoclonal characteristics of organising arterial thrombi: Significance in the origin and growth of human atherosclerotic plaques. Lancet. 1979 Jan 6;1(8106):7–11. doi: 10.1016/s0140-6736(79)90453-7. [DOI] [PubMed] [Google Scholar]
- Pearson T. A., Kramer E. C., Solez K., Heptinstall R. H. The human atherosclerotic plaque. Am J Pathol. 1977 Mar;86(3):657–664. [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. 3. Immune complex arteritis. Am J Pathol. 1973 May;71(2):331–344. [PMC free article] [PubMed] [Google Scholar]
- Rider A. K., Copeland J. G., Hunt S. A., Mason J., Specter M. J., Winkle R. A., Bieber C. P., Billingham M. E., Dong E., Jr, Griepp R. B. The status of cardiac transplantation, 1975. Circulation. 1975 Oct;52(4):531–539. doi: 10.1161/01.cir.52.4.531. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science. 1973 Jun 29;180(4093):1332–1339. doi: 10.1126/science.180.4093.1332. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med. 1976 Aug 12;295(7):369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- Ross R., Harker L. Hyperlipidemia and atherosclerosis. Science. 1976 Sep 17;193(4258):1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- Santerre R. F., Wight T. N., Smith S. C., Brannigan D. Spontaneous atherosclerosis in pigeons. A model system for studying metabolic parameters associated with atherogenesis. Am J Pathol. 1972 Apr;67(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- Satoh C., Duff R., Rapp F., Davidson E. A. Production of mucopolysaccharides by normal and transformed cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):54–56. doi: 10.1073/pnas.70.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma H. M., Geer J. C. Experimental aortic lesions of acute serum sickness in rabbits. Am J Pathol. 1977 Aug;88(2):255–266. [PMC free article] [PubMed] [Google Scholar]
- Spaet T. H., Rhee C., Geiger C. Delayed consequences of endothelial removal from rabbit aortae. Adv Exp Med Biol. 1978;102:165–173. doi: 10.1007/978-1-4757-1217-9_10. [DOI] [PubMed] [Google Scholar]
- Spencer J. L. Marek's disease herpesvirus: comparison of foci (macro) in infected duck embryo fibroblasts under agar medium with foci (micro) in chicken cells. Avian Dis. 1970 Nov;14(4):565–578. [PubMed] [Google Scholar]
- Stemerman M. B., Ross R. Experimental arteriosclerosis. I. Fibrous plaque formation in primates, an electron microscope study. J Exp Med. 1972 Oct 1;136(4):769–789. doi: 10.1084/jem.136.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD P. H., LINDSAY J. R., WARNER N. E. CYTOMEGALIC INCLUSION DISEASE AFFECTING THE TEMPORAL BONE. Laryngoscope. 1965 Apr;75:628–638. doi: 10.1288/00005537-196504000-00005. [DOI] [PubMed] [Google Scholar]
- WELLER T. H., HANSHAW J. B. Virologic and clinical observations on cytomegalic inclusion disease. N Engl J Med. 1962 Jun 14;266:1233–1244. doi: 10.1056/NEJM196206142662401. [DOI] [PubMed] [Google Scholar]
- Woodard J. F., Srinivasan S. R., Zimny M. L., Radhakrishnamurphy B., Berenson G. S. Electron microscopic features of lipoprotein-glycosaminoglycan complexes from human atherosclerotic plaques. Lab Invest. 1976 May;34(5):516–521. [PubMed] [Google Scholar]
- Yoshiki T., Mellors R. C., Strand M., August J. T. The viral envelope glycoprotein of murine leukemia virus and the pathogenesis of immune complex glomerulonephritis of New Zealand mice. J Exp Med. 1974 Oct 1;140(4):1011–1027. doi: 10.1084/jem.140.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAK F. G., HELPERN M., ADLERSBERG D. Stenosing coronary arteritis; its possible role in coronary artery disease. Angiology. 1952 Aug;3(4):289–305. doi: 10.1177/000331975200300404. [DOI] [PubMed] [Google Scholar]
- Zakarian B., Barlow R. M., Rennie J. C., Head K. W. Periarteritis in experimental Border disease of sheep. II. Morphology and histochemistry of the lesion. J Comp Pathol. 1976 Jul;86(3):477–487. doi: 10.1016/0021-9975(76)90016-5. [DOI] [PubMed] [Google Scholar]