Abstract
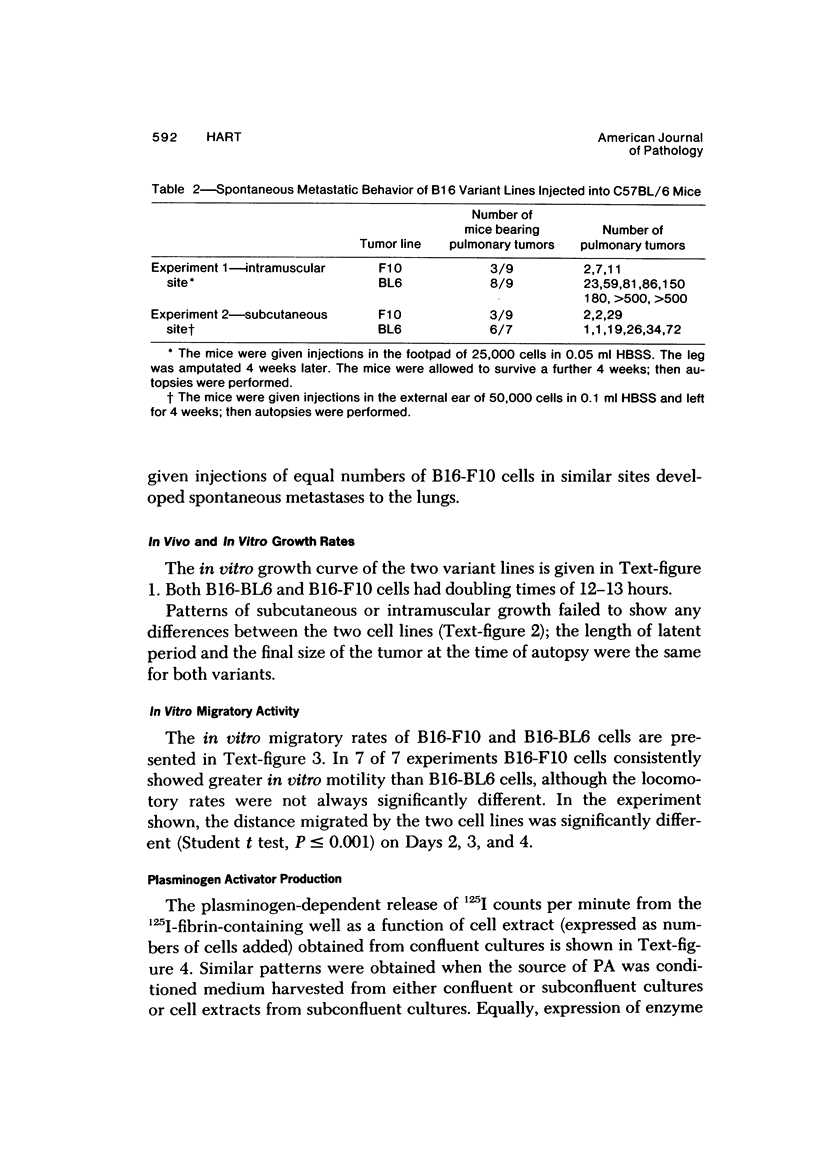
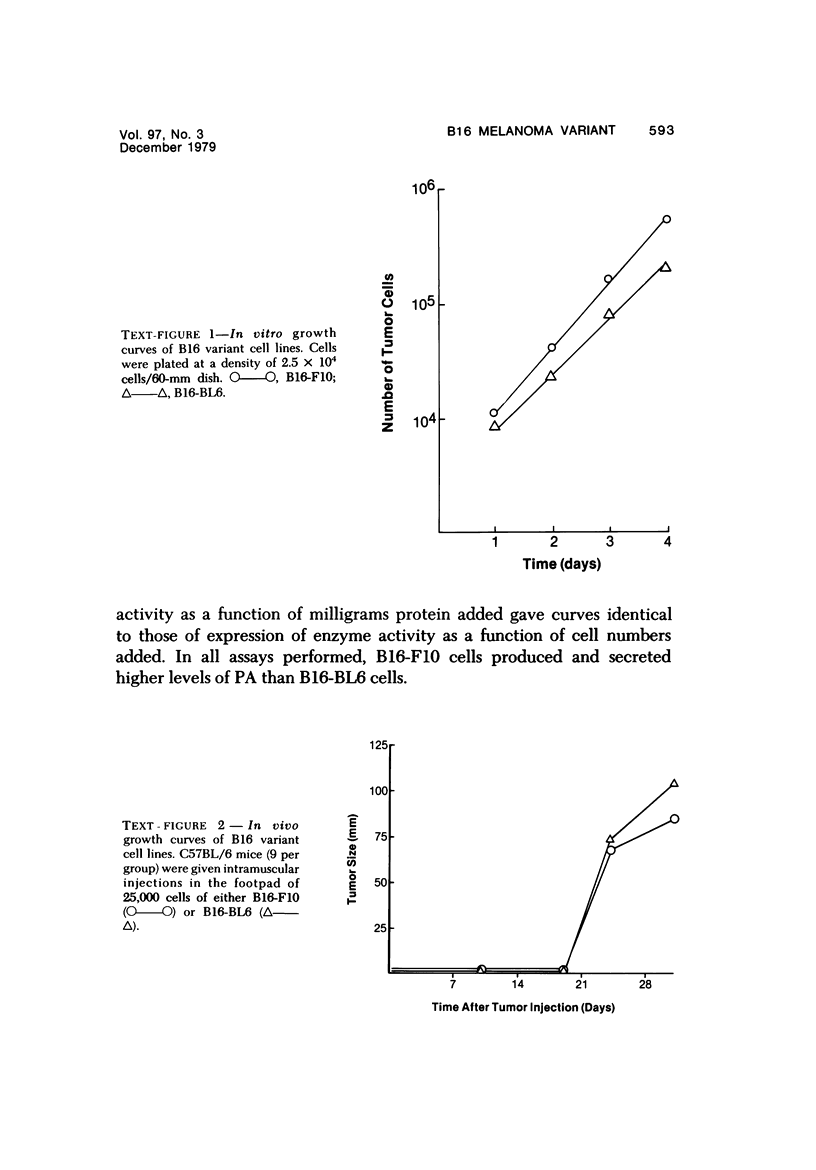
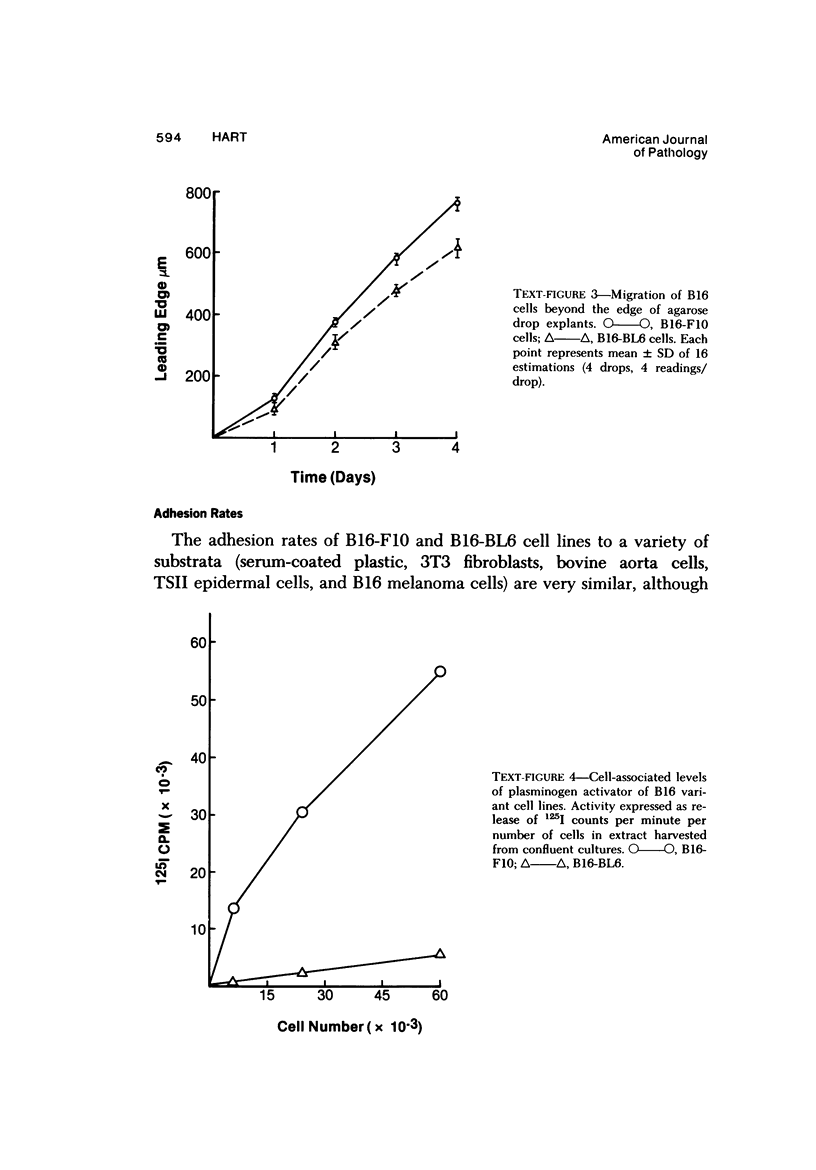
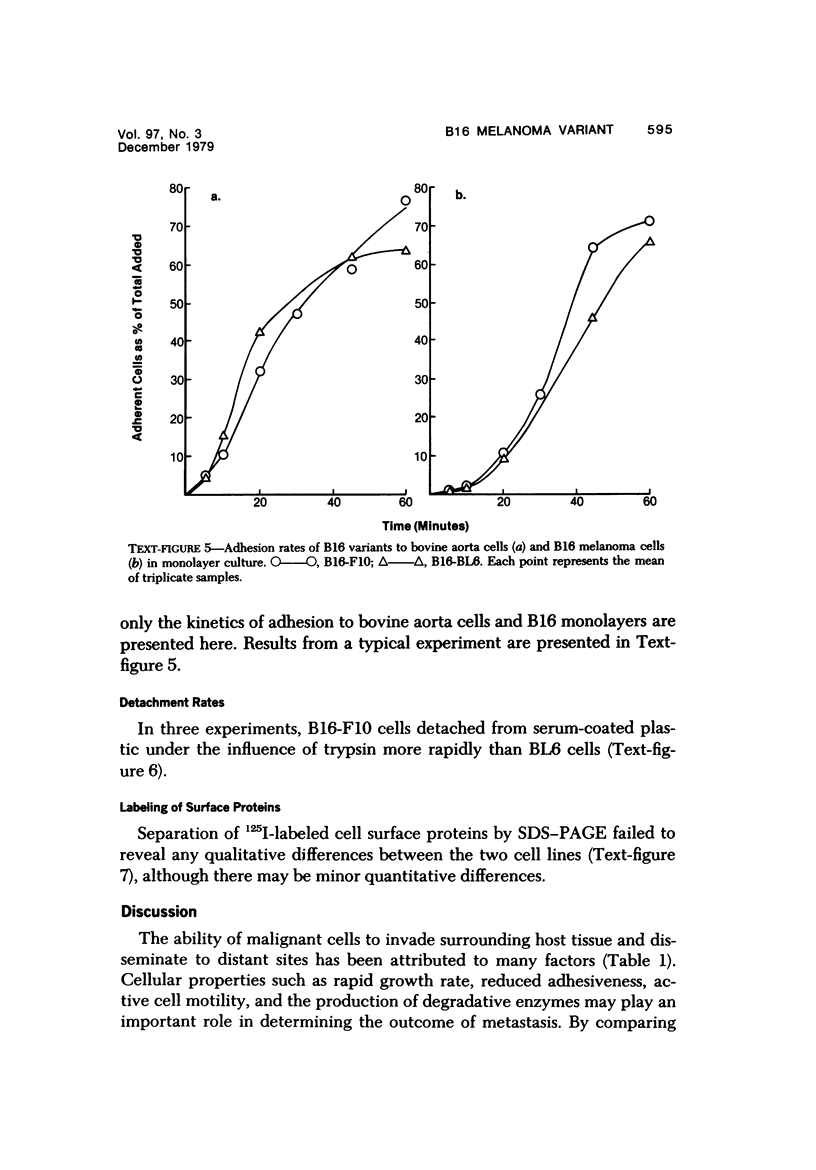
Several in vitro properties of two variant cell lines of the B16 melanoma (B16-F10 and B16-BL6) with markedly different spontaneous metastatic behavior were examined. The two cell lines were compared with regard to their in vitro growth rate, ability to migrate, ability to adhere to a variety of substrata, detachment rates, production of plasminogen activator, and cell surface proteins as determined by lactoperoxidase-catalyzed iodination. Growth rates in vitro, attachment rates, and qualitative patterns of cell surface proteins were almost identical. B16-F10 cells (the less spontaneously metastatic line) produced greater amounts of plasminogen activator, were more motile in vitro, and detached more readily from plastic than the more invasive B16-BL6 cells. The study of tumor cell variants, selected for different biologic behavior, is a valuable approach to the elucidation of those mechanisms responsible for their malignant activity.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briles E. B., Kornfeld S. Isolation and metastatic properties of detachment variants of B16 melanoma cells. J Natl Cancer Inst. 1978 Jun;60(6):1217–1222. doi: 10.1093/jnci/60.6.1217. [DOI] [PubMed] [Google Scholar]
- Brunson K. W., Beattie G., Nicolsin G. L. Selection and altered properties of brain-colonising metastatic melanoma. Nature. 1978 Apr 6;272(5653):543–545. doi: 10.1038/272543a0. [DOI] [PubMed] [Google Scholar]
- Eaves G. The invasive growth of malignant tumours as a purely mechanical process. J Pathol. 1973 Mar;109(3):233–237. doi: 10.1002/path.1711090308. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Kripke M. L. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977 Aug 26;197(4306):893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970 Oct;45(4):773–782. [PubMed] [Google Scholar]
- Fidler I. J., Nicolson G. L. Organ selectivity for implantation survival and growth of B16 melanoma variant tumor lines. J Natl Cancer Inst. 1976 Nov;57(5):1199–1202. doi: 10.1093/jnci/57.5.1199. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Selection of successive tumour lines for metastasis. Nat New Biol. 1973 Apr 4;242(118):148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 1978 Sep;38(9):2651–2660. [PubMed] [Google Scholar]
- Gershman H., Katzin W., Cook R. T. Mobility of cells from solid tumors. Int J Cancer. 1978 Mar 15;21(3):309–316. doi: 10.1002/ijc.2910210310. [DOI] [PubMed] [Google Scholar]
- Hagmar B. Defibrination and metastasis formation: effects of arvin on experimental metastases in mice. Eur J Cancer. 1972 Feb;8(1):17–28. doi: 10.1016/0014-2964(72)90079-5. [DOI] [PubMed] [Google Scholar]
- Haustein D., Marchalonis J. J., Harris A. W. Immunoglobulin of T lymphoma cells. Biosynthesis, surface representation, and partial characterization. Biochemistry. 1975 May 6;14(9):1826–1834. doi: 10.1021/bi00680a004. [DOI] [PubMed] [Google Scholar]
- Kripke M. L., Gruys E., Fidler I. J. Metastatic heterogeneity of cells from an ultraviolet light-induced murine fibrosarcoma of recent origin. Cancer Res. 1978 Sep;38(9):2962–2967. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liotta L. A., Kleinerman J., Catanzaro P., Rynbrandt D. Degradation of basement membrane by murine tumor cells. J Natl Cancer Inst. 1977 May;58(5):1427–1431. doi: 10.1093/jnci/58.5.1427. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Birdwell C. R., Brunson K. W., Robbins J. C., Beattie G., Fidler I. J. Cell interactions in the metastatic process: some cell surface properties associated with successful blood-borne tumor spread. Soc Gen Physiol Ser. 1977;32:225–241. [PubMed] [Google Scholar]
- Nicolson G. L., Brunson K. W., Fidler I. J. Specificity of arrest, survival, and growth of selected metastatic variant cell lines. Cancer Res. 1978 Nov;38(11 Pt 2):4105–4111. [PubMed] [Google Scholar]
- Noguchi P. D., Johnson J. B., O'Donnell R., Petricciani J. C. Chick embryonic skin as a rapid organ culture assay for cellular neoplasia. Science. 1978 Mar 3;199(4332):980–983. doi: 10.1126/science.203036. [DOI] [PubMed] [Google Scholar]
- Roblin R. O., Bell T. E., Young P. L. Assessment of plasminogen synthesis in vitro by mouse tumor cells using a competition radioimmunoassay for mouse plasminogen. Biochim Biophys Acta. 1978 Oct 18;543(3):383–396. doi: 10.1016/0304-4165(78)90056-9. [DOI] [PubMed] [Google Scholar]
- Varani J., Orr W., Ward P. A. A comparison of the migration patterns of normal and malignant cells in two assay systems. Am J Pathol. 1978 Jan;90(1):159–172. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Orr W., Ward P. A. Comparison of subpopulations of tumor cells with altered migratory activity, attachment characteristics, enzyme levels and in vivo behavior. Eur J Cancer. 1979 Apr;15(4):585–592. doi: 10.1016/0014-2964(79)90096-3. [DOI] [PubMed] [Google Scholar]
- WOODS J. R. EXPERIMENTAL STUDIES OF THE INTRAVASCULAR DISSEMINATION OF ASCITIC V2 CARCINOMA CELLS IN THE RABBIT, WITH SPECIAL REFERENCE TO FIBRINOGEN AND FIBRINOLYTIC AGENTS. Bull Schweiz Akad Med Wiss. 1964 May;20:92–121. [PubMed] [Google Scholar]
- Wilder R. L., Yuen C. C., Mage R. G. Lactoperoxidase catalyzed radioiodination of cell surface immunoglobulin: incorporated radioactivity may not reflect relative cell surface Ig density. J Immunol. 1979 Feb;122(2):459–463. [PubMed] [Google Scholar]


